Published online Oct 14, 2014. doi: 10.3748/wjg.v20.i38.13863
Revised: May 24, 2014
Accepted: June 26, 2014
Published online: October 14, 2014
Patients with inflammatory bowel disease (IBD) have an increased risk of vascular complications. Thromboembolic complications, both venous and arterial, are serious extraintestinal manifestations complicating the course of IBD and can lead to significant morbidity and mortality. Patients with IBD are more prone to thromboembolic complications and IBD per se is a risk factor for thromboembolic disease. Data suggest that thrombosis is a specific feature of IBD that can be involved in both the occurrence of thromboembolic events and the pathogenesis of the disease. The exact etiology for this special association between IBD and thromboembolism is as yet unknown, but it is thought that multiple acquired and inherited factors are interacting and producing the increased tendency for thrombosis in the local intestinal microvasculature, as well as in the systemic circulation. Clinicians’ awareness of the risks, and their ability to promptly diagnose and manage tromboembolic complications are of vital importance. In this review we discuss how thromboembolic disease is related to IBD, specifically focusing on: (1) the epidemiology and clinical features of thromboembolic complications in IBD; (2) the pathophysiology of thrombosis in IBD; and (3) strategies for the prevention and management of thromboembolic complications in IBD patients.
Core tip: Thromboembolic complications, both venous and arterial, are serious and challenging complications of inflammatory bowel disease (IBD) and can lead to significant morbidity and mortality. Thrombosis is a specific feature of IBD that can be involved in both the occurrence of thromboembolic events and the pathogenesis of the disease itself. The cause for this association between IBD and thromboembolism is as yet unknown, but multiple acquired and inherited factors have been implicated. Clinicians’ awareness of the risks, and knowledge about the diagnosis and management of thromboembolic complications are of vital importance.
- Citation: Zezos P, Kouklakis G, Saibil F. Inflammatory bowel disease and thromboembolism. World J Gastroenterol 2014; 20(38): 13863-13878
- URL: https://www.wjgnet.com/1007-9327/full/v20/i38/13863.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i38.13863
Thromboembolic events, both venous and arterial, are serious extra-intestinal manifestations complicating the course of inflammatory bowel disease (IBD) and can lead to significant morbidity and mortality. The increasing evidence that patients suffering from both Crohn’s disease (CD) and ulcerative colitis (UC) are more prone to thromboembolic complications compared to the general population implicates the IBD per se as a risk factor[1,2]. Moreover, recent data support the theory that thrombosis and thromboembolism are disease-specific manifestations in IBD, and that they also may be contributing factors in the pathogenesis of the luminal disease. In this review we discuss how thromboembolic disease is related to IBD, specifically, the following: (1) the epidemiology and clinical features of thromboembolic complications in IBD; (2) the pathophysiology of thrombosis in IBD; and (3) strategies for the management, prevention and treatment, of thromboembolic complications in IBD patients.
Historically, in 1936, Bargen and Barker[3] first reported arterial and venous thrombotic complications in UC patients. In a large Mayo Clinic survey, Talbot et al[4] found that 1.3% of their IBD patients manifested thromboembolic complications, while other early studies reported an even higher incidence, up to 7%[5-8]. Moreover, an important and interesting observation came from autopsy studies which found a much higher incidence of venous thromboembolic complications, up to 39%, in UC patients, implying that most of the thromboembolic episodes were either not clinically overt or were overlooked. The thromboembolic complications were the third leading cause of death (10%) in those patients[9].
Thrombosis occurs more often in the deep veins of the legs and the pulmonary circulation[4]; however, arterial thromboembolic complications (ATEs) and numerous other less frequent sites of venous thrombosis have also been described, including cerebrovascular (CVA) disease[10,11], internal carotid artery occlusion[12], mesenteric and portal vein thrombosis[13], Budd-Chiari syndrome[14], cutaneous gangrene secondary to microvascular thrombosis[15], retinal vein occlusion[16-18], and ischemic heart disease (IHD)[19].
Over the past decade in particular, many large case-controlled and cohort studies, in centers throughout the world, have focused on defining the association of IBD with the risk of venous thromboembolism (VTE) and have contributed to significant progress in clarifying the epidemiological and the clinical features of VTEs in IBD[1,2,20].
Some assessed the incidence and the risk of VTE in IBD patients compared to the general population[21-25], while others evaluated the risk of VTE in hospitalized IBD patients compared to hospitalized non-IBD patients[26-30]. A few studies were more focused, and analyzed the risk of VTE in pregnant females with IBD[31,32], the risk of VTE in postoperative IBD patients[33,34] and, finally, one study evaluated the risk of recurrent DVT in adult IBD patients[35].
The overall risk of VTEs, deep vein thrombosis (DVT) and pulmonary embolism (PE), in IBD patients has been estimated in two recent meta-analyses[1,36]. Despite the heterogeneity and the limitations of the studies included, both meta-analyses revealed an approximately 2-fold increased risk for VTEs in IBD patients. Yuhara et al[36], reported that the overall relative risk (RR) for DVT and PE in patients with IBD compared to subjects without IBD was 2.20 (95%CI: 1.83-2.65), and Fumery et al[1] reported that the overall risk of VTE in IBD patients was increased by 96% compared to the general population (RR = 1.96; 95%CI: 1.67-2.30) with no differences between CD and UC patients.
In a recent nationwide multicenter study conducted in Austria, Papay et al[37] investigated the prevalence and the incidence of VTEs in 2811 IBD patients and described many related clinical features. The overall prevalence of all VTEs was 5.6% (157/2811) and the incidence of all VTEs was 6.3/1000 person years. The majority of VTEs were DVT and/or PE (about 90%; 142/157), while other locations of venous thrombosis were rare (about 10%; 15/157) including the portal, the superior mesenteric, the splenic, the internal jugular, and the cerebral veins. No difference was found between CD and UC for the frequency of all VTEs, although the prevalence and incidence for DVT and/or PE was a little higher in CD patients.
VTEs occur earlier in life in IBD patients than in non-IBD thrombotic patients[21,26,38,39]. Bernstein et al[21] analyzed data from IBD and non-IBD hospitalized patients and found that the risk of VTE, DVT and/or PE, was overall higher in hospitalized IBD patients. The most striking difference in the risk was observed in patients who were less than 40 years of age, with an incidence rate ratio (IRR) for VTE of 4.5 for UC and 9.6 for CD compared with the non-IBD patients.
Thromboembolic complications are a significant cause of morbidity and mortality in IBD patients[39-42]. In the study by Talbot et al[4], 25% of IBD patients with thromboembolic complications had a fatal outcome during the thrombotic episode. Recently, Nguyen and Sam[27], also reported higher rates of VTEs in hospitalized IBD patients than in non-IBD hospitalized patients. As was the case in Bernstein’s report, those who were less than 40 years of age were at the greatest risk. Hospitalized IBD patients with thrombosis had a greater in-hospital mortality risk when compared to hospitalized IBD patients without thrombosis (OR = 2.5; 95%CI: 1.83-3.43) and to non-IBD hospitalized patients with thrombosis (OR = 2.1; 95%CI: 1.6-2.9). In addition, the occurrence of VTEs in hospitalized IBD patients significantly increased the length of hospital stay and health resource utilization cost.
Patients with IBD are at increased risk for postoperative VTE[43]. Merill and Millham[33] reported that IBD patients were at increased risk for developing postoperative DVT or PE compared to non-IBD patients, especially after non-intestinal surgery. Furthermore, Wallaert at al[34] studied VTE during the first 30 postoperative days in a large cohort of IBD patients having colorectal surgery (10431 patients, 5001 with UC and 5430 with CD) and found an overall incidence of 2.3% for VTEs (242 VTEs; 178 DVTs and 46 PEs). The rates of VTEs were higher for UC patients compared to CD patients (3.3% vs 1.4% respectively). The thromboembolic episodes occurred at an average of 10 d postoperatively and were associated with significant morbidity and mortality.
Recurrence of thromboembolic events has been previously reported as being 10%-13% in IBD patients[4,39]. Novacek et al[35] reported an approximately 30% probability of VTE recurrence in IBD patients 5 years after discontinuation of the anticoagulant treatment for the first VTE. The risk for recurrent VTE was higher in IBD patients compared to non-IBD patients. IBD, irrespectively of the activity status, was found to be an independent risk factor for recurrent VTE with a relative risk of 2.5 (95%CI: 1.4-4.2). In accordance with that, Papay et al[37] reported a similar incidence of recurrent VTEs (25%) in IBD patients; in the majority, the VTEs occurred in the same location (70%) and were of the same type (DVT or PE) as with the first episode.
Thromboembolic events are more frequent during active phases of IBD and correlate with the extent and location of the disease; most of them occur without evidence of provoking factors[4,22,40,44]. Complicated IBD (fistula, stenosis, abscess)[22,27], use of corticosteroids[24], and recent hospitalization for IBD[24] were all associated with increased risk for VTEs. Solem et al[40] reported that 80% of IBD patients (both CD and UC) had active disease at the time of VTE. Regarding the extent of disease in patients with VTE, 76% of the UC patients had pancolitis and 79% of the CD patients had colonic involvement. In contrast to the above, Talbot et al[4] found that almost 30% of VTEs occurred when the disease was in remission and that 77% of the peripheral VTEs occurred spontaneously. In a recent study, Grainge et al[25] assessed the risk of VTE at various activity phases of IBD (flare, chronic activity, remission) in a retrospective cohort study of 13756 IBD patients and 71672 matched controls from the prospectively generated General Practice Research Database (United Kingdom). They found that there is a significantly increased overall risk of VTE in IBD patients compared to controls during all phases of IBD (HR = 3.4; 95%CI: 2.7-4.3). The risk was most prominently increased during a flare (HR = 8.4; 95%CI: 5.5-12.8) compared with periods of chronic activity (HR = 6.5; 95%CI: 4.6-9) and periods of clinical remission (HR = 2.1; 95%CI: 1.6-2). In this unique study, the overall relative risk of VTEs in ambulatory IBD patients compared to controls appeared to be higher than in hospitalized IBD patients compared to controls (HR = 4.3; 95%CI: 3.3-5.7 vs HR = 2.1; 95%CI: 1.4-3.2). This apparent difference in the risk between ambulatory and hospitalized IBD patients compared to controls was even higher during periods of disease flares (HR = 15.8 vs 3.2, respectively). However, when the data were expressed as absolute risk of VTEs per 1000-person years it was obvious that the hospitalized IBD patients were more prone to thrombosis (25.2) than the non-hospitalized IBD patients (1.8), especially during a flare of the disease (37.5 vs 6.4, respectively). Finally, the risk of VTEs was higher during flares and chronic activity periods compared to periods of remission of the disease, both in ambulatory and hospitalized IBD patients. These data are further supported by the recent study of Papay et al[37] who reported that 77% of VTEs in the IBD cohort occurred spontaneously, 77% occurred in outpatients and 66% occurred during an active period of the disease.
Collectively, all the recent studies discussed above confirm that patients with both CD and UC are at an approximately two-fold increased risk for VTEs compared to the general population or to non-IBD patients. The VTEs, mainly DVT and/or PE, tend to occur spontaneously, at a younger age, and more frequently during periods of active disease, in both ambulatory and hospitalized patients. There is also increased risk during periods of remission, during pregnancy, and postoperatively. They also recur frequently after the first episode. The VTEs in IBD patients are associated with significant morbidity and mortality (Table 1).
| Venous thromboembolism and IBD |
| Prevalence: 1.3%-7% - postmortem about 40% |
| Risk overall: about 2-3-fold |
| Features |
| Deep vein thrombosis (legs) and pulmonary embolism |
| Younger age |
| Spontaneously |
| Recur - 30% (risk about 2.5-fold) |
| Significant morbidity and mortality |
| Risk factors |
| Active disease (ambulatory and hospitalized patients) |
| Complicated disease |
| Corticosteroid use |
| Extensive colonic involvement (UC and CD) |
| Recent hospitalization |
| Surgery |
| Pregnancy |
| Previous history of VTE |
| Family history of VTE |
Numerous cases and case series have reported ATEs in IBD patients. In general, ATEs occur less frequently than VTEs in IBD patients, and may involve the thrombosis and/or occlusion of the cerebral[11,16], retinal[16,17], carotid[12,45,46], coronary[19], splanchnic[47], iliac[48,49], renal[48,49], and limb (upper and lower)[50,51] arteries or the aorta[48,49,52]. They are more common after interventional or surgical procedures, but they can also occur spontaneously[4,51].
Recent studies[53-59] and a meta-analysis[60] provide evidence for an association between IBD and ATE, similar to that which exists with VTEs, despite the fact there are some controversial findings in other studies[30,61,62]. In their meta-analysis Singh et al[60] analyzed data from 9 studies and found that IBD was associated with a modest increase for the risk of cardiovascular morbidity. In particular, 5 studies reported 2424 CVA events in 98240 patients with IBD and six studies reported 6478 occurrences of IHD in 123907 patients with IBD, which translates to a modest 18% increase in the overall risk for both CVA (adjusted OR = 1.18; 95%CI: 1.09-1.27) and IHD (adjusted OR = 1.18; 95%CI: 1.08-1.31). In addition, the risk was higher in females and young patients (age < 40-50 years). There were no differences between UC and CD patients. Finally, 2 studies reported 148 patients with peripheral arterial disease in 25559 patients with IBD, but the analyses showed that IBD was not associated with a significant increase for the risk of peripheral arterial disease (adjusted OR = 1.15; 95%CI: 0.96-1.38).
A nationwide United States study[30], which investigated the association of cardiovascular diseases in 148229 hospitalized subjects with IBD compared to 17261952 controls, showed a significantly increased risk for mesenteric ischemia (adjusted OR = 3.4; 95%CI: 2.9-4.0) and thromboembolic disease in hospitalized IBD patients. Fumery et al[1], in their meta-analysis, concluded that overall IBD was associated with an increased risk of thrombovascular events. The major risks were for VTE and mesenteric ischemia and, to a lesser degree, for arterial thromboembolism and ischemic heart disease. Although they did not find an increase in the risk of cardiovascular mortality in IBD patients, Kristensen et al[54], who investigated the risk of myocardial infarction (MI), stroke, and cardiovascular death in patients with IBD with correlation to disease activity in a nationwide Danish cohort, reported that the IBD is associated with increased risk of MI, stroke, and cardiovascular death during periods with active disease, including acute flares or persistent activity.
To summarize, recent data show that patients with IBD, both CD and UC, are at an increased risk for ATEs, mainly CVA, IHD and mesenteric ischemia, albeit to a lesser degree than for VTEs. The ATEs tend to occur spontaneously or post-surgically, at a younger age, in females, more frequently during periods of active disease and are associated with significant morbidity and mortality (Table 2).
| Arterial thromboembolism and IBD |
| Common sites and risk |
| Cerebrovascular events about 1.2-fold |
| Ischemic heart disease about 1.2-fold |
| Mesenteric ischemia about 3.5-fold |
| Features |
| Younger age |
| Female |
| Post-surgically >> spontaneously |
| Active disease (ambulatory and hospitalized patients) |
| Significant morbidity and mortality |
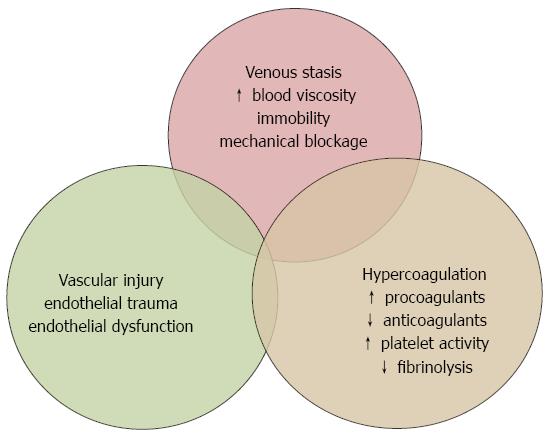
In contrast to hemostasis, which is a normal response to vascular injury, thrombosis is pathological coagulation occurring spontaneously or following a minimal vascular injury. The underlying cause of thrombosis is an imbalance between prothrombotic and antithrombotic mechanisms. The tendency towards thrombosis is related to three basic mechanisms, as defined by the Virchow’s triad: vascular stasis, endothelial injury/vascular damage and hypercoagulability (Figure 1).
Evidence from the literature suggests that thrombosis is a specific feature of IBD that is involved in both the occurrence of thromboembolic events and the pathogenesis of the disease itself[44]. Multifocal vascular infarcts in the intestinal microcirculation, characterized by chronic vasculitis, with focal arteritis and fibrin deposition, have been reported in patients with CD[63]. Histological studies have also found mucosal capillary thrombi in patients with UC[64]. In addition, Thompson et al[65], in a large study involving 129 hemophilia centers in United Kingdom, reported a lower than expected incidence of IBD in 9562 patients with hemophilia or von Willebrand’s disease and concluded that a congenital bleeding diathesis may have a protective role against the development of IBD. Furthermore, Miehsler et al[22] demonstrated that IBD per se is a risk factor for thromboembolic and concluded that thromboembolism is a specific feature of IBD since neither rheumatoid arthritis, another chronic inflammatory disease, nor coeliac disease, another chronic bowel disease, had an increased risk of thromboembolism.
The exact etiology for the higher occurrence of thromboembolism in IBD and the specific association between them is yet unknown, though it seems that multiple acquired and inherited factors may be involved (Table 3).
| Factors | Mechanism |
| Acquired | |
| Inflammation | Hypercoagulation, vascular endothelial injury |
| Immobilization | Stasis |
| Indwelling IV catheters | Vascular injury |
| Dehydration | Stasis |
| Steroid use | Hypercoagulation |
| Oral contraceptives | Hypercoagulation |
| Surgery | Stasis, hypercoagulation, vascular injury |
| Pregnancy | Stasis, hypercoagulation |
| Cancer | Hypercoagulation |
| Infections | Hypercoagulation |
| Age | Hypercoagulation |
| Smoking | Hypercoagulation |
| Hereditary | |
| Proteins C and S deficiencies | Hypercoagulation |
| Antithrombin deficiency | Hypercoagulation |
| Factor V Leiden | Hypercoagulation |
| Hyperhomocysteinemia-MTHFR gene mutation | Hypercoagulation |
| Prothrombin gene mutation G20210A | Hypercoagulation |
| Dysfibrinogenemia | Hypercoagulation |
General acquired prothrombotic factors such as inflammation, older age, surgery, prolonged immobilization, central venous catheters, fluid depletion, steroid therapy, smoking, and oral contraceptives are frequently observed in IBD patients, but their presence cannot adequately explain the increased risk for thromboembolisms in IBD[44]. On the other hand, many studies and reviews have failed to establish a significant association of the inherited thrombophilias, such as factor V Leiden, prothrombin G20210A mutation, MTHFR mutation-related hyperhomocysteinemia, protein C, S and antithrombin deficiencies, with the increased risk of thrombosis in IBD patients, although their co-existence with IBD has a synergistic role in thromboembolic complications[44,66,67]. Multiple risk factors are often present in IBD patients[40]; although none of them is more significant than the others, it seems obvious that as more risk factors accumulate in a patient, thrombosis is more likely to occur in that patient.
Inflammation, both intestinal and systemic, is the prominent feature in IBD. Inflammation and thrombosis are probably interrelated in IBD, through complex and as yet not fully understood pathways. Consequently, local and systemic intravascular hypercoagulable and prothrombotic states or even frank thrombosis, may represent contributing underlying factors in IBD pathogenesis[68].
The hypercoagulable state has been associated particularly with active disease[69]. Several studies have reported abnormalities in various components of hemostasis and the coagulation cascade during exacerbations of IBD (Table 4), as follows: (1) elevated levels of coagulation factors (V, VIII, von Willebrand, and fibrinogen) and products of thrombin and fibrin formation (fibrinopeptide A, prothrombin fragment 1+2 [F1+2], thrombin-antithrombin complex [TAT], and D-Dimers)[70-74]; (2) increased markers of vascular endothelial activation (von Willebrand factor and thrombomodulin)[74-78]; (3) acquired deficiencies and dysfunction of natural anticoagulants (protein C, protein S, and antithrombin)[79-82]; (4) defects in the fibrinolytic system [low levels of tissue plasminogen activator (t-PA), high levels of plasminogen-activator inhibitor type-1 (PAI-1)][83,84]; and (5) elevated number of circulating platelets, platelet activation and increased platelet aggregation tendency[85-87]. However, in other studies, activation of coagulation was observed both in active and inactive IBD[88-90], an observation that is in accordance with the occurrence of thromboembolic complications even in IBD patients with quiescent disease.
| Category | Abnormality |
| Coagulation factors | ↑ V, VIII, vWf, and fibrinogen |
| Products of thrombin generation | ↑ F1 + 2, TAT |
| Products of fibrin formation | ↑ fibrinopeptide A, D-Dimers |
| Vascular endothelium activation | ↑ vWf, thrombomodulin |
| Acquired deficiencies and dysfunction of natural anticoagulants | ↓ protein C, protein S, and AT |
| Defects in fibrinolytic system | ↓ t-PA |
| ↑ PAI-1 | |
| Platelets | ↑ number, activation and aggregation |
The hypercoagulable state in IBD has recently been reviewed thoroughly elsewhere[67]. It can be postulated that, in IBD patients, a persistent latent activation of hemostasis exists in both active and inactive disease states, and is implicated in the thrombotic diathesis and perhaps in disease pathogenesis. Hence, two questions emerge: what is the underlying mechanism for the abnormal hemostasis activation and why do clinically overt thromboembolic complications occur only in a relatively small fraction of IBD patients? A possible explanation for the latter question is the fact that for a thromboembolic event to occur, hypercoagulability alone is not sufficient, and that many other predisposing risk factors have to be present at the same time. On the other hand, for the former question to be answered, one must search deeper into the pathophysiology of intestinal inflammation.
As a result of the chronic inflammation in IBD patients, abnormalities exist in both the local intestinal microvasculature and the systemic circulation. Bargen and Barker, almost 80 years ago, stated that, in a subgroup of UC patients, the disease should be described using the term “thrombo-UC”[3]. Histological studies have revealed vasculitis in a subgroup of UC patients[91], while other studies have shown mucosal capillary thrombi in rectal biopsies from UC and CD patients[64], although this finding is not specific for IBD[92]. Wakefield et al[63], proposed that multifocal infarcts in the intestinal microcirculation caused by arteritis with fibrin deposition due to focal vasculitis, might be implicated in CD pathogenesis. Moreover, the proximal demarcation line between involved and uninvolved colon in UC suggests that a microvascular abnormality may be associated with the pathogenesis and the extent of inflammation in UC[93].
The hemostatic and the inflammatory pathways are closely related in a bi-directional fashion, and the vascular endothelium has been proven to be the interface of their interactions[68,94-97]. The “vascular hypothesis” suggests that endothelial dysfunction in the intestinal microcirculation plays central role in both UC and CD pathogenesis[98-100]. Furthermore, the vascular endothelial dysfunction associated with chronic inflammation is critically involved with the hypercoagulable state and the development of thrombosis and atherosclerosis in IBD patients, and clinically manifested as systemic vascular (venous and arterial) thromboembolic complications or IHD[99-101]. Normally, the “quiescent” intact endothelium exhibits a strong thrombo-resistant surface, expressing antiplatelet, anticoagulant and fibrinolytic properties. An “activated” endothelium is rapidly transformed into a prothrombotic surface, which promotes blood coagulation, inhibits fibrinolysis and activates platelets. The transformation of the vascular endothelial surface from anti-coagulant to pro-coagulant is triggered by mechanical damage, or by perturbation and activation of the vascular endothelial cells. Agents including cytokines, endotoxins, blood mediators, hypoxia, and hemodynamic forces are involved in endothelial cell activation[102].
Inflammation turns the “quiescent” endothelium into a potent pro-coagulant surface. Interleukin-1 (IL-1), tumor-necrosis factor-α (TNF-α) and other cytokines, which are increased in IBD, are responsible for this pro-coagulant, thrombophilic effect, and increase both white cell and platelet adhesion molecules on the endothelial surface. Many studies suggest that IL-1, TNF-α and other pro-inflammatory cytokines, increase various thrombophilic factors and have a significant contribution in intravascular thrombosis[103,104].
On the other hand, both thrombosis and “activated” endothelium can promote inflammation. The central role of the endothelial cell in initiation and propagation of inflammation takes place through the recruitment of leukocytes by cell adhesion molecules. The expression of cell adhesion molecules on the endothelial surface is induced by IL-1, TNF-α, and other proinflammatory cytokines. In IBD, the activated endothelial cells express increased surface levels of various intercellular adhesion molecules, such as ICAM-1 (intercellular adhesion molecule-1) and VCAM-1 (vascular cell adhesion molecule-1)[105]. PECAM-1 (platelet endothelial adhesion molecule-1) is expressed in high levels even in areas of the colon not affected by UC[106]. The selectin family (E-, P-, L- selectins), which is involved in leukocyte rolling on the endothelial surface, is increased in IBD[107]. Furthermore, the CD40/CD40L co-stimulatory pathway, which is involved in inflammation and coagulation, is activated in IBD tissue. In active IBD, CD40 is overexpressed in the microvascular endothelial cells, CD40L is overexpressed in platelets and leukocytes, and the soluble form of CD40L (sCD40L) is increased in the circulation in patients with active disease. The contact of the CD40L+ leukocytes and platelets with the CD40+ endothelial cells in the intestinal microvasculature results in activation of these cells, which in turn promote leukocyte recruitment, platelet aggregation, thrombosis, vascular damage and tissue injury, through a vicious cycle of enhanced production of cytokines and chemokines, and overexpression of adhesion molecules and the tissue factor on endothelial cells[108-110]. All or some of these molecules are possible therapeutic targets in IBD management[107-109,111].
Moreover, the production of potent vasoconstrictors from the activated endothelium, such as endothelin-1 and thromboxanes, may contribute to ischemia-reperfusion vascular and tissue injury[107]. The increased reactive oxygen metabolites (ROMs) found in IBD come from leukocytes and endothelial cells, and may be the products of a recurrent ischemia-reperfusion injury of the vascular epithelium after microthrombi formation[112,113]. ROMs, in turn, may be implicated in the inflammatory reaction and tissue injury in IBD through activation of NF-κB factor, which promotes the production of various pro-inflammatory cytokines[114].
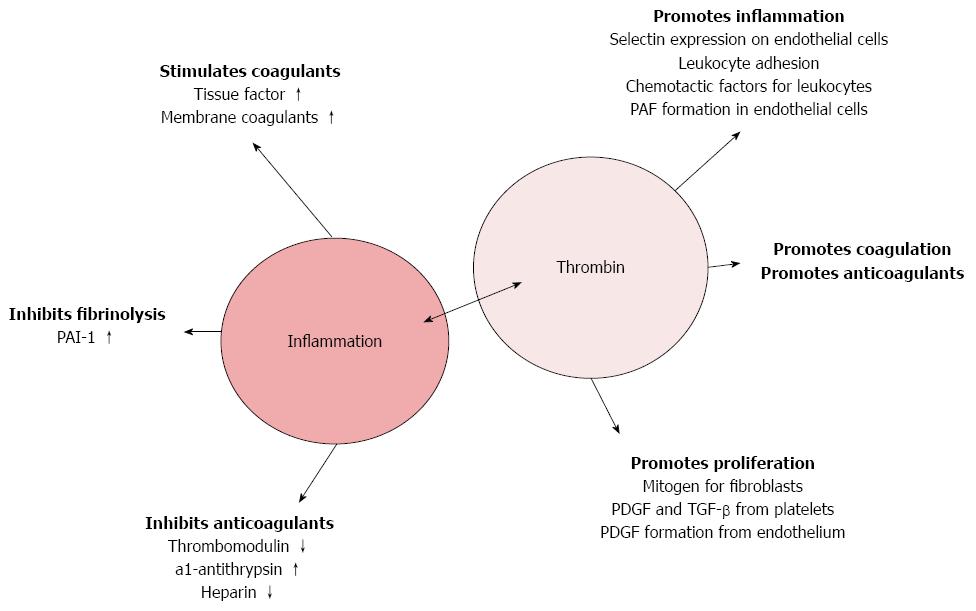
Thrombin, besides its actions in regulating hemostasis, possesses “non-coagulant” functions (Figure 2)[96,115,116]. Thrombin promotes the production of monocyte chemoattractant protein-1 (MCP-1) from monocytes and interleukins-6 and -8 (IL-6 and IL-8) from fibroblasts, epithelial cells, monocytes and endothelial cells. Thrombin enhances leukocyte adhesion on endothelial cells through induction of endothelial PAF (platelet activating factor) formation[117-122].
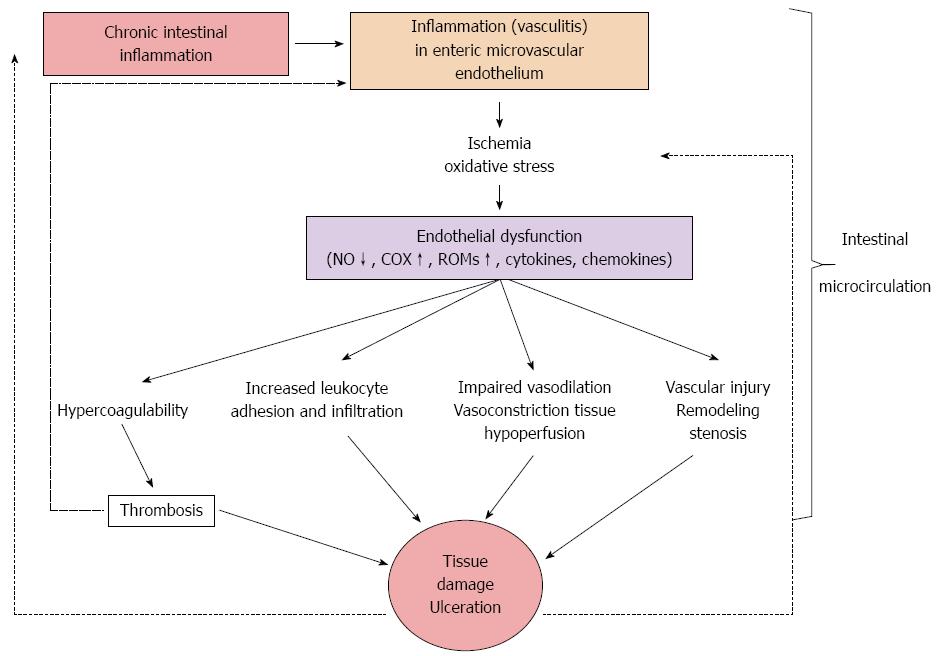
Collectively, a possible pathway in IBD pathogenesis could involve the combination of a “sepsis” model with a persistent, low-grade, controlled and compensated disseminated intravascular coagulation due to infection-induced hemostasis activation[96,97,123-125] and an “endothelial perturbation-inflammation” model due to ischemia-reperfusion injury[98,107,126]. It is well known that the increased intestinal permeability in IBD results in an inflammatory reaction in the bowel wall, due to the dysregulated mucosal uptake of luminal bacterial, toxic and antigenic substances. Both the inflammatory reaction and the endotoxemia might promote hemostasis activation and hypercoagulation[123]. Endotoxin and endotoxin-induced microclots in the systemic circulation have been found in a high proportion of IBD patients[124,125]. Furthermore, ischemia/reperfusion-induced endothelial dysfunction[126] promotes inflammation, thrombosis, vascular anatomic and functional changes, and tissue injury through a self-propagating loop (Figure 3).
The management of thromboembolism in IBD patients includes primary prophylaxis of first Thromboembolic complication, treatment of a Thromboembolic complication and secondary prophylaxis of the recurrence of a thromboembolic complication (Table 5). Currently, there are no universal and specific guidelines for the management of Thromboembolic in IBD patients in various clinical settings, except for the primary prophylaxis of VTE in hospitalized IBD patients with severe active disease[127-132].
| Primary prevention of thromboembolic complications | |
| Ambulatory patients | Hospitalized patients |
| General measures | General measures |
| Physician awareness | Disease activity amelioration |
| Patient education | Early mobilization |
| Active disease treatment and remission maintenance | Judicious use of catheters |
| Recognition, elimination or modification of risk factors | Dehydration or nutritional deficiencies restoration |
| Steroid use | Medication modification |
| Smoking | Peri-operatively or in severely ill non-surgical patients |
| Oral contraceptives | Prophylactic anticoagulation (UH or LMHW) |
| Cardiovascular risk factors and other co-morbidities | Plus mechanical measures when increased thrombosis risk or mechanical measures only, when anticoagulation contraindicated with high bleeding risk |
| Long-distance flights | |
| Post-hospitalization period | |
| Compressive stockings? | |
| Treatment of a thromboembolic event | |
| Amelioration of disease activity | |
| Hematology consultation and thrombophilia screening | |
| Therapeutic anticoagulation - UH or LMWH | |
| Thrombolysis - interventional radiology/surgical consultation | |
| Secondary prevention of thromboembolic complications | |
| After a first TE episode | |
| Active disease - spontaneous event | |
| Short term anticoagulation? - 3 to 6 mo | |
| Plus anticoagulation during subsequent flares? | |
| Inactive disease - spontaneous event | |
| Long term anticoagulation? | |
| Recurrent TE or inherited thrombophilia | |
| Hematology consultation | |
| Long term anticoagulation | |
Hospitalization is an important risk factor for VTEs for many patient groups, including IBD patients[25,27]. According to the American College of Chest Physicians (ACCP) guidelines for the prevention of VTE[127], hospitalized patients with IBD are at a moderate risk (10%-40%) of developing DVT and prophylaxis is the recommended beneficial strategy. The first choice is prophylaxis with a low-molecular-weight heparin (LMWH), low-dose unfractionated heparin (LDUH), or fondaparinux. Mechanical thromboprophylaxis is recommended for patients at high bleeding risk, or if anticoagulants are contraindicated[127]. International and national IBD organizations and societies in North America and Europe have adopted these recommendations in their recent guidelines for the management of IBD patients requiring hospitalization[128-132]. The recommendations are more clearly stated for patients with severe UC[128-131].
However, there are some issues regarding anticoagulation of hospitalized IBD patients which need special consideration. Rectal bleeding is a common symptom in IBD patients (mainly UC) and therefore there is a theoretical concern about worsening the rectal bleeding. Data derived from randomized trials which used UH[133,134] or LMWH[135-138] as a treatment for active IBD (UC) and a meta-analysis[139] showed that although a clear benefit from the heparin use in ameliorating the disease activity was not demonstrated, its use was safe, without major adverse events. Heparin could be an ideal drug for IBD treatment, especially for UC, because of its anticoagulant, anti-inflammatory, immunomodulatory and mucosal healing properties. The failure of the existing trials to prove its efficacy for the UC treatment could be related to the small patient number and the heterogeneity of these studies regarding the compound of LMWH and the dosage administered, the duration of treatment and the definition of response to treatment. Larger studies may be needed to clarify this issue and to reveal the optimal dosing of heparin and the features of a subgroup of patients with active UC who may benefit from LMWH administration.
Recently, Ra et al[140] retrospectively assessed the safety of prophylactic anticoagulation in hospitalized IBD patients. They reported that 80% of the IBD patients received anticoagulation, mainly in the form of LMWH (93%). Anticoagulation administration was more frequent to IBD patients on the surgical service, those with more extensive disease and predominantly those without rectal bleeding. They also found that the rates of major or minor bleeding were not significantly higher in patients who received prophylactic treatment compared to those who did not. The authors concluded that the use of anticoagulation in IBD hospitalized patients is safe, even in the presence rectal bleeding provided that there are no signs of hemodynamic instability[140].
Another issue which needs discussion is whether anticoagulation prophylaxis should be administered only to severely ill hospitalized IBD patients or to all hospitalized IBD patients. Since hospitalization is an independent risk factor for VTEs in IBD patients and these patients may have additional risk factors for thrombosis (inflammation, catheters, immobilization, complicated disease), it would be reasonable to take measures in order to reduce the other risk factors by aiming to: ameliorate disease activity, institute early mobilization, use IV catheters judiciously, avoid/treat dehydration and nutritional deficiencies, and minimize medications predisposing to thrombosis. Finally, it would be prudent to expand the indication for prophylaxis to inpatients with IBD who are not necessarily too ill to be confined to bed[25,27] or even are in remission and hospitalized for other indications, since there is significantly increased risk for VTEs in these groups as well[25]. Prophylaxis, together with the increased awareness for signs VTEs during the routine clinical assessment of the IBD patients admitted to the hospital, may be more feasible and cost-effective in clinical practice than the use of expensive screening tests[141].
Another important question that needs an answer is the duration of prophylactic anticoagulation in non-surgical IBD patients after discharge from the hospital. Studies have demonstrated that many VTEs occur during the immediate post-hospital period both in the general population[142] and in IBD patients[24,25]. Patients with IBD are discharged from the hospital with improved, but not necessarily fully remitted, disease and to date there are no data for extended VTE prophylaxis during this post-hospitalization period, although its use could be justified in patients with increased risk for thrombosis[143-145].
Patients with IBD are at increased risk for postoperative VTE[33,34,43]. According to the data from these studies and to the ACCP guidelines, patients with CD have a moderate risk of VTEs after intestinal surgery, while the patients with UC have a high risk of post-surgical VTEs[146]. Furthermore, Scarpa et al[147] reported that a standard prophylactic dose of LMWH was inadequate to prevent VTEs in IBD patients with major colorectal surgery and in particular in patients with UC. These data suggest that the perioperative prophylactic anticoagulation in IBD patients should include higher doses of LMWH, for longer periods post-operatively, or even be combined with adjunct mechanical methods[146-148].
Recent data have confirmed that ambulatory IBD patients with active disease are at increased risk for VTEs[25,37]. Although, Grainge et al[25] reported that the risk of VTEs was significantly higher during flares and chronic activity periods compared to periods of remission of the disease, both in ambulatory and hospitalized IBD patients, currently there are no guidelines for the primary prevention of VTEs nor is sufficient data for the beneficial use of anticoagulants in the ambulatory setting[145]. However, general prophylactic measures could also be applied in the ambulatory setting and include: patient education about the risks and the presenting symptoms of thromboembolic complications; enhancement of clinician awareness of this ominous extraintestinal manifestation, with attention to the history and clinical signs of TEs in the routine clinical assessment of IBD patients; aggressive treatment of active disease and maintenance of remission; early recognition and elimination or minimization of modifiable risk factors (steroid use, smoking, oral contraceptives, hormone-replacement therapy, long-distance flights)[149-151]. Furthermore, in a recent decision analysis study, Nguyen and Sharma[152] explored the cost-effectiveness of pharmacological VTE prophylaxis in ambulatory IBD patients and concluded that pharmacological VTE prophylaxis in ambulatory IBD patients with acute disease could not be recommended, even though it was beneficial, because it was not cost- effective.
There are no direct data that anticoagulation for VTE prophylaxis in IBD patients actually works since there are no randomized controlled trials that have evaluated this issue yet. However, indirect evidence demonstrated that in acutely ill medical patients pharmacological prophylaxis significantly reduces the incidence of VTE and mortality[127,143,144].
Apart from the general measures mentioned previously and anticoagulation prophylaxis during hospitalization or post-operatively, clinicians should routinely assess IBD patients for cardiovascular risk (hypertension, diabetes, hyperlipidemia, obesity, hyperhomocysteinemia, positive family history) and preventive measures and/or treatment of these risk factors should be applied[2].
The treatment of an acute thromboembolic episode in IBD patients is similar to non-IBD patients. Pharmacological anticoagulation (AC) with UH or LMWH is usually administered in mild to moderate Thromboembolic events, while thrombolysis or catheter-directed thrombolysis (CDT) are reserved for more severe TEs including massive thrombosis and organ- or life- threatening vascular occlusion[2,150]. Therapeutic doses of anticoagulants or thrombolytics for the treatment of TEs in IBD patients presents a major safety concern regarding the risk of gastrointestinal (GI) and systemic hemorrhagic complications, since many of the patients have active disease with rectal bleeding. The management decisions should be individualized according to the clinical setting in each patient and episode, and often requires a multidisciplinary approach[2,150]. The safety of UH and LMWH has been proven in previous studies which evaluated heparin for the treatment of active IBD[139]. Tabibian et al[153] in a systematic review evaluated the clinical outcomes with anticoagulation and CDT in IBD patients with TE, and reported that both CDT and AC were well tolerated by IBD patients with TE. They suggested that CDT may be used preferentially in patients with severe life-threatening TE, while AC may be more suitable in patients with less clinically significant Thromboembolic or patients at higher risk for bleeding. Furthermore, they demonstrated the safety of these treatments, even when they were used in patients with rectal bleeding, provided that there was no concurrent major GI hemorrhage[153,154].
The duration of anticoagulation is another important issue because of the increased risk of recurrence of TEs in IBD patients. The duration of anticoagulation after initial treatment for Thromboembolic ranges from 3 mo to lifelong, depending on the individual case. In cases where a Thromboembolic event occurred during active disease, the anticoagulation must be continued at least until clinical remission occurs[2,154]. In a recent decision analysis study, Nguyen and Bernstein[155] suggested that lifetime anticoagulation was marginally more beneficial than the time-limited (6-mo) anticoagulation after a first unprovoked VTE in the absence of active disease. They also recommended that in the case of VTE during a flare of the disease, time-limited anticoagulation with or without prophylaxis during subsequent flares would be a more suitable option[155]. In general, LMWHs, vitamin K antagonists (VKAs; warfarin) or even the new direct oral anticoagulants (NOACs; rivaroxaban, dabigatran, apixaban, and edoxaban) can be used for the long term treatment of TEs. For NOACs new evidence from studies suggests that they have comparable efficacy to that of VKAs with a more favorable safety profile, but there is no direct evidence for their use in IBD patients yet[156,157].
The risk of thrombosis in IBD patients is high, with significant morbidity and mortality. It is important for the treating physicians to be aware of this serious extraintestinal manifestation and to be able to efficiently recognize and treat the Thromboembolic events. As previously mentioned, international and national IBD organizations and societies in North America and Europe have recently published guidelines for the prevention of TEs in IBD patients[128-132]. However, surveys which have evaluated the practices of gastroenterologists regarding the issue of VTE prohylaxis in IBD patients have shown that although a significant proportion is aware of the increased risk of TEs in hospitalized IBD patients, their practices for VTE prophylaxis is variable[158-161].
Razik et al[158] reported that among 56 Canadian academic gastroenterologists, 55% reported the existence of standard hospital protocols for DVT prophylaxis in hospitalized IBD patients, and more than 80% reported the administration of some form of VTE prophylaxis, but only 50% of them were aware of the existing guidelines. Sam et al[159] in a similar survey among 135 gastroenterologists in United States, practising mainly in an academic setting (77%), reported that although most of them (84%) had IBD patients with VTE and realized the risks of VTEs, only 67% had protocols for VTE prophylaxis, 45% were aware of the guidelines and finally, 14% would never administer prophylaxis in their IBD inpatients. Gastroenterologists with high volumes of IBD patients were more likely to administer VTE prophylaxis. In addition, Tinsley et al[160] reported that the awareness of the heparin use for VTE prophylaxis was more frequent among gastroenterologists who were in academic settings, and those who had high volumes of IBD patients, and those who had less than 5 years of practice experience. Finally, Tinsley et al[161], in another study, investigated retrospectively the rates of pharmacologic VTE prophylaxis in UC inpatients at a tertiary referral center and concluded that pharmacologic prophylaxis was not ordered or was administered inadequately in a substantial proportion of UC patients admitted in the hospital despite the existing guidelines.
To summarize, all these data clearly show that there are significant variations in practice regarding VTE prophylaxis in hospitalized IBD patients due to a high level of unawareness of current guidelines. It is important for Gastroenterology societies and organizations to more aggressively pursue the education of gastroenterologists, especially those with low volumes of IBD patients, so that they better understand the risks and the adverse outcomes of thromboembolism in IBD patients. The goals are to have them routinely incorporate clinical assessment for signs and symptoms of TEs and to have them efficiently prevent or treat TEs[162]. Very recently, during finalization of the present review, the Canadian Association of Gastroenterology (CAG) published (in press-on line first) specific recommendations for the prevention and the treatment of VTE in IBD patients in various clinical settings[163]. The CAG has addressed many of the gaps which exist in the management of VTE in this patient group and has provided a useful and applicable evidence-based guide for the physicians who are involved with the care of IBD patients. The recent CAG guidelines give solid recommendations for some of the important issues we have outlined in Table 5.
Evidence from the literature suggests that thrombosis is a specific feature of IBD that can be involved in both the occurrence of thromboembolic events and the pathogenesis of the disease itself. The precise etiology for the higher rates of thromboembolism in IBD and the specific association is as yet unknown, but multiple acquired and inherited factors are implicated. Hypercoagulability is thought to be involved in IBD pathogenesis and future research may reveal potential therapeutic targets for the IBD management. More importantly, both arterial and venous thromboembolic complications are serious and challenging extra-intestinal manifestations to manage, with significant morbidity and mortality in IBD patients. However, thromboembolism is preventable and, therefore, clinician awareness of the risks, and the knowledge of how to efficiently prevent or treat TEs in patients with IBD are of vital importance. Future clinical trials should clarify the ill-defined issues of the thromboprophylaxis in ambulatory patients with active disease, the thromboprophylaxis in patients during the immediate post-hospitalization period, and the duration of thromboprophylaxis. In addition, clinical trials should provide clinicians with reliable methods or markers for assessing the prothrombotic risk in IBD patients in order to promptly apply preventive measures.
P- Reviewer: Beales ILP S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta-analysis of observational studies. J Crohns Colitis. 2014;8:469-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Tan VP, Chung A, Yan BP, Gibson PR. Venous and arterial disease in inflammatory bowel disease. J Gastroenterol Hepatol. 2013;28:1095-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Bargen JA, Barker NW. Extensive arterial and venous thrombosis complicating chronic ulcerative colitis. Arch Intern Med. 1936;58:17-31. [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 152] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Talbot RW, Heppell J, Dozois RR, Beart RW. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 407] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. III. Complications. Gut. 1964;5:1-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 577] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Koenigs KP, McPhedran P, Spiro HM. Thrombosis in inflammatory bowel disease. J Clin Gastroenterol. 1987;9:627-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Sloan WP, Bargen JA, GAGE RP. Life histories of patients with chronic ulcerative colitis: a review of 2,000 cases. Gastroenterology. 1950;16:25-38. [PubMed] [Cited in This Article: ] |
| 8. | Ricketts WE, Palmer WL. Complications of chronic non-specific ulcerative colitis. Gastroenterology. 1946;7:55-66. [PubMed] [Cited in This Article: ] |
| 9. | Graef V, Baggenstoss AH, Sauer WG, Spittel JA Jr. Venous thrombosis in non-specific ulcerative colitis. A necropsy study. Arch Intern Med. 1965;117:377-382. [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 121] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Johns DR. Cerebrovascular complications of inflammatory bowel disease. Am J Gastroenterol. 1991;86:367-370. [PubMed] [Cited in This Article: ] |
| 11. | Katsanos AH, Kosmidou M, Giannopoulos S, Katsanos KH, Tsivgoulis G, Kyritsis AP, Tsianos EV. Cerebral arterial infarction in inflammatory bowel diseases. Eur J Intern Med. 2014;25:37-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Prior A, Strang FA, Whorwell PJ. Internal carotid artery occlusion in association with Crohn’s disease. Dig Dis Sci. 1987;32:1047-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Landman C, Nahon S, Cosnes J, Bouhnik Y, Brixi-Benmansour H, Bouguen G, Colombel JF, Savoye G, Coffin B, Abitbol V. Portomesenteric vein thrombosis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:582-589. [PubMed] [Cited in This Article: ] |
| 14. | Maccini DM, Berg JC, Bell GA. Budd-Chiari syndrome and Crohn‘s disease. An unreported association. Dig Dis Sci. 1989;34:1933-1936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Stapleton SR, Curley RK, Simpson WA. Cutaneous gangrene secondary to focal thrombosis--an important cutaneous manifestation of ulcerative colitis. Clin Exp Dermatol. 1989;14:387-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Schneiderman JH, Sharpe JA, Sutton DM. Cerebral and retinal vascular complications of inflammatory bowel disease. Ann Neurol. 1979;5:331-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Keyser BJ, Hass AN. Retinal vascular disease in ulcerative colitis. Am J Ophthalmol. 1994;118:395-396. [PubMed] [Cited in This Article: ] |
| 18. | Vayalambrone D, Ivanova T, Misra A. Nonischemic central retinal vein occlusion in an adolescent patient with ulcerative colitis. Case Rep Ophthalmol Med. 2011;2011:963583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Mutlu B, Ermeydan CM, Enç F, Fotbolcu H, Demirkol O, Bayrak F, Basaran Y. Acute myocardial infarction in a young woman with severe ulcerative colitis. Int J Cardiol. 2002;83:183-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol. 2011;106:713-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Bernstein CN, Blanchard JF, Houston DS, Wajda A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost. 2001;85:430-434. [PubMed] [Cited in This Article: ] |
| 22. | Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, Machold K, Scholz S, Vogelsang H. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 23. | Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167:935-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 287] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 24. | Kappelman MD, Horvath-Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, Baron JA, Sørensen HT. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. 2011;60:937-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 208] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 492] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 26. | Bernstein CN, Nabalamba A. Hospitalization-based major comorbidity of inflammatory bowel disease in Canada. Can J Gastroenterol. 2007;21:507-511. [PubMed] [Cited in This Article: ] |
| 27. | Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:2272-2280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 28. | Saleh T, Matta F, Yaekoub AY, Danescu S, Stein PD. Risk of venous thromboembolism with inflammatory bowel disease. Clin Appl Thromb Hemost. 2011;17:254-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Rothberg MB, Lindenauer PK, Lahti M, Pekow PS, Selker HP. Risk factor model to predict venous thromboembolism in hospitalized medical patients. J Hosp Med. 2011;6:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Sridhar AR, Parasa S, Navaneethan U, Crowell MD, Olden K. Comprehensive study of cardiovascular morbidity in hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2011;5:287-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Nguyen GC, Boudreau H, Harris ML, Maxwell CV. Outcomes of obstetric hospitalizations among women with inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol. 2009;7:329-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Bröms G, Granath F, Linder M, Stephansson O, Elmberg M, Kieler H. Complications from inflammatory bowel disease during pregnancy and delivery. Clin Gastroenterol Hepatol. 2012;10:1246-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Merrill A, Millham F. Increased risk of postoperative deep vein thrombosis and pulmonary embolism in patients with inflammatory bowel disease: a study of National Surgical Quality Improvement Program patients. Arch Surg. 2012;147:120-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Wallaert JB, De Martino RR, Marsicovetere PS, Goodney PP, Finlayson SR, Murray JJ, Holubar SD. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum. 2012;55:1138-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 35. | Novacek G, Weltermann A, Sobala A, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139:779-787, 787.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 36. | Yuhara H, Steinmaus C, Corley D, Koike J, Igarashi M, Suzuki T, Mine T. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:953-962. [PubMed] [Cited in This Article: ] |
| 37. | Papay P, Miehsler W, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, Fuchssteiner H. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J Crohns Colitis. 2013;7:723-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Grip O, Svensson PJ, Lindgren S. Inflammatory bowel disease promotes venous thrombosis earlier in life. Scand J Gastroenterol. 2000;35:619-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Jackson LM, O’Gorman PJ, O’Connell J, Cronin CC, Cotter KP, Shanahan F. Thrombosis in inflammatory bowel disease: clinical setting, procoagulant profile and factor V Leiden. QJM. 1997;90:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 141] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Solem CA, Loftus EV, Tremaine WJ, Sandborn WJ. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99:97-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Jess T, Gamborg M, Munkholm P, Sørensen TI. Overall and cause-specific mortality in ulcerative colitis: meta-analysis of population-based inception cohort studies. Am J Gastroenterol. 2007;102:609-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Duricova D, Pedersen N, Elkjaer M, Gamborg M, Munkholm P, Jess T. Overall and cause-specific mortality in Crohn’s disease: a meta-analysis of population-based studies. Inflamm Bowel Dis. 2010;16:347-353. [PubMed] [Cited in This Article: ] |
| 43. | O’Connor OJ, Cahill RA, Kirwan WO, Redmond HP. The incidence of postoperative venous thrombosis among patients with ulcerative colitis. Ir J Med Sci. 2005;174:20-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 45. | Nogami H, Iiai T, Maruyama S, Tani T, Hatakeyama K. Common carotid arterial thrombosis associated with ulcerative colitis. World J Gastroenterol. 2007;13:1755-1757. [PubMed] [Cited in This Article: ] |
| 46. | Richard S, Mione G, Perrin J, Toussaint-Hacquard M, Lacour JC, Ducrocq X. Internal carotid thrombus in patients with inflammatory bowel disease: two cases. World J Gastroenterol. 2013;19:773-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Irving PM, Alstead EM, Greaves RR, Feakins RM, Pollok RC, Rampton DS. Acute mesenteric infarction: an important cause of abdominal pain in ulcerative colitis. Eur J Gastroenterol Hepatol. 2005;17:1429-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Szychta P, Reix T, Sevestre MA, Brazier F, Pietri J. Aortic thrombosis and ulcerative colitis. Ann Vasc Surg. 2001;15:402-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 49. | Novacek G, Haumer M, Schima W, Müller C, Miehsler W, Polterauer P, Vogelsang H. Aortic mural thrombi in patients with inflammatory bowel disease: report of two cases and review of the literature. Inflamm Bowel Dis. 2004;10:430-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Levy PJ, Tabares AH, Olin JW. Lower extremity arterial occlusions in young patients with Crohn’s colitis and premature atherosclerosis: report of six cases. Am J Gastroenterol. 1997;92:494-497. [PubMed] [Cited in This Article: ] |
| 51. | Haumer M, Teml A, Dirisamer A, Vogelsang H, Koppensteiner R, Novacek G. Severe ulcerative colitis complicated by an arterial thrombus in the brachiocephalic trunk. Inflamm Bowel Dis. 2007;13:937-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Di Fabio F, Obrand D, Satin R, Gordon PH. Intra-abdominal venous and arterial thromboembolism in inflammatory bowel disease. Dis Colon Rectum. 2009;52:336-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Ha C, Magowan S, Accortt NA, Chen J, Stone CD. Risk of arterial thrombotic events in inflammatory bowel disease. Am J Gastroenterol. 2009;104:1445-1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 54. | Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp-Pedersen C, Nielsen OH, Gislason GH, Hansen PR. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death--a Danish nationwide cohort study. PLoS One. 2013;8:e56944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 55. | Andersohn F, Waring M, Garbe E. Risk of ischemic stroke in patients with Crohn’s disease: a population-based nested case-control study. Inflamm Bowel Dis. 2010;16:1387-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Bernstein CN, Wajda A, Blanchard JF. The incidence of arterial thromboembolic diseases in inflammatory bowel disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:41-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 58. | Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62:689-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 59. | Zöller B, Li X, Sundquist J, Sundquist K. Risk of subsequent ischemic and hemorrhagic stroke in patients hospitalized for immune-mediated diseases: a nationwide follow-up study from Sweden. BMC Neurol. 2012;12:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 60. | Singh S, Singh H, Loftus EV, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:382-393.e1: quiz e22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 193] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 61. | Dorn SD, Sandler RS. Inflammatory bowel disease is not a risk factor for cardiovascular disease mortality: results from a systematic review and meta-analysis. Am J Gastroenterol. 2007;102:662-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Osterman MT, Yang YX, Brensinger C, Forde KA, Lichtenstein GR, Lewis JD. No increased risk of myocardial infarction among patients with ulcerative colitis or Crohn‘s disease. Clin Gastroenterol Hepatol. 2011;9:875-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 63. | Wakefield AJ, Sawyerr AM, Dhillon AP, Pittilo RM, Rowles PM, Lewis AA, Pounder RE. Pathogenesis of Crohn’s disease: multifocal gastrointestinal infarction. Lancet. 1989;2:1057-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 424] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 64. | Dhillon AP, Anthony A, Sim R, Wakefield AJ, Sankey EA, Hudson M, Allison MC, Pounder RE. Mucosal capillary thrombi in rectal biopsies. Histopathology. 1992;21:127-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Thompson NP, Wakefield AJ, Pounder RE. Inherited disorders of coagulation appear to protect against inflammatory bowel disease. Gastroenterology. 1995;108:1011-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 95] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Tsiolakidou G, Koutroubakis IE. Thrombosis and inflammatory bowel disease-the role of genetic risk factors. World J Gastroenterol. 2008;14:4440-4444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Owczarek D, Cibor D, Głowacki MK, Rodacki T, Mach T. Inflammatory bowel disease: epidemiology, pathology and risk factors for hypercoagulability. World J Gastroenterol. 2014;20:53-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 64] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Yoshida H, Granger DN. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis. 2009;15:1245-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 69. | van Bodegraven AA. Haemostasis in inflammatory bowel diseases: clinical relevance. Scand J Gastroenterol Suppl. 2003;51-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Souto JC, Martínez E, Roca M, Mateo J, Pujol J, González D, Fontcuberta J. Prothrombotic state and signs of endothelial lesion in plasma of patients with inflammatory bowel disease. Dig Dis Sci. 1995;40:1883-1889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Lee LC, Spittell JA, Sauer WG, Owen CA, Thompson JH. Hypercoagulability associated with chronic ulcerative colitis: changes in blood coagulation factors. Gastroenterology. 1968;54:76-85. [PubMed] [Cited in This Article: ] |
| 72. | Lam A, Borda IT, Inwood MJ, Thomson S. Coagulation studies in ulcerative colitis and Crohn’s disease. Gastroenterology. 1975;68:245-251. [PubMed] [Cited in This Article: ] |
| 73. | Conlan MG, Haire WD, Burnett DA. Prothrombotic abnormalities in inflammatory bowel disease. Dig Dis Sci. 1989;34:1089-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Hudson M, Hutton RA, Wakefield AJ, Sawyerr AM, Pounder RE. Evidence for activation of coagulation in Crohn’s disease. Blood Coagul Fibrinolysis. 1992;3:773-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Stevens TR, James JP, Simmonds NJ, McCarthy DA, Laurenson IF, Maddison PJ, Rampton DS. Circulating von Willebrand factor in inflammatory bowel disease. Gut. 1992;33:502-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Sawyerr AM, Smith MS, Hall A, Hudson M, Hay CR, Wakefield AJ, Brook MG, Tomura H, Pounder RE. Serum concentrations of von Willebrand factor and soluble thrombomodulin indicate alteration of endothelial function in inflammatory bowel diseases. Dig Dis Sci. 1995;40:793-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Meucci G, Pareti F, Vecchi M, Saibeni S, Bressi C, de Franchis R. Serum von Willebrand factor levels in patients with inflammatory bowel disease are related to systemic inflammation. Scand J Gastroenterol. 1999;34:287-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Zezos P, Papaioannou G, Nikolaidis N, Vasiliadis T, Giouleme O, Evgenidis N. Elevated plasma von Willebrand factor levels in patients with active ulcerative colitis reflect endothelial perturbation due to systemic inflammation. World J Gastroenterol. 2005;11:7639-7645. [PubMed] [Cited in This Article: ] |
| 79. | Jorens PG, Hermans CR, Haber I, Kockx MM, Vermylen J, Parizel GA. Acquired protein C and S deficiency, inflammatory bowel disease and cerebral arterial thrombosis. Blut. 1990;61:307-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Aadland E, Odegaard OR, Røseth A, Try K. Free protein S deficiency in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1992;27:957-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Aadland E, Odegaard OR, Røseth A, Try K. Free protein S deficiency in patients with Crohn’s disease. Scand J Gastroenterol. 1994;29:333-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Wyshock E, Caldwell M, Crowley JP. Deep venous thrombosis, inflammatory bowel disease, and protein S deficiency. Am J Clin Pathol. 1988;90:633-635. [PubMed] [Cited in This Article: ] |
| 83. | de Jong E, Porte RJ, Knot EA, Verheijen JH, Dees J. Disturbed fibrinolysis in patients with inflammatory bowel disease. A study in blood plasma, colon mucosa, and faeces. Gut. 1989;30:188-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Hayat M, Ariëns RA, Moayyedi P, Grant PJ, O’Mahony S. Coagulation factor XIII and markers of thrombin generation and fibrinolysis in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2002;14:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Collins CE, Cahill MR, Newland AC, Rampton DS. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994;106:840-845. [PubMed] [Cited in This Article: ] |
| 86. | Collins CE, Rampton DS. Review article: platelets in inflammatory bowel disease--pathogenetic role and therapeutic implications. Aliment Pharmacol Ther. 1997;11:237-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Danese S, Katz JA, Saibeni S, Papa A, Gasbarrini A, Vecchi M, Fiocchi C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52:1435-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 198] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 88. | van Bodegraven AA, Schoorl M, Baak JP, Linskens RK, Bartels PC, Tuynman HA. Hemostatic imbalance in active and quiescent ulcerative colitis. Am J Gastroenterol. 2001;96:487-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | van Bodegraven AA, Schoorl M, Linskens RK, Bartels PC, Tuynman HA. Persistent activation of coagulation and fibrinolysis after treatment of active ulcerative colitis. Eur J Gastroenterol Hepatol. 2002;14:413-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Zezos P, Papaioannou G, Nikolaidis N, Patsiaoura K, Vassiliadis T, Mpoumponaris A, Giouleme O, Evgenidis N. Elevated markers of thrombin generation and fibrinolysis in patients with active and quiescent ulcerative colitis. Med Sci Monit. 2009;15:CR563-CR572. [PubMed] [Cited in This Article: ] |
| 91. | Warren S, Sommers SC. Pathogenesis of ulcerative colitis. Am J Pathol. 1949;25:657-679. [PubMed] [Cited in This Article: ] |
| 92. | Brandt LJ, Gomery P, Mitsudo SM, Chandler P, Boley SJ. Disseminated intravascular coagulation in nonocclusive mesenteric ischemia: the lack of specificity of fibrin thrombi in intestinal infarction. Gastroenterology. 1976;71:954-957. [PubMed] [Cited in This Article: ] |
| 93. | Hamilton MI, Dick R, Crawford L, Thompson NP, Pounder RE, Wakefield AJ. Is proximal demarcation of ulcerative colitis determined by the territory of the inferior mesenteric artery? Lancet. 1995;345:688-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Korzenik JR. IBD: A Vascular Disorder? The Case for Heparin Therapy. Inflamm Bowel Dis. 1997;3:87-94. [PubMed] [Cited in This Article: ] |
| 95. | Levi M, ten Cate H, van der Poll T. Endothelium: interface between coagulation and inflammation. Crit Care Med. 2002;30:S220-S224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 96. | Esmon CT, Fukudome K, Mather T, Bode W, Regan LM, Stearns-Kurosawa DJ, Kurosawa S. Inflammation, sepsis, and coagulation. Haematologica. 1999;84:254-259. [PubMed] [Cited in This Article: ] |
| 97. | Levi M, van der Poll T, Schultz M. New insights into pathways that determine the link between infection and thrombosis. Neth J Med. 2012;70:114-120. [PubMed] [Cited in This Article: ] |
| 98. | Hatoum OA, Miura H, Binion DG. The vascular contribution in the pathogenesis of inflammatory bowel disease. Am J Physiol Heart Circ Physiol. 2003;285:H1791-H1796. [PubMed] [Cited in This Article: ] |
| 99. | Hatoum OA, Binion DG. The vasculature and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Inflamm Bowel Dis. 2005;11:304-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 100. | Papa A, Scaldaferri F, Danese S, Guglielmo S, Roberto I, Bonizzi M, Mocci G, Felice C, Ricci C, Andrisani G. Vascular involvement in inflammatory bowel disease: pathogenesis and clinical aspects. Dig Dis. 2008;26:149-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Roifman I, Sun YC, Fedwick JP, Panaccione R, Buret AG, Liu H, Rostom A, Anderson TJ, Beck PL. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2009;7:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 102. | Makrides SC, Ryan US. Overview of the endothelium. In Loscalzo J, Schafer Al (eds): Thrombosis and hemorrhage. 2nd ed. Baltimore: Williams and Wilkins 1998; 295-305. [Cited in This Article: ] |
| 103. | Joseph L, Fink LM, Hauer-Jensen M. Cytokines in coagulation and thrombosis: a preclinical and clinical review. Blood Coagul Fibrinolysis. 2002;13:105-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 104. | Danese S. Inflammation and the mucosal microcirculation in inflammatory bowel disease: the ebb and flow. Curr Opin Gastroenterol. 2007;23:384-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 105. | Jones SC, Banks RE, Haidar A, Gearing AJ, Hemingway IK, Ibbotson SH, Dixon MF, Axon AT. Adhesion molecules in inflammatory bowel disease. Gut. 1995;36:724-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 149] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 106. | Schuermann GM, Aber-Bishop AE, Facer P, Lee JC, Rampton DS, Doré CJ, Polak JM. Altered expression of cell adhesion molecules in uninvolved gut in inflammatory bowel disease. Clin Exp Immunol. 1993;94:341-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Panés J, Granger DN. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 287] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 108. | Danese S, Sans M, Scaldaferri F, Sgambato A, Rutella S, Cittadini A, Piqué JM, Panes J, Katz JA, Gasbarrini A. TNF-alpha blockade down-regulates the CD40/CD40L pathway in the mucosal microcirculation: a novel anti-inflammatory mechanism of infliximab in Crohn’s disease. J Immunol. 2006;176:2617-2624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 109. | Danese S, Sans M, Fiocchi C. The CD40/CD40L costimulatory pathway in inflammatory bowel disease. Gut. 2004;53:1035-1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 110. | Zhou L, Stordeur P, de Lavareille A, Thielemans K, Capel P, Goldman M, Pradier O. CD40 engagement on endothelial cells promotes tissue factor-dependent procoagulant activity. Thromb Haemost. 1998;79:1025-1028. [PubMed] [Cited in This Article: ] |
| 111. | van Assche G, Rutgeerts P. Antiadhesion molecule therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2002;8:291-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 112. | Granger DN. Ischemia-reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Hsieh HJ, Liu CA, Huang B, Tseng AH, Wang DL. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J Biomed Sci. 2014;21:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 114. | Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247-2258. [PubMed] [Cited in This Article: ] |
| 115. | Saibeni S, Saladino V, Chantarangkul V, Villa F, Bruno S, Vecchi M, de Franchis R, Sei C, Tripodi A. Increased thrombin generation in inflammatory bowel diseases. Thromb Res. 2010;125:278-282. [PubMed] [Cited in This Article: ] |
| 116. | Scaldaferri F, Lancellotti S, Pizzoferrato M, De Cristofaro R. Haemostatic system in inflammatory bowel diseases: new players in gut inflammation. World J Gastroenterol. 2011;17:594-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 117. | Degen JL. Hemostatic factors and inflammatory disease. Thromb Haemost. 1999;82:858-864. [PubMed] [Cited in This Article: ] |
| 118. | Johnson K, Choi Y, DeGroot E, Samuels I, Creasey A, Aarden L. Potential mechanisms for a proinflammatory vascular cytokine response to coagulation activation. J Immunol. 1998;160:5130-5135. [PubMed] [Cited in This Article: ] |
| 119. | Johnson K, Aarden L, Choi Y, De Groot E, Creasey A. The proinflammatory cytokine response to coagulation and endotoxin in whole blood. Blood. 1996;87:5051-5060. [PubMed] [Cited in This Article: ] |
| 120. | Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103:879-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 602] [Cited by in F6Publishing: 588] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 121. | Zimmerman GA, McIntyre TM, Prescott SM. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985;76:2235-2246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 310] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 122. | Zimmerman GA, McIntyre TM, Prescott SM. Thrombin stimulates neutrophil adherence by an endothelial cell-dependent mechanism: characterization of the response and relationship to platelet-activating factor synthesis. Ann N Y Acad Sci. 1986;485:349-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Levi M, van der Poll T, Schultz M. Infection and inflammation as risk factors for thrombosis and atherosclerosis. Semin Thromb Hemost. 2012;38:506-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 124. | Juhlin L, Krause U, Shelley WB. Endotoxin-induced microclots in ulcerative colitis and Crohn’s disease. Scand J Gastroenterol. 1980;15:311-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | Gardiner KR, Halliday MI, Barclay GR, Milne L, Brown D, Stephens S, Maxwell RJ, Rowlands BJ. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897-901. [PubMed] [Cited in This Article: ] |
| 126. | Ibrahim CB, Aroniadis OC, Brandt LJ. On the role of ischemia in the pathogenesis of IBD: a review. Inflamm Bowel Dis. 2010;16:696-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:381S-453S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3095] [Cited by in F6Publishing: 2861] [Article Influence: 178.8] [Reference Citation Analysis (0)] |
| 128. | Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-523; quiz 524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 899] [Cited by in F6Publishing: 891] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 129. | Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 933] [Cited by in F6Publishing: 893] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 130. | Bitton A, Buie D, Enns R, Feagan BG, Jones JL, Marshall JK, Whittaker S, Griffiths AM, Panaccione R. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012;107:179-194; author reply 195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 131. | Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, Beaugerie L, Gomollón F, Häuser W, Herrlinger K. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 329] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 132. | Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis. 2010;4:63-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 526] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 133. | Folwaczny C, Wiebecke B, Loeschke K. Unfractioned heparin in the therapy of patients with highly active inflammatory bowel disease. Am J Gastroenterol. 1999;94:1551-1555. [PubMed] [Cited in This Article: ] |
| 134. | Ang YS, Mahmud N, White B, Byrne M, Kelly A, Lawler M, McDonald GS, Smith OP, Keeling PW. Randomized comparison of unfractionated heparin with corticosteroids in severe active inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:1015-1022. [PubMed] [Cited in This Article: ] |
| 135. | Bloom S, Kiilerich S, Lassen MR, Forbes A, Leiper K, Langholz E, Irvine EJ, O’Morain C, Lowson D, Orm S. Low molecular weight heparin (tinzaparin) vs. placebo in the treatment of mild to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2004;19:871-878. [PubMed] [Cited in This Article: ] |
| 136. | Zezos P, Papaioannou G, Nikolaidis N, Patsiaoura K, Papageorgiou A, Vassiliadis T, Giouleme O, Evgenidis N. Low-molecular-weight heparin (enoxaparin) as adjuvant therapy in the treatment of active ulcerative colitis: a randomized, controlled, comparative study. Aliment Pharmacol Ther. 2006;23:1443-1453. [PubMed] [Cited in This Article: ] |
| 137. | de Bièvre MA, Vrij AA, Schoon EJ, Dijkstra G, de Jong AE, Oberndorff-Klein Woolthuis AH, Hemker HC, Stockbrügger RW. Randomized, placebo-controlled trial of low molecular weight heparin in active ulcerative colitis. Inflamm Bowel Dis. 2007;13:753-758. [PubMed] [Cited in This Article: ] |
| 138. | Pastorelli L, Saibeni S, Spina L, Signorelli C, Celasco G, de Franchis R, Vecchi M. Oral, colonic-release low-molecular-weight heparin: an initial open study of Parnaparin-MMX for the treatment of mild-to-moderate left-sided ulcerative colitis. Aliment Pharmacol Ther. 2008;28:581-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 139. | Shen J, Ran ZH, Tong JL, Xiao SD. Meta-analysis: The utility and safety of heparin in the treatment of active ulcerative colitis. Aliment Pharmacol Ther. 2007;26:653-663. [PubMed] [Cited in This Article: ] |
| 140. | Ra G, Thanabalan R, Ratneswaran S, Nguyen GC. Predictors and safety of venous thromboembolism prophylaxis among hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2013;7:e479-e485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 141. | Nguyen GC, Wu H, Gulamhusein A, Rosenberg M, Thanabalan R, Yeo EL, Bernstein CN, Steinhart AH, Margolis M. The utility of screening for asymptomatic lower extremity deep venous thrombosis during inflammatory bowel disease flares: a pilot study. Inflamm Bowel Dis. 2013;19:1053-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167:1471-1475. [PubMed] [Cited in This Article: ] |
| 143. | Kaatz S, Spyropoulos AC. Venous thromboembolism prophylaxis after hospital discharge: transition to preventive care. Hosp Pract (1995). 2011;39:7-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 144. | Mahan CE, Fisher MD, Mills RM, Fields LE, Stephenson JJ, Fu AC, Spyropoulos AC. Thromboprophylaxis patterns, risk factors, and outcomes of care in the medically ill patient population. Thromb Res. 2013;132:520-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 145. | Bryant RV, Jairath V, Curry N, Travis SP. Thrombosis in inflammatory bowel disease: are we tailoring prophylaxis to those most at risk? J Crohns Colitis. 2014;8:166-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 146. | Nguyen GC. IBD: postoperative VTE prophylaxis in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:5-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 147. | Scarpa M, Pilon F, Pengo V, Romanato G, Ruffolo C, Erroi F, Elisa B, Frego M, Ossi E, Manzato E. Deep venous thrombosis after surgery for inflammatory bowel disease: is standard dose low molecular weight heparin prophylaxis enough? World J Surg. 2010;34:1629-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 148. | Kumar A, Auron M, Aneja A, Mohr F, Jain A, Shen B. Inflammatory bowel disease: perioperative pharmacological considerations. Mayo Clin Proc. 2011;86:748-757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 149. | Nguyen GC, Yeo EL. Prophylaxis of venous thromboembolism in IBD. Lancet. 2010;375:616-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 150. | Zitomersky NL, Verhave M, Trenor CC. Thrombosis and inflammatory bowel disease: a call for improved awareness and prevention. Inflamm Bowel Dis. 2011;17:458-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 151. | Huang V, Mishra R, Thanabalan R, Nguyen GC. Patient awareness of extraintestinal manifestations of inflammatory bowel disease. J Crohns Colitis. 2013;7:e318-e324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | Nguyen GC, Sharma S. Feasibility of venous thromboembolism prophylaxis during inflammatory bowel disease flares in the outpatient setting: a decision analysis. Inflamm Bowel Dis. 2013;19:2182-2189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 153. | Tabibian JH, Streiff MB. Inflammatory bowel disease-associated thromboembolism: a systematic review of outcomes with anticoagulation versus catheter-directed thrombolysis. Inflamm Bowel Dis. 2012;18:161-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 154. | Nguyen GC. Treatment of thromboembolism in IBD: more questions than answers. Inflamm Bowel Dis. 2012;18:172-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 155. | Nguyen GC, Bernstein CN. Duration of anticoagulation for the management of venous thromboembolism in inflammatory bowel disease: a decision analysis. Am J Gastroenterol. 2013;108:1486-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 156. | Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S-e494S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2565] [Cited by in F6Publishing: 2445] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 157. | van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:320-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 339] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 158. | Razik R, Bernstein CN, Sam J, Thanabalan R, Nguyen GC. Survey of perceptions and practices among Canadian gastroenterologists regarding the prevention of venous thromboembolism for hospitalized inflammatory bowel disease patients. Can J Gastroenterol. 2012;26:795-798. [PubMed] [Cited in This Article: ] |
| 159. | Sam JJ, Bernstein CN, Razik R, Thanabalan R, Nguyen GC. Physicians’ perceptions of risks and practices in venous thromboembolism prophylaxis in inflammatory bowel disease. Dig Dis Sci. 2013;58:46-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 160. | Tinsley A, Naymagon S, Trindade AJ, Sachar DB, Sands BE, Ullman TA. A survey of current practice of venous thromboembolism prophylaxis in hospitalized inflammatory bowel disease patients in the United States. J Clin Gastroenterol. 2013;47:e1-e6. [PubMed] [Cited in This Article: ] |
| 161. | Tinsley A, Naymagon S, Enomoto LM, Hollenbeak CS, Sands BE, Ullman TA. Rates of pharmacologic venous thromboembolism prophylaxis in hospitalized patients with active ulcerative colitis: results from a tertiary care center. J Crohns Colitis. 2013;7:e635-e640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 162. | Allen JI. Mind the gap: venous thromboembolism prophylaxis in patients hospitalized with inflammatory bowel disease. Dig Dis Sci. 2013;58:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 163. | Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, Leontiadis GI, Geerts W, Bressler B, Butzner JD, Carrier M. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835-848.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |