Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.16858
Revised: August 27, 2014
Accepted: October 14, 2014
Published online: December 7, 2014
Twenty years after its introduction, computed tomographic colonography (CTC) has reached its maturity, and it can reasonably be considered the best radiological diagnostic test for imaging colorectal cancer (CRC) and polyps. This examination technique is less invasive than colonoscopy (CS), easy to perform, and standardized. Reduced bowel preparation and colonic distention using carbon dioxide favor patient compliance. Widespread implementation of a new image reconstruction algorithm has minimized radiation exposure, and the use of dedicated software with enhanced views has enabled easier image interpretation. Integration in the routine workflow of a computer-aided detection algorithm reduces perceptual errors, particularly for small polyps. Consolidated evidence from the literature shows that the diagnostic performances for the detection of CRC and large polyps in symptomatic and asymptomatic individuals are similar to CS and are largely superior to barium enema, the latter of which should be strongly discouraged. Favorable data regarding CTC performance open the possibility for many different indications, some of which are already supported by evidence-based data: incomplete, failed, or unfeasible CS; symptomatic, elderly, and frail patients; and investigation of diverticular disease. Other indications are still being debated and, thus, are recommended only if CS is unfeasible: the use of CTC in CRC screening and in surveillance after surgery for CRC or polypectomy. In order for CTC to be used appropriately, contraindications such as acute abdominal conditions (diverticulitis or the acute phase of inflammatory bowel diseases) and surveillance in patients with a long-standing history of ulcerative colitis or Crohn’s disease and in those with hereditary colonic syndromes should not be overlooked. This will maximize the benefits of the technique and minimize potential sources of frustration or disappointment for both referring clinicians and patients.
Core tip: Computed tomographic colonography (CTC) is easy to perform, standardized, and patient-friendly. Radiation exposure is minimized and image interpretation is facilitated by the use of a computer-aided detection algorithm. The diagnostic accuracies for colorectal cancer (CRC) and large polyps are similar to that of colonoscopy (CS) and are largely superior to that of barium enema. Incomplete, failed, or unfeasible CS and investigation of symptomatic, elderly, and frail patients and diverticular disease are clear indications. CTC is recommended for CRC screening and in surveillance after surgery or polypectomy if CS is unfeasible. Acute abdominal conditions and surveillance in patients with ulcerative colitis, Crohn’s disease, and hereditary colonic syndromes are known contraindications.
- Citation: Laghi A. Computed tomography colonography in 2014: An update on technique and indications. World J Gastroenterol 2014; 20(45): 16858-16867
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/16858.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.16858
Twenty years after the introduction of computed tomographic colonography (CTC)[1], this technique has reached its maturity, and it can reasonably be considered the best radiological diagnostic test for imaging CRC and polyps.
Recent evidence in the literature shows that the diagnostic performance for the detection of CRC both in symptomatic[2,3] and asymptomatic subjects[4,5] is similar to that of colonoscopy (CS) and is largely superior to that of barium enema (BE), thus leading the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) and the European Society of Gastrointestinal Endoscopy (ESGE) “¡ to recommend CT colonography as the radiological examination of choice in the context of colorectal neoplasia”[6], and to discourage the use of BE if CTC is available.
In this paper, this technique will be reviewed and the performance and current indications and contraindications to CTC will be discussed.
The technique is easy, standardized[7], and much less labor-intensive and invasive than BE and CS. The process consists of four consecutive steps: bowel cleansing, colon distention, CT protocol, and image analysis.
Similar to CS, in which an optimal examination requires a clean colon, bowel cleansing is critical for CTC. Residual stools may mimic cancer or a polyp or, alternatively, may hide a real colonic lesion; the same is true for fluid residues. Bowel preparation is an evolving area of research since the ultimate goal is to reduce or abolish the use of laxative agents in order to improve patient compliance compared with CS, but not at the expense of diagnostic quality. The ideal colonic preparation is still being debated, but general agreement exists regarding the need for dietary restriction, fecal tagging, and bowel purgation[7]. Dietary restriction (e.g., a low fiber diet) for one to three days before CTC examination is aimed at reducing fecal volume and stool inhomogeneity, thus favoring optimal tagging[8]. Bowel purgation should normally be included in CTC preparation; however, in order to improve patient compliance, aggressive catharsis should be restricted to 24 h or less and a reduced volume of laxative agent should be preferred[9-12]. The choice of the agent reflects local experiences, with no clear preference for any agent.
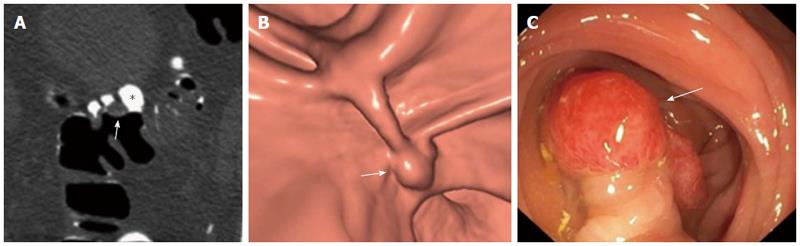
In order to improve polyp detection and to reduce the number of false-positive examinations, “labelling” or “tagging” of residual stools and fluids is strongly recommended and is considered mandatory unless contraindicated. Fecal tagging consists of oral administration of either water-soluble iodinated contrast medium or, alternatively, a diluted barium sulfate suspension. Because such oral contrast agents are hyperattenuating to X-rays, the tagged residual bowel content appears hyperdense and can be readily distinguished from true colonic lesions. The use of electronic stool subtraction software allows the colon to be cleansed of tagged residues and virtual navigation to be performed[13] (Figure 1). Although robust data are not available in the literature comparing the two tagging agents[14-16], iodinated contrasts offer more homogeneous tagging and the possibility for same-day CS; the risk of allergic reactions, due to iodine absorption through the gastrointestinal tract, is extremely rare, or even anecdotal[17].
A further step to improve patient compliance is the utilization of reduced bowel preparations[18] using only high doses of hyperosmolar contrast agents, without the need for any additional laxative agent[19,20]. This approach, which is associated with better patient compliance and with reduced cathartic effects compared with a conventional colon cleansing for CS, can now be considered routine practice, particularly in screening[5] and in elderly and frail patients[21,22].
The colon is inflated with air or carbon dioxide by using a thin and flexible rectal catheter by a radiologist, resident, technician, or nurse specifically trained in the technique[23]. Although automatic insufflation with carbon dioxide is the method of choice to optimize colonic distention and to maximize patient comfort, a higher cost is a drawback of this technique[24]. No sedation is required. The use of a spasmolytic agent, and in particular hyoscine butylbromide (Buscopan®)[25], may provide better distension, especially of the sigmoid colon in the case of diverticular disease or severely stenosing cancer, although a clear improvement in the accuracy of polyp detection has not been demonstrated[26,27]. The intravenous injection of iodinated contrast medium is reserved for the following situations: in patients with known CRC, to improve staging, and in symptomatic patients based on the clinical indication, and for the need to investigate the extracolonic organs fully[28].
The use of multidetector row CT (MDCT) scanners (≥ 16 rows) is considered an essential prerequisite to achieve high-quality (max collimation ≤ 2.5 mm; single breath-hold) and low-dose examinations. The use of dose modulation devices[29], which reduce does exposure by 30%-35% (except in obese patients) is strongly recommended; if available, adaptive statistical iterative reconstruction and model-based iterative reconstructions[30], which reduce dose exposure up to 50%, are preferred.
Image post-processing is performed on dedicated off-line workstations suitable for 3D data management and reconstruction. CTC interpretation should be based on both 2D and 3D images, and the use of either a primary 3D or 2D approach depends on personal preference and availability[31]. Computer-aided detection (CAD) software is useful to reduce perceptual errors during CTC interpretation, if employed in a second-reader paradigm. Particularly, CAD improves sensitivity for small (6-9 mm) polyps, even in expert readers[32]. However, the use of CAD should not preclude adequate reader training, since the interpretation of CAD markings is under the control of the radiologist.
All of the relevant data are provided in Table 1.
| Ref. | Year | Design | Polyp > 6 mm | Polyp > 6 mm, < 10 mm | Polyp > 10 mm | CancerSe | |||
| Se | Sp | Se | Sp | Se | Sp | ||||
| Pickhardt et al[33] | 2011 | Meta-analysis | 96.1% | ||||||
| de Haan et al[4] | 2011 | Meta-analysis | 75.9% | 94.6% | 68.1% | 96.5% | 83.3% | 98.7% | |
| Sosna et al[43] | 2003 | Meta-analysis | 84% | 88% | 95% | ||||
| Chaparro et al[41] | 2009 | Meta-analysis | 63% | 90% | 83% | 92% | |||
| Mulhall et al[44] | 2005 | Meta-analysis | 70% | 93% | 85% | 97% | |||
| Halligan et al[42] | 2005 | Meta-analysis | 86% | 86% | 93% | 97% | |||
| Rosman et al[45] | 2007 | Meta-analysis | 63% | 82% | |||||
| Sosna et al[69] | 2008 | Meta-analysis | 70.7% | 82.3% | 95.4% | ||||
| Plumb et al[46] | 2014 | Meta-analysis | 89.7% | 74% | 92% | 95% | 95.8% | ||
| Atkin et al[2] | 2013 | RCT | 96.5% | ||||||
| Halligan et al[3] | 2013 | RCT | 93.3% | ||||||
| Stoop et al[5] | 2012 | RCT | |||||||
| Johnson et al[37] | 2008 | Obs Multic | 78% | 88% | 90% | 86% | |||
| Regge et al[38] | 2009 | Obs Multic | 85.3% | 87.8% | 90.8% | 84.5% | 95.1% | ||
| Neri et al[7] | 2013 | Obs Single | 95.6% | 93.9% | 100% | 98% | |||
| Graser et al[40] | 2009 | Obs Single | 91.3% | 93.1% | 92% | 97.9% | |||
| Pickhardt et al[39] | 2003 | Obs Single | 88.7% | 79.6% | 93.8% | 96% | |||
| Mean | 86.3% | 87.1% | 69.8% | 93.1% | 88.8% | 94.3% | 95.4% | ||
Recent meta-analyses[4,33] and randomized clinical trials have reported that the diagnostic performance of CTC for the detection of CRC and clinically significant adenomatous polyps (≥ 10 mm) both in symptomatic[2,3] and asymptomatic subjects[5] is similar to CS and is largely superior to BE. In fact, the sensitivity of CTC and CS for CRC were shown to be 96% and 95%, respectively, in a recent meta-analysis[33]. The importance of this study is that it showed that sensitivity is maintained, despite a wide variation in technique, demonstrating potential generalizability and widespread implementation of CTC. In the first randomized trial[2], which compared CTC and CS in symptomatic patients, the overall performances were not statistically significant different for either CRC or large (≥ 10 mm) polyps. Given the relatively low prevalence of CRC, even among symptomatic cohorts, primary CTC may be more suitable than CS for the initial investigation of suspected CRC. In fact, the cancer miss rate of CTC is low. In a study on 3800 patients who were followed-up by using the National Cancer Registry database after CTC[34], seven cancers were missed (five because of technical limitations and two because of perceptive errors; systems errors and severe patient co-morbidity contributed to three of the cases), with an overall missed rate of around 5.3%. These data are similar to those collected for CS (miss rate, 5.9%)[35] and were better than those collected for BE (missed rate, 6.7%)[36].
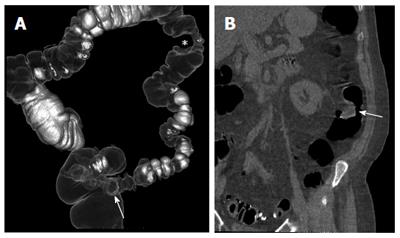
Several studies have demonstrated the accuracy of CTC in identifying and characterizing colonic polyps (Figure 2). Two randomized clinical trials[2,3,5], two multicenter trials[37,38], two single-center trials[39,40], and eight different meta-analyses[4,33,41-46] came to similar conclusions: CTC sensitivity for clinically significant polyps (larger than 10 mm) is high and is similar to that of CS; the sensitivity for small (6-9 mm) polyps is slightly lower than that of CS; the specificity and negative predictive value, even for small polyps, are good, especially if fecal/fluid tagging techniques are used; and the variability of the results among different series is mostly due to perceptual errors and consequently to reader inexperience[47]. The importance of reader experience was also confirmed by a multicenter study[48] designed to assess the accuracy of CTC in detecting polyps or cancers after a preliminary training and qualifying program for radiologists. The most relevant finding of the study was that radiologist’s polyp detection rate during training was the only significant factor in predicting the accuracy of CTC for detecting polyps.
This result leads to the issue of training and the learning curve. Although the debate is on-going, one study[49] attempted to determine how many CTC training datasets had to be evaluated by a novice reader to reach an adequate level of diagnostic confidence, which was defined as a sensitivity of 95% for lesions 10 mm or larger, a sensitivity of 90% for lesions 6 mm or larger, and a per-patient specificity of 80% for lesions 6 mm or larger. This study showed that at least 175 CTC studies with colonoscopic verification are necessary for most trainees, although a sub-group may require additional training because of the inability to reach the predefined threshold levels. However, once adequately trained, radiologists can obtain consistent performance for adenoma and advanced neoplasia detection, as well as other clinically relevant endpoints, in a CTC screening program[50].
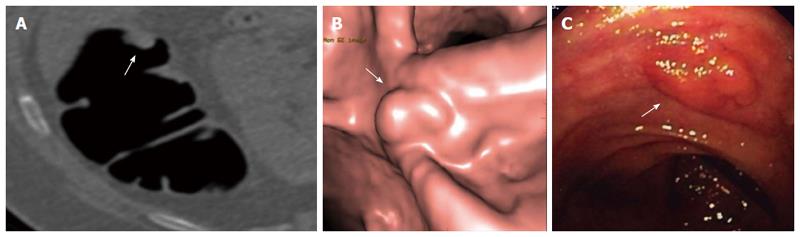
Non-polypoid lesions represent a sub-group of colonic neoplasms, which are classified into the following types: slightly elevated (type 0-IIa), completely flat (type 0-IIb), and depressed (type 0-IIc)[51]. Type 0-IIa lesions are sometimes misclassified as sessile polyps because they are slightly elevated from the mucosal surface. In order to avoid this confusion, the height of a flat lesion should not exceed ½ of its diameter[52]. In CTC, a lesion is defined as flat when the vertical elevation above the surrounding mucosa is less than 3 mm[53] (Figure 3). Another sub-type of non-polypoid tumors is the so-called “carpet” lesion, which is defined as a flat, laterally spreading colorectal mass measuring 3 cm or greater in size[54].
Although the epidemiology is not completely clear, recent data suggest that the prevalences of non-polypoid lesions in Europe[55] and the United States[56] are close to that of Japan[57]. The likelihood of malignancy is directly related to morphology rather than to size: types 0-IIa and 0-IIb have a risk of harboring high-grade dysplasia or cancer similar to polyps, whereas type 0-IIc has a definitely higher likelihood of malignancy[57]. CTC can potentially detect flat and depressed adenomas, which result in focal thickening of the colonic wall, but it is probably not suitable for evaluating the presence of completely flat lesions[58]; however, the latter are exceedingly rare[56,57]. At the time of this paper, data regarding CTC sensitivity for flat lesions are sparse, and the patient series are small for the available studies. The largest studies report sensitivity in the range of 80%-90% for flat adenocarcinomas[59,60], and excellent results were also described for lateral spreading tumors[61].
A possible advocated advantage of CTC is the detection of extracolonic findings (ECF). ECF are common in symptomatic patients as well as in screening patients referred for CTC, with a prevalence ranging between 25% to more than 50%[62]. They increase with age and are not clinically significant in most cases; however, they are reported as important in 10% of patients, requiring further work-up[63]. Important ECF include extracolonic cancers (with the most common being renal and lung cancers and lymphoma), abdominal aortic aneurysms, and adrenal lesions. The work-up for ECF produces additional costs that should be considered when calculating the cost-effectiveness of CTC, particularly for screening. In a mathematic model, the detection of an ECF such as abdominal aortic aneurysm and extracolonic cancers in addition to CRC makes CTC a dominant screening strategy over both CS and CS with one-time ultrasonography[64].
The availability of robust and evidence-based data in the literature has increased the indications for CTC. Today, incomplete or failed CS, the evaluation of elderly, frail, and symptomatic patients, and the investigation of diverticular disease are clear clinical situations where CTC can be safely proposed. However, CTC can also be recommended in CRC screening and surveillance despite the lack of robust evidence, if CS is unfeasible. Further, potential contraindications should not be overlooked in order to ensure the appropriate use of CTC.
Incomplete or failed CS: Incomplete or failed CS is not an uncommon event. The caecal intubation rate, a quality metric recorded by the endoscopists, is extremely variable in daily practice and it can be far from high-quality standards[65].
CS can be incomplete for a variety of reasons other than poor bowel preparation: obstructing masses, neoplastic or inflammatory strictures, redundant colon, patient discomfort, colonic spasm, severe diverticulosis, and adherences from previous surgery. Further, incomplete CS is more common in elderly patients and in women[66]. The experience of the endoscopist is another extremely important variable because up to 10% of CS procedures are considered technically challenging, even for experts[67].
In the case of incomplete/failed CS, examination of the entire colon is recommended in order to avoid missing an advanced lesion[68]. Because the performance of CTC is superior to BE[3,46,69], CTC is the examination of choice[70]. In the case of neoplastic obstruction, CTC can be used for excluding synchronous lesions in the remaining colon and for whole body staging of primary colonic neoplasm, if contrast-enhanced CTC is performed[71,72]. Moreover, CTC can define predictive factors for a potentially difficult CS, such as colonic looping, acute angle flexures, and tortuosity, because it reveals colonic anatomy[73].
Elderly and frail patients: CS, apart from being incomplete or failed, can be unfeasible because of the patient’s condition, including advanced age, poor compliance to bowel preparation, or associated severe co-morbidities. CS has an increased risk of perforation and bleeding in elderly patients[74] and in those undergoing anti-coagulant therapy[75], respectively. Because of the progressive aging of the population, this sub-group of patients is likely to increase in the future, with consequently higher frequencies of colonic cancer and diverticular disease[76,77]. CTC has the advantage of being technically feasible, well tolerated, and safe. Only patients with a positive finding will be referred for more invasive and risky examinations because of the high positive and negative predictive values for cancer and large polyps[78,79].
Investigation of diverticular disease: Diverticular disease is the most common colonic disorder in the Western world, with a prevalence of over 60% at 80 years[77]. The clinical diagnosis is challenging because patient symptoms and laboratory findings are unspecific and overlap with other gastroenterological entities (e.g., irritable bowel syndrome) and in young women (< 40 years of age) with gynecological disorders[80]. Thus, imaging tests are necessary for the diagnosis. The choice of either CTC or CS as the first-line imaging examination depends mostly on the patient’s age, risk factors, clinical status, and preference, as well as on imaging availability and local expertise[81]. In elderly individuals, especially those who are frail and have potential contraindications to CS and sedation, CTC is preferred because it is minimally invasive. On the other hand, CS should be the first choice in younger patients in whom symptoms might be related to colic inflammatory changes because the diagnosis would be challenging with CTC. The use of BE should be discouraged because of poorer patient compliance[82], longer examination time, higher risk of complications[83,84], and radiation exposure[85].
In patients with established diverticular disease, CTC can provide a balanced view of the disease by incorporating visual analysis with quantitative analysis by using a CTC-based diverticular disease severity score; this score appears to correlate with relevant coexisting lesions and can potentially influence therapeutic decision-making[86].
Symptomatic patients: Gastrointestinal alarm symptoms potentially suggestive of CRC such as abdominal pain or discomfort, rectal bleeding, iron-deficiency anemia, and unintended weight loss are highly non-specific; further, these symptoms are, unfortunately, common in the general population, particularly in the elderly[87]. The accuracy for identifying patients with underlying structural disease is disappointing, and many of these patients who are referred for CS will ultimately prove to be normal[88]. Elderly patients are often frail and more likely to have an incomplete or difficult CS, with a greater risk of adverse events[65,66] and an increased workload for endoscopy centers. For these reasons, CTC might be used as a triage technique, with only patients with positive findings being referred for CS. Practically, proposing CS as the best indication for symptomatic patients presenting with bleeding and diarrhea and proposing CTC for those with pain or weight loss may be reasonable.
Other indications: New and emerging indications have been explored, with some of them being entered de facto in clinical use: CTC for tumor localization before laparoscopic surgery because of the inaccuracy of CS in determining the precise localization and extent of the lesion[89]; investigation of patients with colonic stoma[90]; and assessment of colonic involvement in patients affected by deep pelvic endometriosis[91].
CRC screening: CRC screening is complex, and this is particularly true in Europe, where population-based programs using fecal occult blood tests are already organized. Within the framework of an established screening program, the role of CTC is to replace BE in the case of incomplete CS[92] and to evaluate patients with a positive fecal occult blood test who refuse CS[93].
Despite the current situation, research is committed to exploring the potential use of CTC as a screening modality alternative to the available tests. Before implementing CTC, data on patient adherence to CTC-based screening programs and cost-effectiveness are necessary. The recent results of a Dutch trial[5] showed that the participation rate for the screening program was increased with CTC compared with CS (34% vs 22%; P < 0.0001). Although these results are extremely interesting, they must be confirmed by other on-going trials[94,95].
The available data on the cost-effectiveness of CTC for screening based on mathematical models are discordant: in the majority of the models, CTC is dominated by either CS or a combination of flexible sigmoidoscopy and fecal occult blood test[96]. However, the costs of CT-screening in the Dutch trial[97] were substantially lower than the cost assumptions used in published cost-effectiveness analyses, indicating the need for re-evaluation of those data.
Apart from population screening, on an individual basis, CTC can be suggested as a CRC screening test provided the individual is adequately informed about the test characteristics, benefits, and possible risks[98].
Surveillance: In patients who have undergone previous surgery for CRC, colonic and extracolonic surveillance are needed because more than 50% of recurrent tumors will present as extracolonic metastatic disease (particularly in the liver parenchyma), and many local recurrences lack an intraluminal colonic component[99]. The current surveillance guidelines include a combination of clinical assessment, serum carcinoembryonic antigen (CEA) testing, CS, and contrast-enhanced CT[100]. CTC, if performed by using a different technical strategy (i.e., during the intravenous administration of iodinated contrast medium), combines the ability of detecting polyps and cancer with an accuracy similar to CS, while simultaneously offering an evaluation of extracolonic findings[101]. However, this approach is presently recommended only if a patient is unable or unwilling to undergo CS because of the lack of robust and evidence-based data.
Patients who have previously undergone endoscopic resection of colonic adenoma are likely to develop a metachronous lesion; therefore, these patients must be included in a surveillance program using CS. The frequency intervals for follow-up remain controversial[102]; these are based on the findings at index CS (size, number, and histology of the removed polyp/s). Unfortunately, despite the official recommendations, patient adherence to follow-up is extremely variable and is generally poor in clinical practice[103]. For those patients unwilling to undergo CS, follow-up CTC can be suggested as an alternative option.
Acute abdominal conditions, like diverticulitis or the acute phase of IBDs, are contraindications to CTC because of the high risk of complications (i.e., perforations)[84].
Further, precautions should be taken when performing CTC after endoscopic resection. A two-week delay is suggested[6], although clear scientific evidence is lacking to confirm this. In fact, a study on patients who underwent CTC within 24 h following CS with either polypectomy or biopsy sampling did not find colonic perforation[27]. On the other hand, endoscopic biopsy is not considered a contraindication and same-day CTC can be safely performed.
CTC should be also avoided as a surveillance test in patients with a long-standing history of ulcerative colitis or Crohn’s disease and in those with hereditary non-polypoid colorectal cancer (HNPCC), Lynch syndrome, and APC-associated polyposis conditions. In all of these cases, CS is the preferred diagnostic option at a timing and interval different from screening in average- or higher-than-average risk individuals because of the highly increased risk of developing CRC[104,105].
In conclusion, CTC is a mature and robust imaging modality, with an accuracy very close to CS. CTC has completely replaced BE because of its superior performance, higher patient compliance, and lower dose exposure. CTC can be considered the leading modality for colonic imaging in many clinical situations, although CS is still preferred for several indications. A clear understanding of the exact role of CTC will be beneficial to maximize the benefits and minimize the potential sources of frustration or disappointment for both referring clinicians and patients.
P- Reviewer: Nagata K, Nash GF, Patai AV, Sabbagh LC, Shehata MMM, Steele SR S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Vining DJ, Gelfand DW, Bechtold RE, Scharding ES, Grishaw EK, Shifrin RY. Technical feasibility of colon imaging with helical CT and virtual reality. AJR Am J Roentgenol. 1994;162:104. [Cited in This Article: ] |
| 2. | Atkin W, Dadswell E, Wooldrage K, Kralj-Hans I, von Wagner C, Edwards R, Yao G, Kay C, Burling D, Faiz O. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet. 2013;381:1194-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 3. | Halligan S, Wooldrage K, Dadswell E, Kralj-Hans I, von Wagner C, Edwards R, Yao G, Kay C, Burling D, Faiz O. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. Lancet. 2013;381:1185-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | de Haan MC, van Gelder RE, Graser A, Bipat S, Stoker J. Diagnostic value of CT-colonography as compared to colonoscopy in an asymptomatic screening population: a meta-analysis. Eur Radiol. 2011;21:1747-1763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, van de Vijver MJ, Biermann K, Thomeer M, van Leerdam ME. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13:55-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 263] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Spada C, Stoker J, Alarcon O, Barbaro F, Bellini D, Bretthauer M, De Haan MC, Dumonceau JM, Ferlitsch M, Halligan S. Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline. Eur Radiol. 2014;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Neri E, Halligan S, Hellström M, Lefere P, Mang T, Regge D, Stoker J, Taylor S, Laghi A. The second ESGAR consensus statement on CT colonography. Eur Radiol. 2013;23:720-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Liedenbaum MH, Denters MJ, de Vries AH, van Ravesteijn VF, Bipat S, Vos FM, Dekker E, Stoker J. Low-fiber diet in limited bowel preparation for CT colonography: Influence on image quality and patient acceptance. AJR Am J Roentgenol. 2010;195:W31-W37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Keedy AW, Yee J, Aslam R, Weinstein S, Landeras LA, Shah JN, McQuaid KR, Yeh BM. Reduced cathartic bowel preparation for CT colonography: prospective comparison of 2-L polyethylene glycol and magnesium citrate. Radiology. 2011;261:156-164. [PubMed] [Cited in This Article: ] |
| 10. | Pollentine A, Mortimer A, McCoubrie P, Archer L. Evaluation of two minimal-preparation regimes for CT colonography: optimising image quality and patient acceptability. Br J Radiol. 2012;85:1085-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Campanella D, Morra L, Delsanto S, Tartaglia V, Asnaghi R, Bert A, Neri E, Regge D. Comparison of three different iodine-based bowel regimens for CT colonography. Eur Radiol. 2010;20:348-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Iafrate F, Iannitti M, Ciolina M, Baldassari P, Pichi A, Laghi A. Bowel cleansing before CT colonography: comparison between two minimal-preparation regimens. Eur Radiol. 2014;Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 13. | Oda M, Kitasaka T, Mori K, Suenaga Y, Takayama T, Takabatake H, Mori M, Natori H, Nawano S. Digital bowel cleansing free colonic polyp detection method for fecal tagging CT colonography. Acad Radiol. 2009;16:486-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Liedenbaum MH, de Vries AH, Gouw CI, van Rijn AF, Bipat S, Dekker E, Stoker J. CT colonography with minimal bowel preparation: evaluation of tagging quality, patient acceptance and diagnostic accuracy in two iodine-based preparation schemes. Eur Radiol. 2010;20:367-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Davis W, Nisbet P, Hare C, Cooke P, Taylor SA. Non-laxative CT colonography with barium-based faecal tagging: is additional phosphate enema beneficial and well tolerated? Br J Radiol. 2011;84:120-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Nagata K, Singh AK, Sangwaiya MJ, Näppi J, Zalis ME, Cai W, Yoshida H. Comparative evaluation of the fecal-tagging quality in CT colonography: barium vs. iodinated oral contrast agent. Acad Radiol. 2009;16:1393-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Ridley LJ. Allergic reactions to oral iodinated contrast agents: reactions to oral contrast. Australas Radiol. 1998;42:114-117. [PubMed] [Cited in This Article: ] |
| 18. | Ghanouni A, Smith SG, Halligan S, Taylor SA, Plumb A, Boone D, von Wagner C. An interview study analysing patients’ experiences and perceptions of non-laxative or full-laxative preparation with faecal tagging prior to CT colonography. Clin Radiol. 2013;68:472-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Zalis ME, Blake MA, Cai W, Hahn PF, Halpern EF, Kazam IG, Keroack M, Magee C, Näppi JJ, Perez-Johnston R. Diagnostic accuracy of laxative-free computed tomographic colonography for detection of adenomatous polyps in asymptomatic adults: a prospective evaluation. Ann Intern Med. 2012;156:692-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Iannaccone R, Laghi A, Catalano C, Mangiapane F, Lamazza A, Schillaci A, Sinibaldi G, Murakami T, Sammartino P, Hori M. Computed tomographic colonography without cathartic preparation for the detection of colorectal polyps. Gastroenterology. 2004;127:1300-1311. [PubMed] [Cited in This Article: ] |
| 21. | Iafrate F, Hassan C, Zullo A, Stagnitti A, Ferrari R, Spagnuolo A, Laghi A. CT colonography with reduced bowel preparation after incomplete colonoscopy in the elderly. Eur Radiol. 2008;18:1385-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Keeling AN, Slattery MM, Leong S, McCarthy E, Susanto M, Lee MJ, Morrin MM. Limited-preparation CT colonography in frail elderly patients: a feasibility study. AJR Am J Roentgenol. 2010;194:1279-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Burling D; International Collaboration for CT Colonography Standards. CT colonography standards. Clin Radiol. 2010;65:474-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Boellaard TN, de Haan MC, Venema HW, Stoker J. Colon distension and scan protocol for CT-colonography: an overview. Eur J Radiol. 2013;82:1144-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Rogalla P, Lembcke A, Rückert JC, Hein E, Bollow M, Rogalla NE, Hamm B. Spasmolysis at CT colonography: butyl scopolamine versus glucagon. Radiology. 2005;236:184-188. [PubMed] [Cited in This Article: ] |
| 26. | Bruzzi JF, Moss AC, Brennan DD, MacMathuna P, Fenlon HM. Efficacy of IV Buscopan as a muscle relaxant in CT colonography. Eur Radiol. 2003;13:2264-2270. [PubMed] [Cited in This Article: ] |
| 27. | Kim SY, Park SH, Choi EK, Lee SS, Lee KH, Kim JC, Yu CS, Kim HC, Kim AY, Ha HK. Automated carbon dioxide insufflation for CT colonography: effectiveness of colonic distention in cancer patients with severe luminal narrowing. AJR Am J Roentgenol. 2008;190:698-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Sosna J, Morrin MM, Kruskal JB, Farrell RJ, Nasser I, Raptopoulos V. Colorectal neoplasms: role of intravenous contrast-enhanced CT colonography. Radiology. 2003;228:152-156. [PubMed] [Cited in This Article: ] |
| 29. | Graser A, Wintersperger BJ, Suess C, Reiser MF, Becker CR. Dose reduction and image quality in MDCT colonography using tube current modulation. AJR Am J Roentgenol. 2006;187:695-701. [PubMed] [Cited in This Article: ] |
| 30. | Yoon MA, Kim SH, Lee JM, Woo HS, Lee ES, Ahn SJ, Han JK. Adaptive statistical iterative reconstruction and Veo: assessment of image quality and diagnostic performance in CT colonography at various radiation doses. J Comput Assist Tomogr. 2012;36:596-601. [PubMed] [Cited in This Article: ] |
| 31. | Neri E, Mang T, Hellstrom M, Mantarro A, Faggioni L, Bartolozzi C. How to read and report CTC. Eur J Radiol. 2013;82:1166-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Iussich G, Correale L, Senore C, Segnan N, Laghi A, Iafrate F, Campanella D, Neri E, Cerri F, Hassan C. CT colonography: preliminary assessment of a double-read paradigm that uses computer-aided detection as the first reader. Radiology. 2013;268:743-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology. 2011;259:393-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 34. | Sabanli M, Balasingam A, Bailey W, Eglinton T, Hider P, Frizelle FA. Computed tomographic colonography in the diagnosis of colorectal cancer. Br J Surg. 2010;97:1291-1294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Leaper M, Johnston MJ, Barclay M, Dobbs BR, Frizelle FA. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy. 2004;36:499-503. [PubMed] [Cited in This Article: ] |
| 36. | McDonald S, Lyall P, Israel L, Coates R, Frizelle F. Why barium enemas fail to identify colorectal cancers. ANZ J Surg. 2001;71:631-633. [PubMed] [Cited in This Article: ] |
| 37. | Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, Menias CO, Siewert B, Cheema JI, Obregon RG. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 775] [Cited by in F6Publishing: 670] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 38. | Regge D, Laudi C, Galatola G, Della Monica P, Bonelli L, Angelelli G, Asnaghi R, Barbaro B, Bartolozzi C, Bielen D. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA. 2009;301:2453-2461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 39. | Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191-2200. [PubMed] [Cited in This Article: ] |
| 40. | Graser A, Stieber P, Nagel D, Schäfer C, Horst D, Becker CR, Nikolaou K, Lottes A, Geisbüsch S, Kramer H. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 41. | Chaparro M, Gisbert JP, Del Campo L, Cantero J, Maté J. Accuracy of computed tomographic colonography for the detection of polyps and colorectal tumors: a systematic review and meta-analysis. Digestion. 2009;80:1-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Halligan S, Altman DG, Taylor SA, Mallett S, Deeks JJ, Bartram CI, Atkin W. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology. 2005;237:893-904. [PubMed] [Cited in This Article: ] |
| 43. | Sosna J, Morrin MM, Kruskal JB, Lavin PT, Rosen MP, Raptopoulos V. CT colonography of colorectal polyps: a metaanalysis. AJR Am J Roentgenol. 2003;181:1593-1598. [PubMed] [Cited in This Article: ] |
| 44. | Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005;142:635-650. [PubMed] [Cited in This Article: ] |
| 45. | Rosman AS, Korsten MA. Meta-analysis comparing CT colonography, air contrast barium enema, and colonoscopy. Am J Med. 2007;120:203-210.e4. [PubMed] [Cited in This Article: ] |
| 46. | Plumb AA, Halligan S, Pendsé DA, Taylor SA, Mallett S. Sensitivity and specificity of CT colonography for the detection of colonic neoplasia after positive faecal occult blood testing: systematic review and meta-analysis. Eur Radiol. 2014;24:1049-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Doshi T, Rusinak D, Halvorsen RA, Rockey DC, Suzuki K, Dachman AH. CT colonography: false-negative interpretations. Radiology. 2007;244:165-173. [PubMed] [Cited in This Article: ] |
| 48. | Heresbach D, Djabbari M, Riou F, Marcus C, Le Sidaner A, Pierredon-Foulogne MA, Ponchon T, Boudiaf M, Seyrig JA, Laumonier H. Accuracy of computed tomographic colonography in a nationwide multicentre trial, and its relation to radiologist expertise. Gut. 2011;60:658-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Liedenbaum MH, Bipat S, Bossuyt PM, Dwarkasing RS, de Haan MC, Jansen RJ, Kauffman D, van der Leij C, de Lijster MS, Lute CC. Evaluation of a standardized CT colonography training program for novice readers. Radiology. 2011;258:477-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Pooler BD, Kim DH, Hassan C, Rinaldi A, Burnside ES, Pickhardt PJ. Variation in diagnostic performance among radiologists at screening CT colonography. Radiology. 2013;268:127-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [PubMed] [Cited in This Article: ] |
| 52. | Tsuda S, Veress B, Tóth E, Fork FT. Flat and depressed colorectal tumours in a southern Swedish population: a prospective chromoendoscopic and histopathological study. Gut. 2002;51:550-555. [PubMed] [Cited in This Article: ] |
| 53. | Zalis ME, Barish MA, Choi JR, Dachman AH, Fenlon HM, Ferrucci JT, Glick SN, Laghi A, Macari M, McFarland EG. CT colonography reporting and data system: a consensus proposal. Radiology. 2005;236:3-9. [PubMed] [Cited in This Article: ] |
| 54. | Tanaka S, Haruma K, Oka S, Takahashi R, Kunihiro M, Kitadai Y, Yoshihara M, Shimamoto F, Chayama K. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62-66. [PubMed] [Cited in This Article: ] |
| 55. | Bianco MA, Cipolletta L, Rotondano G, Buffoli F, Gizzi G, Tessari F; Flat Lesions Italian Network (FLIN). Prevalence of nonpolypoid colorectal neoplasia: an Italian multicenter observational study. Endoscopy. 2010;42:279-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, Matsui S, Friedland S. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 484] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 57. | Kudo Se, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 58. | Ignjatovic A, Burling D, Ilangovan R, Clark SK, Taylor SA, East JE, Saunders BP. Flat colon polyps: what should radiologists know? Clin Radiol. 2010;65:958-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR Am J Roentgenol. 2004;183:1343-1347. [PubMed] [Cited in This Article: ] |
| 60. | Park SH, Kim SY, Lee SS, Bogoni L, Kim AY, Yang SK, Myung SJ, Byeon JS, Ye BD, Ha HK. Sensitivity of CT colonography for nonpolypoid colorectal lesions interpreted by human readers and with computer-aided detection. AJR Am J Roentgenol. 2009;193:70-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Pickhardt PJ, Lam VP, Weiss JM, Kennedy GD, Kim DH. Carpet lesions detected at CT colonography: clinical, imaging, and pathologic features. Radiology. 2014;270:435-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Yee J, Sadda S, Aslam R, Yeh B. Extracolonic findings at CT colonography. Gastrointest Endosc Clin N Am. 2010;20:305-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Pickhardt PJ, Hanson ME, Vanness DJ, Lo JY, Kim DH, Taylor AJ, Winter TC, Hinshaw JL. Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology. 2008;249:151-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 64. | Hassan C, Pickhardt PJ, Laghi A, Kim DH, Zullo A, Iafrate F, Di Giulio L, Morini S. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: model simulation with cost-effectiveness analysis. Arch Intern Med. 2008;168:696-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Rathgaber SW, Wick TM. Colonoscopy completion and complication rates in a community gastroenterology practice. Gastrointest Endosc. 2006;64:556-562. [PubMed] [Cited in This Article: ] |
| 66. | Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007;132:2297-2303. [PubMed] [Cited in This Article: ] |
| 67. | Anderson ML, Heigh RI, McCoy GA, Parent K, Muhm JR, McKee GS, Eversman WG, Collins JM. Accuracy of assessment of the extent of examination by experienced colonoscopists. Gastrointest Endosc. 1992;38:560-563. [PubMed] [Cited in This Article: ] |
| 68. | Neerincx M, Terhaar sive Droste JS, Mulder CJ, Räkers M, Bartelsman JF, Loffeld RJ, Tuynman HA, Brohet RM, van der Hulst RW. Colonic work-up after incomplete colonoscopy: significant new findings during follow-up. Endoscopy. 2010;42:730-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 69. | Sosna J, Sella T, Sy O, Lavin PT, Eliahou R, Fraifeld S, Libson E. Critical analysis of the performance of double-contrast barium enema for detecting colorectal polyps & gt; or = 6 mm in the era of CT colonography. AJR Am J Roentgenol. 2008;190:374-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | AGA Clinical Practice and Economics Committee. Position of the American Gastroenterological Association (AGA) Institute on computed tomographic colonography. Gastroenterology. 2006;131:1627-1628. [PubMed] [Cited in This Article: ] |
| 71. | Hong N, Park SH. CT colonography in the diagnosis and management of colorectal cancer: emphasis on pre- and post-surgical evaluation. World J Gastroenterol. 2014;20:2014-2022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Sali L, Falchini M, Taddei A, Mascalchi M. Role of preoperative CT colonography in patients with colorectal cancer. World J Gastroenterol. 2014;20:3795-3803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Eickhoff A, Pickhardt PJ, Hartmann D, Riemann JF. Colon anatomy based on CT colonography and fluoroscopy: impact on looping, straightening and ancillary manoeuvres in colonoscopy. Dig Liver Dis. 2010;42:291-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230-236. [PubMed] [Cited in This Article: ] |
| 75. | Ko CW, Riffle S, Michaels L, Morris C, Holub J, Shapiro JA, Ciol MA, Kimmey MB, Seeff LC, Lieberman D. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin Gastroenterol Hepatol. 2010;8:166-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 76. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1965] [Cited by in F6Publishing: 2135] [Article Influence: 213.5] [Reference Citation Analysis (1)] |
| 77. | Young-Fadok TM, Roberts PL, Spencer MP, Wolff BG. Colonic diverticular disease. Curr Probl Surg. 2000;37:457-514. [PubMed] [Cited in This Article: ] |
| 78. | Iafrate F, Hassan C, Ciolina M, Lamazza A, Baldassari P, Pichi A, Zullo A, Stagnitti A, Iannitti M, Rengo M. High positive predictive value of CT colonography in a referral centre. Eur J Radiol. 2011;80:e289-e292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Saunders JH, Miskovic D, Bowman C, Panto P, Menon A. Colorectal cancer is reliably excluded in the frail and elderly population by minimal preparation CT. Tech Coloproctol. 2014;18:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Stollman NH, Raskin JB. Diagnosis and management of diverticular disease of the colon in adults. Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:3110-3121. [PubMed] [Cited in This Article: ] |
| 81. | Halligan S, Saunders B. Imaging diverticular disease. Best Pract Res Clin Gastroenterol. 2002;16:595-610. [PubMed] [Cited in This Article: ] |
| 82. | von Wagner C, Smith S, Halligan S, Ghanouni A, Power E, Lilford RJ, Morton D, Dadswell E, Atkin W, Wardle J. Patient acceptability of CT colonography compared with double contrast barium enema: results from a multicentre randomised controlled trial of symptomatic patients. Eur Radiol. 2011;21:2046-2055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Iafrate F, Iussich G, Correale L, Hassan C, Regge D, Neri E, Baldassari P, Ciolina M, Pichi A, Iannitti M. Adverse events of computed tomography colonography: an Italian National Survey. Dig Liver Dis. 2013;45:645-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Bellini D, Rengo M, De Cecco CN, Iafrate F, Hassan C, Laghi A. Perforation rate in CT colonography: a systematic review of the literature and meta-analysis. Eur Radiol. 2014;24:1487-1496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 85. | Neri E, Faggioni L, Cerri F, Turini F, Angeli S, Cini L, Perrone F, Paolicchi F, Bartolozzi C. CT colonography versus double-contrast barium enema for screening of colorectal cancer: comparison of radiation burden. Abdom Imaging. 2010;35:596-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Flor N, Rigamonti P, Pisani Ceretti A, Romagnoli S, Balestra F, Sardanelli F, Cornalba G, Pickhardt PJ. Diverticular disease severity score based on CT colonography. Eur Radiol. 2013;23:2723-2729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 258] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 88. | Wald A. Clinical manifestations and diagnosis of irritable bowel syndrome. Accessed June 2, 2010. Available from: http://www.uptodate.com/contents/topic.do?topicKey =GAST/2630. [Cited in This Article: ] |
| 89. | Neri E, Turini F, Cerri F, Faggioni L, Vagli P, Naldini G, Bartolozzi C. Comparison of CT colonography vs. conventional colonoscopy in mapping the segmental location of colon cancer before surgery. Abdom Imaging. 2010;35:589-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Lee JH, Park SH, Lee SS, Kim AY, Kim JC, Yu CS, Ha HK. CT colonography in patients who have undergone sigmoid colostomy: a feasibility study. AJR Am J Roentgenol. 2011;197:W653-W657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Koutoukos I, Langebrekke A, Young V, Qvigstad E. Imaging of endometriosis with computerized tomography colonography. Fertil Steril. 2011;95:259-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Sali L, Falchini M, Bonanomi AG, Castiglione G, Ciatto S, Mantellini P, Mungai F, Menchi I, Villari N, Mascalchi M. CT colonography after incomplete colonoscopy in subjects with positive faecal occult blood test. World J Gastroenterol. 2008;14:4499-4504. [PubMed] [Cited in This Article: ] |
| 93. | Sali L, Grazzini G, Ventura L, Falchini M, Borgheresi A, Castiglione G, Grimaldi M, Ianniciello N, Mallardi B, Zappa M. Computed tomographic colonography in subjects with positive faecal occult blood test refusing optical colonoscopy. Dig Liver Dis. 2013;45:285-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Sali L, Grazzini G, Carozzi F, Castiglione G, Falchini M, Mallardi B, Mantellini P, Ventura L, Regge D, Zappa M. Screening for colorectal cancer with FOBT, virtual colonoscopy and optical colonoscopy: study protocol for a randomized controlled trial in the Florence district (SAVE study). Trials. 2013;14:74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 95. | Regge D, Iussich G, Senore C, Correale L, Hassan C, Bert A, Montemezzi S, Segnan N. Population screening for colorectal cancer by flexible sigmoidoscopy or CT colonography: study protocol for a multicenter randomized trial. Trials. 2014;15:97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Hassan C, Pickhardt PJ. Cost-effectiveness of CT colonography. Radiol Clin North Am. 2013;51:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | de Haan MC, Thomeer M, Stoker J, Dekker E, Kuipers EJ, van Ballegooijen M. Unit costs in population-based colorectal cancer screening using CT colonography performed in university hospitals in The Netherlands. Eur Radiol. 2013;23:897-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, Pickhardt P, Rex DK, Thorson A, Winawer SJ; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1169] [Cited by in F6Publishing: 1163] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 99. | Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93:1115-1122. [PubMed] [Cited in This Article: ] |
| 100. | Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465-4470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 101. | Kim HJ, Park SH, Pickhardt PJ, Yoon SN, Lee SS, Yee J, Kim DH, Kim AY, Kim JC, Yu CS. CT colonography for combined colonic and extracolonic surveillance after curative resection of colorectal cancer. Radiology. 2010;257:697-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Hassan C, Quintero E, Dumonceau JM, Regula J, Brandão C, Chaussade S, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Gimeno-García A. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 392] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 103. | Rapuri S, Spencer J, Eckels D. Importance of postpolypectomy surveillance and postpolypectomy compliance to follow-up screening--review of literature. Int J Colorectal Dis. 2008;23:453-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 104. | Bojarski C. Malignant transformation in inflammatory bowel disease: prevention, surveillance and treatment - new techniques in endoscopy. Dig Dis. 2009;27:571-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 754] [Cited by in F6Publishing: 764] [Article Influence: 54.6] [Reference Citation Analysis (0)] |