Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12621
Revised: March 18, 2014
Accepted: June 14, 2014
Published online: September 21, 2014
AIM: To determine the optimal b value of diffusion-weighted imaging for detecting active inflammation in Crohn’s disease.
METHODS: Thirty-one patients clinically diagnosed with active Crohn’s disease were referred for magnetic resonance examination. All patients were scanned on a 3.0T magnetic resonance scanner using the same protocol involving four different b values (800, 1500, 2000 and 2500 s/mm2). The diagnostic effect of diffusion-weighted imaging was evaluated and compared with endoscopic findings. The diffusion-weighted image quality of four b value groups was evaluated and apparent diffusion coefficient was measured for both normal and inflammatory intestinal segments.
RESULTS: The contrast-to-noise ratio and signal-to-noise ratio were not satisfied when b value 2000 or 2500 s/mm2 was adopted (36.52 ± 14.95 vs 34.78 ± 24.83, P > 0.05; 53.58 ± 23.45 vs 47.58 ± 29.67, P > 0.05). The qualitative image quality was not enough to meet diagnostic requirement. No matter which b value was chosen, the apparent diffusion coefficient of inflammatory intestinal segments was significantly lower than that of normal intestinal segments (1.38 ± 0.28 vs 2.00 ± 0.38, P < 0.01; 1.09 ± 0.20 vs 1.50 ± 0.28, P < 0.01; 0.95 ± 0.19 vs 1.34 ± 0.28, P < 0.01; 0.88 ± 0.14 vs 1.20 ± 0.21, P < 0.01). The lesion detection rate (90.32%), diagnostic sensitivity (81.18%) and specificity (95.10%) would be appropriate when b value 1500 s/mm2 was adopted.
CONCLUSION: High b value is suitable for intestinal DW examination on a high field MR scanner.
Core tip: To date, nearly all the articles regarding diffusion-weighted imaging (DWI) utility in active Crohn’s disease chose relatively lower b values, and the examinations were finished on 1.5T magnetic resonance (MR) scanners. In our study, we try to investigate the appropriate b value of DWI on a high field MR scanner. During scanning, we adopted a low b value (800 s/mm2), a high b value (1500 s/mm2) and two very high b values (2000 and 2500 s/mm2). Our findings suggested that the b value of 1500 s/mm2 would be appropriate and when DWI was adopted on a 3.0T MR scanner, a high b value should be applied.
-
Citation: Feng Q, Yan YQ, Zhu J, Tong JL, Xu JR. Optimal
b value of diffusion-weighted imaging on a 3.0T magnetic resonance scanner in Crohn’s disease. World J Gastroenterol 2014; 20(35): 12621-12627 - URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12621.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12621
Crohn’s disease (CD) is a chronic and relapsing inflammatory disorder of unknown cause that affects mainly young people[1]. Because of its broad availability and high spatial resolution, computed tomography (CT) based imaging, especially CT enterography, has become the most widely used cross-sectional imaging technology for Crohn’s disease[2]. Growing concern about the flaw intrinsic to CT technology is the radiation utilized to generate the images. Magnetic resonance enterography, which offers the advantage of multi-planar capability and lacks ionizing radiation has proven to be a clinically useful technique for the evaluation of CD, particularly in younger patients[3]. Diffusion-weighted imaging (DWI) has been widely used for intracranial diseases and for the evaluation of various abdominal organs with increasing frequency in recent years[4-6]. It derives its image contrast from differences in the motion of water molecules between tissues[7]. The b value refers to the strength of the diffusion sensitizing gradient. The sensitivity of the diffusion sequence is adjusted by varying the b value, which is most readily achieved by altering the gradient amplitude.
High b value DWI was commonly used in the evaluation of the central nervous system. The b values applied in the stroke studies with DWI could reach 1500 to 5000 s/mm2[8]. In abdominal MR examinations, b value 2000 s/mm2 was reported in prostate cancer detection[9,10] and 1000 s/mm2 was reported in pancreatic neuroendocrine tumor diagnosis[11]. To date, there were only a few articles about the utility of DWI for patients with active Crohn’s disease. Each of them chose a relatively lower b value, and the examinations were finished on 1.5-T MR scanners. None of these articles adopted a b value higher than 1000 s/mm2[12-14].
The purpose of our study was to determine the optimal b value when DWI was adopted in 3.0-T MR scanning for detecting active inflammation in patients suffering from Crohn’s disease.
From September 2010 to October 2011, 31 CD patients clinically diagnosed with active inflammation were referred for MR examination. There were 20 men and 11 women enrolled in this study with a mean age of 28 years (range 17 to 63 years). Clinical activity was assessed based on the following criteria: (1) Crohn’s disease activity index > 150 (Table 1); (2) C-reactive protein (CRP) > 8 mg/L; and (3) erythrocyte sedimentation rate (ESR) > 20 mm/h. All the patients accepted small and large intestinal endoscopy. The small intestinal endoscopy was conducted via a per-anal route. Institutional review board approval was obtained and informed consent was waived for this retrospective study, which complied with the Health Insurance Portability and Accountability Act of 1996.
| Signs and symptoms | Multiplication factor |
| Number of liquid or very soft stools per day in a week | 2 |
| Abdominal pain (0 = none; 1 = mild; 2 = moderate; 3 = severe) | 5 |
| Number of complications | 20 |
| Taking loperamide/opiates for diarrhea (0 = no, 1 = yes) | 30 |
| Abdominal mass (0 = none, 2 = questionable, 5 = definite) | 10 |
| Hematocrit: male (47-Hct); female (42-Hct) | 6 |
| Percentage below predicted body weight | 1 |
| Evaluation | < 150: remission |
| 150-450: moderate-severe | |
| > 450: very serious |
The MR imaging examination was performed using a 3.0-T GE scanner (Signa Excite; GE HealthCare, Milwaukee, WI, United States). Patients fasted for 8 h before MR examinations and took 1000 mL solution of Polyethylene Glycol Electrolyte Powder (Wanhe Inc., Shenzhen, China) to clean the small and large intestine. About an hour before MR scanning, another 1000 mL solution of Polyethylene Glycol Electrolyte Powder was administered orally to each patient. Scopolamine 10 mg (Shanghai Xinyi Pharmaceuticals Co., Ltd., Shanghai, China) was administered intramuscularly 10 min before the examination started. All patients were routinely scanned with an 8-channel, phased-array body coil in the supine position. All sequences except T1W imaging were scanned using the respiratory-triggering technique.
T2-weighted single shot fast spin-echo images were acquired in axial and coronal planes. The scan parameters were as follows: TR/TE, 2000-3400/68 ms; slice thickness, 5 mm; interslice gap, 0 mm; matrix, 384 × 192; FOV, 42 cm × 25 cm; NEX, 0.6; SENSE factor, 2. Axial T1-weighted three-dimensional fast spoiled gradient echo was acquired with the breath-holding technique: TR/TE, 310/2.5 ms; slice thickness, 6 mm; interslice gap, 0 mm; matrix, 288 × 192; FOV, 35 cm × 25 cm; NEX, 0.5; SENSE factor, 2.
Diffusion-weighted MR images were acquired in the transverse plane using the single-shot echo planar imaging technique with parallel imaging and fat suppression (spectral attenuation inversion recovery). Scan parameters were as follows: TR/TE, 5820-6200/74-78 ms; slice thickness, 6 mm; interslice gap, 2 mm; matrix, 128 × 96; FOV, 40 cm × 28 cm; NEX, 4. The frequency direction was left to right. Diffusion-encoding gradients were applied as 5 b values from 0 to 2500 s/mm2 (0, 800, 1500, 2000 and 2500 s/mm2) along the three orthogonal directions of motion-probing gradients. The apparent diffusion coefficient (ADC) maps were automatically constructed on a pixel-by-pixel basis. Each DWI acquisition time was less than 5 min. The whole acquisition time of the entire examination for each patient was approximately 40 min depending on the patients’ respiratory rates.
Image evaluation was performed on a workstation (GE Healthcare, AW4.2). All images were evaluated by two experienced gastrointestinal radiologists (4 and 6 years of experience) who were both blinded to clinical details, as well as endoscopic results. For any disagreement in the data analysis, a joint reading session was performed to reach consensus.
Eight intestinal segments were evaluated for each patient: proximal ileum (30-130 cm up to the ileocecal valve), distal ileum (10-30 cm up to the ileocecal valve), terminal ileum (10 cm up to the ileocecal valve), ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. On DW images, the intestinal segment was evaluated as positive when it demonstrated significant high signal intensity on DWI and low signal intensity on ADC map. The DWI findings were compared with endoscopic results.
The manifestation of intestinal anatomic structure on DWI was evaluated on a three-point scale as follows: 0 = both inflammatory and normal intestinal wall structures were clear, 1 = inflammatory intestinal wall structure was clear while partial normal intestinal wall structure was vague, 2 = only partial positive or negative intestinal wall structure could be identified.
The signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) in all four different b value groups were measured. Image noise was measured from a large area (approximately 260 cm2) outside the abdominal parenchyma and defined as the standard deviation of background signal intensity. The signal contrast and ADCs were be measured on both normal and inflammatory bowel walls when these images were properly magnified, and region of interest (ROI) areas were be determined as large as possible. On DW images, ROIs were placed on the segments where the signal was the brightest.
Statistical analysis was performed using SPSS 11.5. Continuous variables are expressed as mean ± standard deviation and categorical variables as frequencies or percentages. The comparisons of CNR, SNR and ADCs were performed using one-way ANOVA. The diagnostic effect between different b value groups was assessed using the χ2 test.
In the current study, a total of 248 intestinal segments were evaluated, including 85 positive segments and 163 negative segments. The positive segments included 4 proximal ileum segments, 7 distal ileum segments, 15 terminal ileum segments, 14 ascending colon segments, 10 transverse colon segments, 11 descending colon segments, 11 sigmoid colon, and 13 rectum segments.
Considering the visual image quality of DWI, when b value 800 s/mm2 was adopted, all cases were evaluated as scale 0. When b value 1500 s/mm2 was chosen, 4 cases were evaluated as scale 1 and the other 27 cases as scale 0. When b value 2000 s/mm2 was chosen, 8 cases were evaluated as scale 1, while the other 23 cases were evaluated as scale 2. When b value 2500 s/mm2 was chosen, all cases were evaluated as scale 2.
Mean and standard deviation values of measured SNR and CNR are summarized in Tables 2 and 3. The SNR was decreased by 25.63% on b value 1500 s/mm2, 52.99% on b value 2000 s/mm2 and 58.26% on b value 2500 s/mm2 images vs that on b value 800 s/mm2 images. The CNR was decreased by 27.28% on b value 1500 s/mm2, 46.05% on b value 2000 s/mm2 and 56.14% on b value 2500 s/mm2 images vs that on b value 800 s/mm2 images. This finding suggested that significant differences did exist in mean SNR (F = 17.074, P < 0.01) and mean CNR (F = 14.920, P < 0.01), except for the group comparison between b value 2000 s/mm2 and 2500 s/mm2.
| b = 800 | b = 1500 | b = 2000 | b = 2500 | |
| b = 800 | 79.30 ± 34.59 | P = 0.006 | P < 0.01 | P < 0.01 |
| b = 1500 | P = 0.006 | 57.66 ± 23.95 | P = 0.007 | P = 0.003 |
| b = 2000 | P < 0.01 | P = 0.007 | 36.52 ± 14.95 | P = 0.820 |
| b = 2500 | P < 0.01 | P = 0.003 | P = 0.820 | 34.78 ± 24.83 |
| b = 800 | b = 1500 | b = 2000 | b = 2500 | |
| b = 800 | 113.98 ± 48.92 | P = 0.007 | P < 0.01 | P < 0.01 |
| b = 1500 | P = 0.007 | 84.77 ± 33.24 | P = 0.004 | P = 0.001 |
| b = 2000 | P < 0.01 | P = 0.004 | 53.58 ± 23.45 | P = 0.567 |
| b = 2500 | P < 0.01 | P = 0.001 | P = 0.567 | 47.58 ± 29.67 |
The ADCs are shown in Table 4. No matter which b value was chosen, the ADCs of the inflammatory intestinal segments were significantly lower than those of the normal segments.
| b value (s/mm2) | ADC value (×10-3mm2/s) | P value | |
| Inflammatory segments | Normal segments | ||
| 800 | 1.38 ± 0.28 | 2.00 ± 0.38 | < 0.01 |
| 1500 | 1.09 ± 0.20 | 1.50 ± 0.28 | < 0.01 |
| 2000 | 0.95 ± 0.19 | 1.34 ± 0.28 | < 0.01 |
| 2500 | 0.88 ± 0.14 | 1.20 ± 0.21 | < 0.01 |
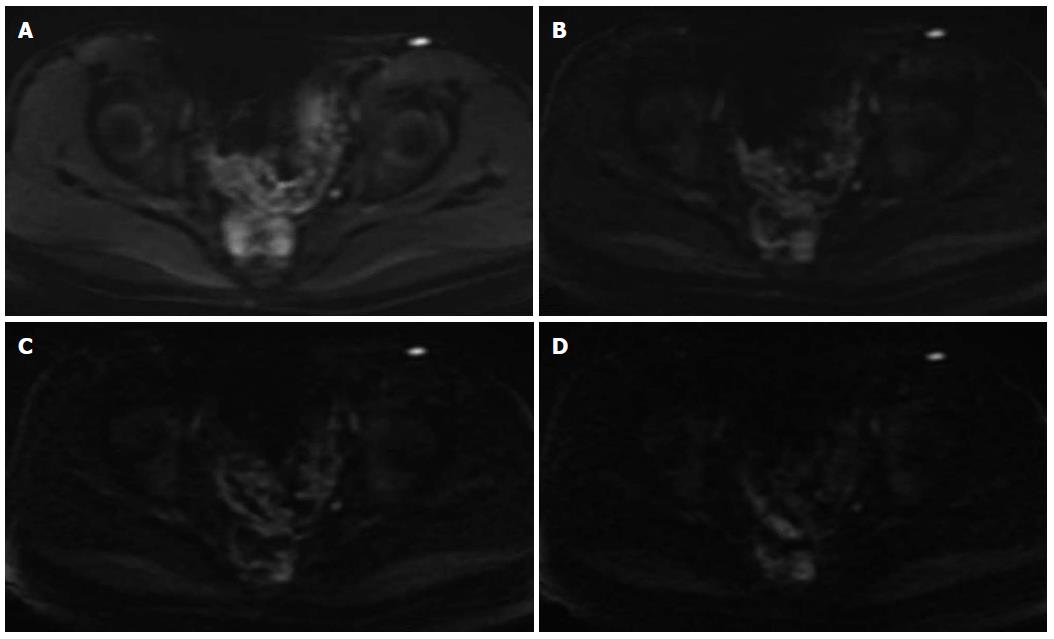
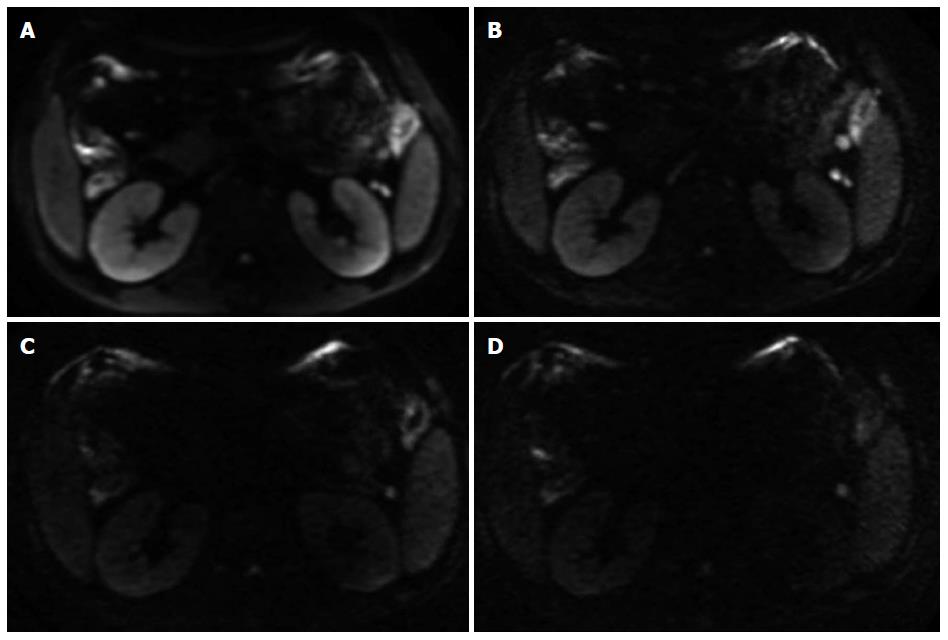
The diagnostic assessment of inflammatory segments on DW images is shown in Table 5. There was no significant difference in lesion detection rate between b value 800 s/mm2 and b value 1500 s/mm2 (χ2 = 1.288, P = 0.256), between b value 800 s/mm2 and b value 2000 s/mm2 (χ2 = 0, P = 1.0), or between b value 800 s/mm2 and b value 2500 s/mm2 (χ2 = 0.599, P = 0.439). There was also no significant difference between b value 1500 s/mm2 and b value 2000 s/mm2 (χ2 = 1.288, P = 0.256), or between b value 2000 s/mm2 and b value 2500 s/mm2 (χ2 = 0.599, P = 0.439) (Figures 1 and 2).
| b value (s/mm2) | TP | FP | FN | TN | Sensitivity | Specificity | Lesion detection rate |
| 800 | 71 | 20 | 12 | 145 | 83.53% | 88.96% | 87.10% |
| 1500 | 69 | 8 | 16 | 155 | 81.18% | 95.10% | 90.32% |
| 2000 | 62 | 9 | 23 | 154 | 72.94% | 94.48% | 87.10% |
| 2500 | 55 | 8 | 30 | 155 | 64.71% | 95.10% | 84.68% |
There was no significant difference in diagnostic sensitivity between b value 800 s/mm2 and b value 1500 s/mm2 (χ2 = 0.162, P = 0.687), between b value 800 s/mm2 and b value 2000 s/mm2 (χ2 = 2.798, P = 0.094), between b value 1500 s/mm2 and b value 2000 s/mm2 (χ2 = 1.630, P = 202), or between b value 2000 s/mm2 and b value 2500 s/mm2 (χ2 = 1.343, P = 0.246), while a significant difference did exist between b value 800 s/mm2 and b value 2500 s/mm2 (χ2 = 7.850, P = 0.005), and between b value 1500 s/mm2 and b value 2500 s/mm2 (χ2 = 5.842, P = 0.016). There was no significant difference in diagnostic specificity between b value 800 s/mm2 and b value 2000 s/mm2 (χ2 = 3.271, P = 0.071), between b value 1500 s/mm2 and b value 2000 s/mm2 (χ2 = 0.062, P = 0.803), between b value 1500 s/mm2 and b value 2500 s/mm2 (χ2 = 0, P = 1.0), or between b value 2000 s/mm2 and b value 2500 s/mm2 (χ2 = 0.062, P = 0.803), while a significant difference did exist between b value 800 s/mm2 and b value 1500 s/mm2, and between b value 800 s/mm2 and b value 2500 s/mm2 (χ2 = 4.179, P = 0.041).
The utility of DWI for gastrointestinal examination relatively falls behind the utility for other parenchymal organs in abdomen. So far, DWI is usually acquired in rectal cancer evaluation at relatively high b value (1000 s/mm2), especially during postoperative follow-up for the detection of local recurrence[15-17]. As far as the bowel inflammation was concerned, most examinations were completed using a 1.0- or 1.5-T MR scanner and the b values of DWI sequences were relatively lower. Oto et al[12] reported that dynamic contrast-enhanced MRI and DWI provide quantitative measures of small bowel inflammation that can differentiate active inflammatory small bowel segments from normal segments in CD patients with b values 0 and 600 s/mm2. It was also reported that three b values (0, 100 and 800 s/mm2) could be used with axial images through the upper and lower abdomen obtained in Crohn’s disease[18]. Neubauer et al[19] reported the utility of DWI in children and young adults suffering from Crohn’s disease with b values 50 and 800 s/mm2. It is obvious that the optimal b value of MR intestinal scanning was still in argument and no trial of b value higher than 1000 s/mm2 was conducted, especially on a high field MR scanner.
In this study, the ADCs of the active inflammatory segments were lower than those of the normal bowel segments in all DWI sequences. It was demonstrated that no matter which b value was chosen, a significant difference was detected (P < 0.01), although it is also suggested that there was no way to tell which b value was better through ADC measurement individually.
When quantitative analysis of image quality was concerned, it was confirmed that SNR and CNR were the best when b value 800 was chosen. When higher b values (2000 or 2500 s/mm2) were adopted, SNR and CNR of DWI images apparently declined. Burdette increased the number of excitations to maintain SNR at high b values[20], while we did not change the number of excitations in order to make reliable comparisons. When qualitative analysis of image quality was concerned, b = 2000 or 2500 s/mm2 was not satisfied for diagnostic requirement.
It was suggested that when low b value was chosen, the diagnostic sensitivity was higher while the specificity was lower. The diagnostic sensitivity decreased and the specificity increased when higher b value was adopted.
Kim et al[10] reported that DW imaging with a b value of 1000 s/mm2 was more sensitive and more accurate in localizing prostate cancer than DW imaging with a b value of 2000 s/mm2 on a 3.0-T MR scanner. However, some studies focused on high b value DWI in ischemic stroke supported that high b values might increase the diagnostic sensitivity in patients with hyper-acute infarction[21-23]. In our study, a b value of 800 s/mm2 had the best diagnostic sensitivity, while its false positive rate was also higher, which was due to the fact that the signal intensity of normal intestine was not suppressed enough. Therefore, although the image quality and diagnostic sensitivity were pretty good when b value 800 s/mm2 was adopted, the lesion detection rate was not good because its diagnostic specificity was not satisfied. The false high signal intensity of normal intestinal segments might be partially due to the mucus covering the intestinal mucosa. It is possible that the utility of higher b values might increase diffusion specificity by diminishing the hyper-intensity in intestinal lumen with long T2 relaxation times and suppress the high signal of intestinal wall caused by normal mucosa. When b value became higher and higher (1500 s/mm2 to 2500 s/mm2), diagnostic specificity was not apparently increased and diagnostic accuracy deceased because of the unsatisfied sensitivity. The reason might be that in some high b value cases (especially 2000 s/mm2 or 2500 s/mm2), the signal intensity, even that of inflammatory lesion was suppressed. The poor image quality could be part of the reasons, while the major contributor was still required to be explored. Overall, it was concluded that when a b value of 1500 s/mm2 was chosen, the diagnostic specificity and sensitivity were appropriate and the diagnostic accuracy was the highest (Figures 1 and 2).
In conclusion, a b value of 1500 s/mm2 offered the best balance of image quality and diagnostic requirement in this current study.
A limitation of this study was its relatively small sample size. Only 31 patients were included. Furthermore, the small intestinal endoscopy was conducted via a per-anal route, and the farthest reach was the proximal ileum not including the jejunum. So the diagnostic effect of DWI on active CD lesions in the upper digestive tract was still required to be discussed. In addition, only four b values were chosen in this study and the average interval was 500 to 700 s/mm2. It is possible that the best b value could be found around 1500 s/mm2 with a smaller interval.
Crohn’s disease (CD) is a chronic and relapsing inflammatory disorder of unknown cause that affects mainly young people. Because of its broad availability and high spatial resolution, computed tomography (CT) based imaging, especially CT enterography, has become the most widely used cross-sectional imaging technology for Crohn’s disease.
The utility of diffusion-weighted imaging (DWI) for gastrointestinal examination relatively falls behind the utility for other parenchymal organs in abdomen. So far, DWI is usually acquired in rectal cancer evaluation at relatively high b values (1000 s/mm2), especially during postoperative follow-up for the detection of local recurrence.
Authors try to investigate the appropriate b value of DWI on a high field MR scanner. During scanning, we adopted a low b value (800 s/mm2), a high b value (1500 s/mm2) and two very high b values (2000 and 2500 s/mm2). It was suggested that the b value of 1500 s/mm2 would be appropriate and when DWI was adopted on a 3.0-T MR scanner, a high b value should be applied. High b value is suitable for intestinal DW examination on high field MR scanners.
This is a small study of 30 patients who were scanned on a 3.0-T MR scanner for detecting colonic inflammation in patients with active CD. The author’s goal is to determine the optimal b value for those patients.
P- Reviewer: Economopoulos K, Liu F S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Scaldaferri F, Fiocchi C. Inflammatory bowel disease: progress and current concepts of etiopathogenesis. J Dig Dis. 2007;8:171-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Huprich JE, Fletcher JG. CT enterography: principles, technique and utility in Crohn’s disease. Eur J Radiol. 2009;69:393-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Tolan DJ, Greenhalgh R, Zealley IA, Halligan S, Taylor SA. MR enterographic manifestations of small bowel Crohn disease. Radiographics. 2010;30:367-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Taouli B, Tolia AJ, Losada M, Babb JS, Chan ES, Bannan MA, Tobias H. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007;189:799-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 287] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 5. | Matsuki M, Inada Y, Nakai G, Tatsugami F, Tanikake M, Narabayashi I, Masuda D, Arisaka Y, Takaori K, Tanigawa N. Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging. 2007;32:481-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Zhang J, Tehrani YM, Wang L, Ishill NM, Schwartz LH, Hricak H. Renal masses: characterization with diffusion-weighted MR imaging--a preliminary experience. Radiology. 2008;247:458-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Laurent V, Trausch G, Bruot O, Olivier P, Felblinger J, Régent D. Comparative study of two whole-body imaging techniques in the case of melanoma metastases: advantages of multi-contrast MRI examination including a diffusion-weighted sequence in comparison with PET-CT. Eur J Radiol. 2010;75:376-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Cihangiroglu M, Citci B, Kilickesmez O, Firat Z, Karlıkaya G, Uluğ AM, Bingol CA, Kovanlikaya I. The utility of high b-value DWI in evaluation of ischemic stroke at 3T. Eur J Radiol. 2011;78:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Ohgiya Y, Suyama J, Seino N, Hashizume T, Kawahara M, Sai S, Saiki M, Munechika J, Hirose M, Gokan T. Diagnostic accuracy of ultra-high-b-value 3.0-T diffusion-weighted MR imaging for detection of prostate cancer. Clin Imaging. 2012;36:526-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Kim CK, Park BK, Kim B. High-b-value diffusion-weighted imaging at 3 T to detect prostate cancer: comparisons between b values of 1,000 and 2,000 s/mm2. AJR Am J Roentgenol. 2010;194:W33-W37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Brenner R, Metens T, Bali M, Demetter P, Matos C. Pancreatic neuroendocrine tumor: added value of fusion of T2-weighted imaging and high b-value diffusion-weighted imaging for tumor detection. Eur J Radiol. 2012;81:e746-e749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol. 2009;16:597-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 13. | Kiryu S, Dodanuki K, Takao H, Watanabe M, Inoue Y, Takazoe M, Sahara R, Unuma K, Ohtomo K. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging. 2009;29:880-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Oto A, Kayhan A, Williams JT, Fan X, Yun L, Arkani S, Rubin DT. Active Crohn’s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging. 2011;33:615-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Kilickesmez O, Atilla S, Soylu A, Tasdelen N, Bayramoglu S, Cimilli T, Gurmen N. Diffusion-weighted imaging of the rectosigmoid colon: preliminary findings. J Comput Assist Tomogr. 2009;33:863-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Sani F, Foresti M, Parmiggiani A, D’Andrea V, Manenti A, Amorotti C, Scotti R, Gallo E, Torricelli P. 3-T MRI with phased-array surface coil in the local staging of rectal cancer. Radiol Med. 2011;116:375-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Lambregts DM, Cappendijk VC, Maas M, Beets GL, Beets-Tan RG. Value of MRI and diffusion-weighted MRI for the diagnosis of locally recurrent rectal cancer. Eur Radiol. 2011;21:1250-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Griffin N, Grant LA, Anderson S, Irving P, Sanderson J. Small bowel MR enterography: problem solving in Crohn’s disease. Insights Imaging. 2012;3:251-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Neubauer H, Pabst T, Dick A, Machann W, Evangelista L, Wirth C, Köstler H, Hahn D, Beer M. Small-bowel MRI in children and young adults with Crohn disease: retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr Radiol. 2013;43:103-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Burdette JH, Elster AD. Diffusion-weighted imaging of cerebral infarctions: are higher B values better? J Comput Assist Tomogr. 2002;26:622-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Kim HJ, Choi CG, Lee DH, Lee JH, Kim SJ, Suh DC. High-b-value diffusion-weighted MR imaging of hyperacute ischemic stroke at 1.5T. AJNR Am J Neuroradiol. 2005;26:208-215. [PubMed] [Cited in This Article: ] |
| 22. | Meyer JR, Gutierrez A, Mock B, Hebron D, Prager JM, Gorey MT, Homer D. High-b-value diffusion-weighted MR imaging of suspected brain infarction. AJNR Am J Neuroradiol. 2000;21:1821-1829. [PubMed] [Cited in This Article: ] |
| 23. | Toyoda K, Kitai S, Ida M, Suga S, Aoyagi Y, Fukuda K. Usefulness of high-b-value diffusion-weighted imaging in acute cerebral infarction. Eur Radiol. 2007;17:1212-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |