Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5582
Peer-review started: October 6, 2014
First decision: October 29, 2014
Revised: November 16, 2014
Accepted: January 21, 2015
Article in press: January 21, 2015
Published online: May 14, 2015
AIM: To compare the prognostic ability of inflammation scores for patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) undergoing transarterial chemoembolization (TACE).
METHODS: Data of 224 consecutive patients who underwent TACE for unresectable HBV-related HCC from September 2009 to November 2011 were retrieved from a prospective database. The association of inflammation scores with clinicopathologic variables and overall survival (OS) were analyzed, and receiver operating characteristic curves were generated, and the area under the curve (AUC) was calculated to evaluate the discriminatory ability of each inflammation score and staging system, including tumor-node-metastasis, Barcelona Clinic Liver Cancer, and Cancer of the Liver Italian Program (CLIP) scores.
RESULTS: The median follow-up period was 390 d, the one-, two-, and three-year OS were 38.4%, 18.3%, and 11.1%, respectively, and the median OS was 390 d. The Glasgow Prognostic Score (GPS), modifed GPS, neutrophil-lymphocyte ratio, and Prognostic Index were associated with OS. The GPS consistently had a higher AUC value at 6 mo (0.702), 12 mo (0.676), and 24 mo (0.687) in comparison with other inflammation scores. CLIP consistently had a higher AUC value at 6 mo (0.656), 12 mo (0.711), and 24 mo (0.721) in comparison with tumor-node-metastasis and Barcelona Clinic Liver Cancer staging systems. Multivariate analysis revealed that alanine aminotransferase, GPS, and CLIP were independent prognostic factors for OS. The combination of GPS and CLIP (AUC = 0.777) was superior to CLIP or GPS alone in prognostic ability for OS.
CONCLUSION: The prognostic ability of GPS is superior to other inflammation scores for HCC patients undergoing TACE. Combining GPS and CLIP improved the prognostic power for OS.
Core tip: This study compared the inflammation scores [including the Glasgow Prognostic Score (GPS), modified GPS, neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, Prognostic Index, and Prognostic Nutritional Index] in patients with hepatitis B virus-related hepatocellular carcinoma undergoing transarterial chemoembolization, and concluded that GPS was superior to others. To improve the prognostic power, we proposed a new combined score containing GPS and Cancer of the Liver Italian Program, and the results showed that the combined scores enhanced the predictive ability. Thus, our study provides evidence for individualized treatment in clinical practice.
- Citation: Zhou DS, Xu L, Luo YL, He FY, Huang JT, Zhang YJ, Chen MS. Inflammation scores predict survival for hepatitis B virus-related hepatocellular carcinoma patients after transarterial chemoembolization. World J Gastroenterol 2015; 21(18): 5582-5590
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5582
Hepatocellular carcinoma (HCC) is the fifth most frequent cancer worldwide, and the third leading cause of cancer-related deaths, with an estimated 748300 new HCC cases and 695900 HCC-related deaths occurring in 2008[1]. Only 10%-30% of HCCs are amenable to curative treatments, including surgical resection, liver transplantation and local ablation, at the time of diagnosis because of advanced tumor stage and/or underlying advanced liver cirrhosis[2]. Transarterial chemoembolization (TACE) is considered to be the standard care for unresectable and unablatable intermediate- and advanced-stage HCC worldwide[3-5], and shows survival benefit in select patients[6,7]. However not all patients benefit from TACE, and some patients even suffer detrimental effects. Thus, it is important to recruit patients who would most likely benefit from TACE in such a heterogeneous HCC population[8]. Studies have demonstrated that the utility of most of available staging systems in predicting survival in patients undergoing TACE was often limited[9-11]. The characteristics of patients with unresectable HCC might substantially differ from a population of patients balanced between early and advanced disease, for which the available staging systems were originally developed.
There is increasing interest in the role of systemic inflammation as a predictor of outcome in HCC. The Glasgow Prognostic Score (GPS), a combination of serum C-reactive protein (CRP) and albumin is one of the useful inflammation scores for HCC and other cancer patients[12-15]. Moreover, the neutrophil-lymphocyte ratio (NLR) is associated with survival in patients with HCC[16-19]. Pinato et al[20] suggested the Prognostic Nutritional Index (PNI), which combines albumin and lymphocyte levels, as an independent and externally validated predictor of poor survival in patients with HCC. The platelet-lymphocyte ratio (PLR) and Prognostic Index (PI) have also been associated with outcome of other cancers[21,22]. However, which inflammation score is more suitable for predicting outcome in patients with hepatitis B virus (HBV)-related HCC undergoing TACE has not been fully elucidated.
The aims of present study were to validate the prognostic value of inflammation-based prognostic scores, including the GPS, modifed GPS (mGPS), NLR, PLR, PI, and PNI, for patients with HBV-related HCC undergoing TACE, and to validate the combination of staging system and inflammation score to improve the prognostic power.
Patients who received TACE treatment for unresectable HCC from September 2009 to November 2011 at the Department of Hepatobiliary Surgery, Sun Yat-Sen University Cancer Canter (Guangzhou, China) were identified from our prospective database. The research was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center, and written informed consent was obtained.
The diagnosis of HCC was based on the diagnostic criteria for HCC used by the American Association for the Study of the Liver guidelines[23]. HCC was diagnosed by at least two radiologic images showing characteristic features of HCC or one radiologic image showing characteristic features of HCC associated with elevated serum alpha-fetoprotein (AFP; ≥ 400 ng/mL) or histopathologic evidence. Patients that met all of the following criteria were included in analysis: (1) no previous treatment before TACE; (2) HBV positive; (3) HCV and HIV negative; (4) liver function Child-Pugh A or B; (5) follow-up period ≥ 3 mo; and (6) HCC was considered to be unresectable by our multidisciplinary team including surgeons, oncologists, radiologists, hepatologists, and pathologists. Unresectable disease was defined as extensive bilobular involvement of the liver by a large solitary lesion or by multiple lesions, or invasion of major blood vessels including the main portal vein, hepatic veins, inferior vena cava, and main hepatic artery. Patients with any of the following were excluded: (1) obstructive jaundice; (2) hepatic encephalopathy; (3) liver function Child-Pugh C; and (4) poor data integrity.
All the parameters were recorded and evaluated as possible predictors of survivals including sex, age, CRP, white blood cell count, neutrophil count, lymphocyte count, platelet count, AFP, alkaline phosphatase (ALP), alpha-L-fucosidase (AFU), total bilirubin level, albumin, tumor size and number, and tumor thrombus. The inflammation-based prognostic scores, including GPS, mGPS, NLR, PLR, PI, and PNI, were determined as described in Table 1.
| Scoring systems | Score |
| Glasgow Prognostic Score | |
| CRP ( ≤ 10 mg/L) and albumin (≥ 35 g/L) | 0 |
| CRP ( ≤ 10 mg/L) and albumin (< 35 g/L) | 1 |
| CRP (> 10 mg/L) and albumin (≥ 35 g/L) | 1 |
| CRP (> 10 mg/L) and albumin (< 35 g/L) | 2 |
| Modified Glasgow Prognostic Score | |
| CRP ( ≤ 10 mg/L) and albumin (≥ 35 g/L) | 0 |
| CRP ( ≤ 10 mg/L) and albumin (< 35 g/L) | 0 |
| CRP (> 10 mg/L) | 1 |
| CRP (> 10 mg/L) and albumin (< 35 g/L) | 2 |
| Neutrophil-lymphocyte ratio | |
| Neutrophil count:lymphocyte count < 3:1 | 0 |
| Neutrophil count:lymphocyte count ≥ 3:1 | 1 |
| Plt-lymphocyte ratio | |
| Plt count:lymphocyte count < 150:1 | 0 |
| Plt count:lymphocyte count ≥ 150:1 | 1 |
| Plt count:lymphocyte count > 300:1 | 2 |
| Prognostic index | |
| CRP ( ≤ 10 mg/L) and WBC ( ≤ 11 × 109/L) | 0 |
| CRP ( ≤ 10 mg/L) and WBC (> 11 × 109/L) | 1 |
| CRP (> 10 mg/L) and WBC ( ≤ 11 × 109/L) | 1 |
| CRP (> 10 mg/L) and WBC (> 11 × 109/L) | 2 |
| Prognostic Nutritional Index | |
| Albumin (g/L) × total lymphocyte count × 109/L ≥ 45 | 0 |
| Albumin (g/L) × total lymphocyte count × 109/L < 45 | 1 |
TACE was performed using the protocol that we have previously reported[24]. A selective 5 Fr catheter was introduced into the hepatic artery and visceral angiography was carried out to assess the arterial blood supply to the liver. Depending on the size, location, and arterial supply of the tumor, the tip of the catheter was advanced into the right or left hepatic artery; if all the tumors were fed by one enlarged independent hepatic artery branch, the tip of catheter was introduced into this tumor-feeding artery. If the conventional catheter could not enter the hepatic artery because of technical reasons, a 2.9 Fr micro catheter (Terumo Corp., Tokyo, Japan) was used. Hepatic artery infusion chemotherapy was performed using carboplatin 300 mg (Bristol-Myers Squibb, New York, NY, United States). After that, chemolipiodolization was performed using epirubicin 50 mg (Pharmorubicin; Pfizer, Inc., New York, NY, United States), and mitomycin C 6 mg (Zhejiang Hisun Pharmaceutical Co. Ltd., Taizhou, China) mixed with 5 mL of Lipiodol (Lipiodol Ultra-Fluide; Andre’ Guerbet Laboratories, Aulnay-Sous-Bois, France). If the chemolipiodolized arterial territory did not show stagnant flow, pure Lipiodol was then injected. In some patients, we were unable to reach stasis in a tumor-feeding artery even with the injection of the maximum amount of iodized oil (25 mL) because of the large size of the tumor. Embolization was then performed in these patients with injection of absorbable gelatin sponge particles (Gelfoam; Hanzhou alc Ltd, China), 1-2 mm in diameter, through the angiographic catheter. This treatment regimen was used consistently, regardless of tumor type and size.
Patients were followed carefully after treatment. Patients underwent liver CT scans one month after TACE, and liver CT scans were performed at three-month intervals during the first two years, then every 6 mo thereafter with physical examination and blood tests for AFP and liver function. When metastasis was suspected, chest CT, bone scintigraphy, and biopsy if indicated were also performed to confirm metastasis. The end of follow-up was the time of last follow-up (December 2013) or death.
Another session of TACE was performed every 4-10 wk until CT scans and AFP levels suggested stabilization of the tumor, or until it was not technically feasible either because of hepatic artery occlusion or impaired liver function. Overall survival (OS) was defined as the interval between treatment and death or last follow-up of surviving patients. Causes of death were determined from death certificates, medical interviews, and radiologic findings.
The definition and calculation for all inflammation scores (GPS, mGPS, NLR, PLR, PNI, PI) are listed in Table 1, and the universal cutoff values reported by literatures were utilized in the present study. Comparisons between two groups were conducted using the Student’s t test for continuous data, and the χ2 test for categorical data. The OS was calculated by the Kaplan-Meier method and compared by a log-rank test. The prognostic variables in predicting OS were assessed by multivariate Cox proportional hazards regression analysis. Variables that proved to be significant in the univariate analysis were subsequently tested with the multivariate Cox proportional hazard model using a forward selection method. The hazard ratio of survival by Cox proportional hazard model was calculated to compare the strength of predictors of survival. To evaluate the discriminatory ability of each scoring system and each staging, the receiver operating characteristics (ROC) curve and the area under the curve (AUC) were constructed at 6-mo, 12-mo, and 24-mo follow-up. The AUC was also used to assess the discrimination ability of the new combined scoring with other scorings. Results are given as mean ± SD. All statistical tests were two-sided, and a significant difference was considered at P < 0.05. All the statistical analyses were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, United States).
A total of 224 consecutive patients who met our criteria were included in this study. Patients’ baseline characteristics are summarized in Table 2. There were 199 male (89%) and 25 female (11%) patients with a median age of 53 years (range: 23-80 years). The majority of our patients had a good liver functional reserve with Child-Pugh A (93.0%).
| Variables | Value |
| Age (yr) | 53 (23-80) |
| Sex (M/F) | 199/25 |
| WBC (× 109/L) | 6.6 (2.1-24.6) |
| Neutrophil count (× 109/L) | 4.2 (0.7-13.6) |
| Lymphocyte count (× 109/L) | 1.5 (0.3-4.8) |
| CRP (mg/L) | 21.1 (0.2-218.3) |
| PLT count (× 109/L) | 182 (23-548) |
| ALT (U/L) | 55.6 (8.0-304.0) |
| AST (U/L) | 76.3 (20.2-472.6) |
| Albumin (g/L) | 38.9 (7.6-79.4) |
| Total serum bilirubin (mmol/L) | 18.0 (4.8-222.9) |
| ALP (IU/L) | 148.7 (13.0-574.6) |
| AFP (ng/mL) | 25828.4 (1.3-1210000.0) |
| AFU (U/L) | 43.8 (12.6-992.0) |
| Diameter of largest lesion (cm) | 9.2 (1.4-20.0) |
| Tumor number (solitary/multiple) | 71/153 |
| Vascular invasion (absent/present) | 149/75 |
| Child-Pugh grade (A/B) | 208/16 |
| GPS (0/1/2) | 99/101/24 |
| Modified GPS (0/1/2) | 115/85/24 |
| NLR (0/1) | 108/116 |
| PLR (0/1/2) | 156/57/11 |
| PI (0/1/2) | 115/102/7 |
| PNI (0/1) | 154/70 |
| TNM stage (I/II/IIIa/IIIb/IVa/IVb) | 44/24/71/52/5/28 |
| CLIP score (0/1/2/3/4/5) | 19/56/70/43/34/2 |
| BCLC stage (A/B/C) | 10/124/90 |
Among the 224 patients, 126 (56%) patients had an elevated CRP level (> 10 mg/L), 40 (18%) had hypoalbuminemia (< 35 g/L), and 24 (11%) had both elevated CRP level and hypoalbuminemia. Sixty-eight (30%) patients had PLR ≥ 150; 116 (52%) patients had NLR ≥ 3; 70 (31%) patients had PNI > 45, and 109 (49%) patients were allocated to PI 1 or 2. The relationships between inflammatory scores and clinicopathologic features were analyzed (data not shown). Both GPS and mGPS were associated with tumor size, vascular invasion, AST, ALP, Child-Pugh grade, and Cancer of the Liver Italian Program (CLIP); NLR with age, tumor size, vascular invasion, AST, ALP, CLIP, and BCLC stage; PI with tumor size, AST, ALP, and CLIP, PLR with age, tumor size, and ALP; PNI with Child-Pugh grade only.
The median follow-up period was 390 d (range: 90-1527), and at the time of analysis, 198 patients had died. The one-, two-, and three-year OS was 38.4%, 18.3%, and 11.1% respectively, and the median OS was 390 d.
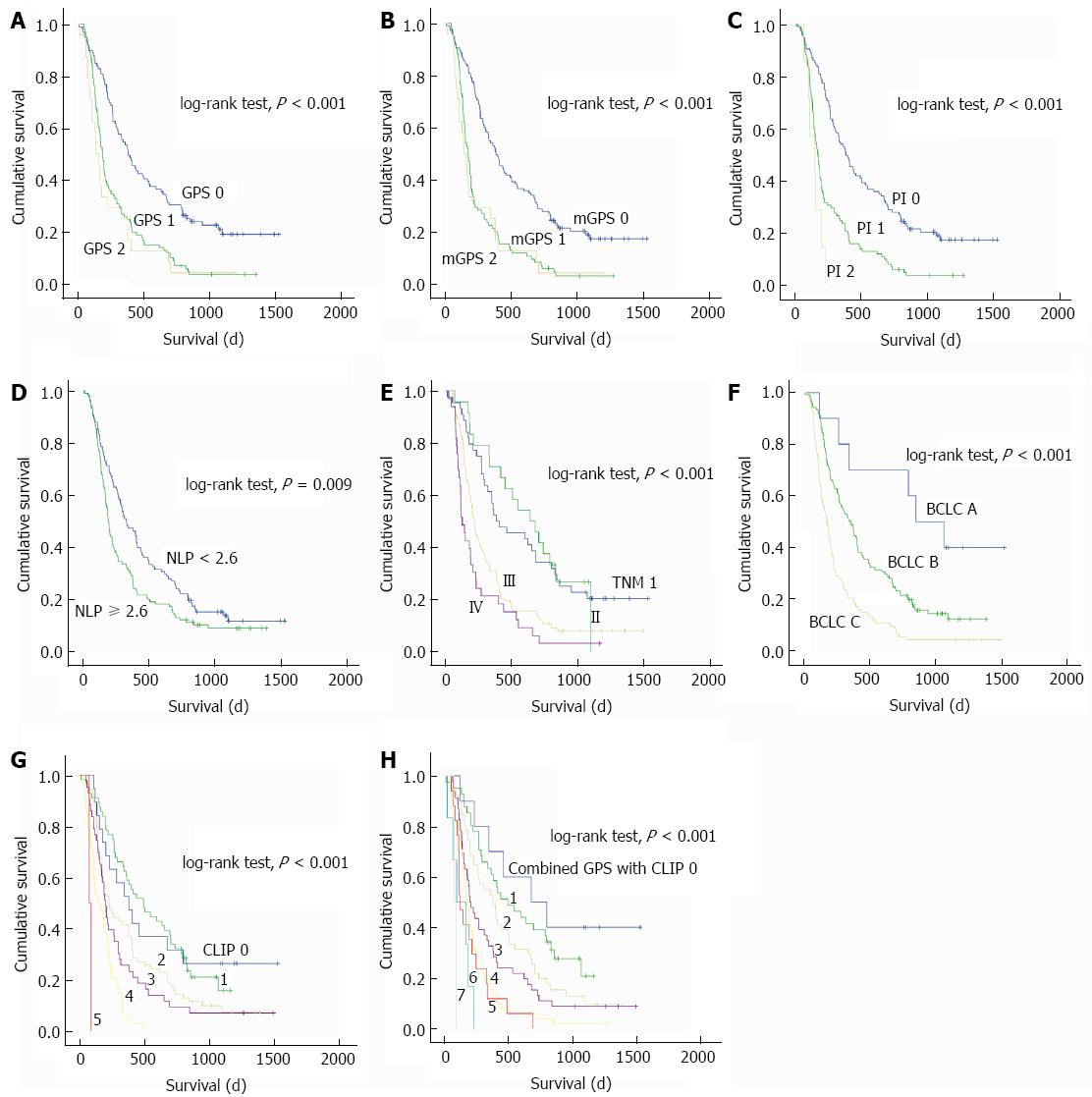
The univariate and multivariate analyses of prognostic factors for OS are shown in Table 3. In univariate analysis, age, neutrophil count, CRP, ALT, AST, ALP, AFU, diameter of the largest lesion, total bilirubin, AFP, tumor size, tumor number, vascular invasion, Child-Pugh score, and inflammatory scores including GPS, mGPS, NLR, and PI were associated with OS (all P < 0.05). The BCLC stage, CLIP score, and TNM stage were also confirmed as significant predictors of OS (all P < 0.001) (Figure 1).
| Variable | Univariate analysis | Multivariate analysis | |
| P value | HR (95%CI) | P value | |
| Age (< 60/≥ 60 yr) | 0.008 | ||
| Sex (M/F) | 0.118 | ||
| WBC ( ≤ 10/> 10 × 109/L) | 0.094 | ||
| Neutrophil count ( ≤ 7/> 7 × 109/L) | 0.038 | ||
| Lymphocyte count ( ≤ 0.8/> 0.8 × 109/L) | 0.088 | ||
| CRP ( ≤ 8/> 8 mg/L) | < 0.001 | ||
| PLT count ( ≤ 100/> 100 × 109/L) | 0.472 | ||
| ALT ( ≤ 40/> 40 U/L) | 0.002 | 1.005 (1.001-1.009) | 0.008 |
| AST ( ≤ 45/> 45 U/L) | < 0.001 | ||
| Albumin ( ≤ 35/> 35 g/L) | 0.208 | ||
| Total bilirubin ( ≤ 20.5/> 20.5 mmol/L) | < 0.001 | ||
| ALP ( ≤ 110/> 110 IU/L) | < 0.001 | ||
| AFP ( ≤ 400/> 400 ng/ml) | < 0.001 | ||
| AFU ( ≤ 40/> 40 U/L) | 0.042 | ||
| Diameter of largest lesion (< 8/≥ 8 cm) | < 0.001 | ||
| Tumor number (solitary/multiple) | 0.025 | ||
| Vascular invasion (absent/present) | < 0.001 | ||
| Child-Pugh grade (A/B) | 0.030 | ||
| GPS (0/1/2) | < 0.001 | 1.697 (1.325-2.174) | < 0.001 |
| Modified GPS (0/1/2) | < 0.001 | ||
| NLR (0/1) | 0.009 | ||
| PLR (0/1/2) | 0.553 | ||
| PI (0/1/2) | < 0.001 | ||
| PNI (0/1) | 0.573 | ||
| TNM stage (I/II/IIIa/IIIb/IVa/IVb) | < 0.001 | ||
| CLIP score (0/1/2/3/4/5) | < 0.001 | 1.297 (1.1-1.53) | 0.002 |
| BCLC stage (A/B/C) | < 0.001 | ||
Multivariate analysis showed that ALT, GPS, and CLIP were independent prognostic factors for OS (all P < 0.01). When GPS and CLIP were replaced by the combined scores, multivariate analysis showed that the combined GPS and CLIP scores (HR = 1.724, 95%CI: 1.347-2.285; P < 0.001) were independent prognostic factors for OS along with ALT.
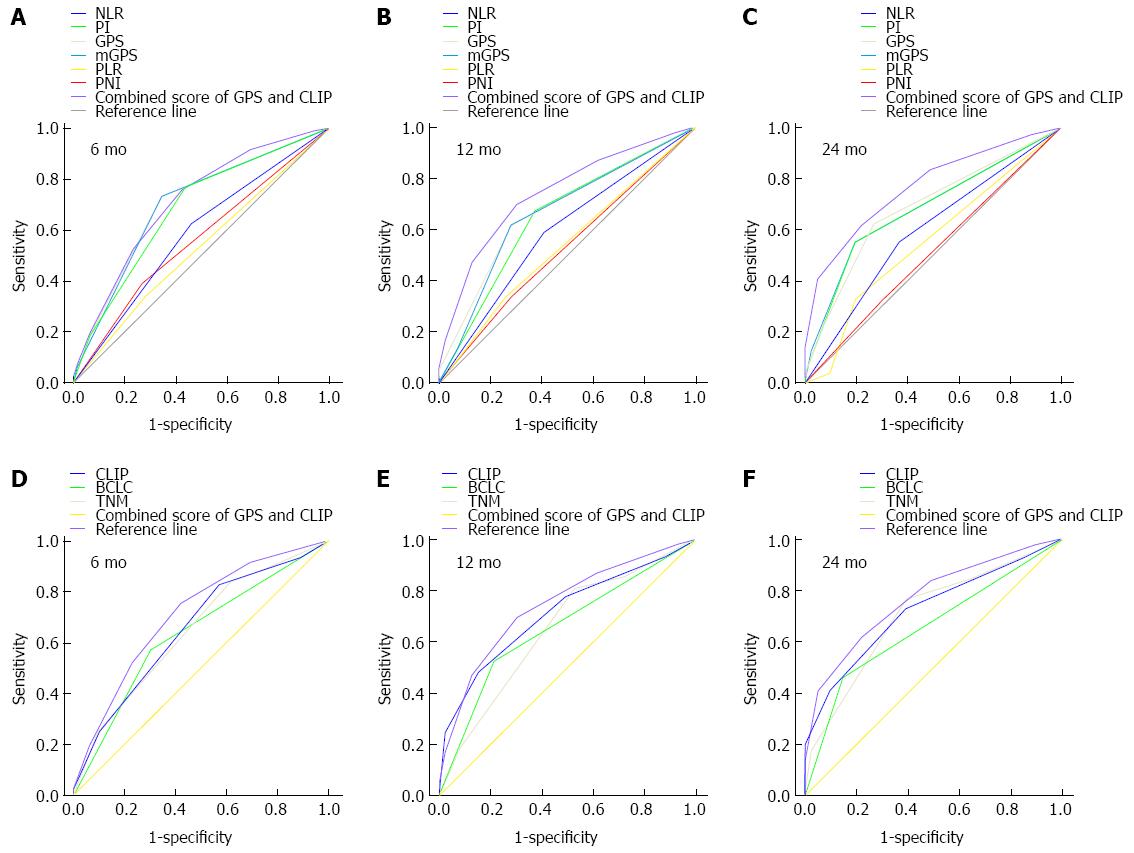
The prognostic power of inflammation scores and staging systems was compared by means of AUC analysis. ROC curves were calculated for survival status at 6-mo, 12-mo, and 24-mo follow-up, and the AUC were compared to assess the discrimination ability of each inflammation scores and staging systems. The GPS consistently had a higher AUC value at 6 mo, 12 mo, and 24 mo in comparison with other inflammation-based prognostic scores (all P < 0.001), and the CLIP consistently had a higher AUC value at 6 mo, 12 mo, and 24 mo in comparison with TNM and BCLC staging systems (all P < 0.001) (Table 4, Figure 2).
| Period | AUC | 95%CI | P value |
| 6-mo | |||
| GPS | 0.702 | 0.630-0.773 | < 0.001 |
| mGPS | 0.699 | 0.627-0.770 | < 0.001 |
| PI | 0.681 | 0.609-0.754 | < 0.001 |
| NLR | 0.582 | 0.505-0.659 | 0.410 |
| PNI | 0.561 | 0.482-0.640 | 0.126 |
| PLR | 0.531 | 0.385-0.629 | 0.436 |
| TNM | 0.649 | 0.575-0.723 | < 0.001 |
| BCLC | 0.635 | 0.559-0.712 | 0.001 |
| CLIP | 0.656 | 0.582-0.730 | < 0.001 |
| 12-mo | |||
| GPS | 0.676 | 0.604-0.747 | < 0.001 |
| mGPS | 0.665 | 0.592-0.738 | < 0.001 |
| PI | 0.652 | 0.577-0.726 | < 0.001 |
| NLR | 0.590 | 0.513-0.667 | 0.024 |
| PNI | 0.527 | 0.450-0.605 | 0.495 |
| PLR | 0.537 | 0.460-0.615 | 0.346 |
| TNM | 0.651 | 0.576-0.725 | < 0.001 |
| BCLC | 0.656 | 0.584-0.729 | < 0.001 |
| CLIP | 0.711 | 0.644-0.778 | < 0.001 |
| 24-mo | |||
| GPS | 0.687 | 0.601-0.772 | < 0.001 |
| mGPS | 0.684 | 0.601-0.767 | < 0.001 |
| PI | 0.682 | 0.599-0.766 | < 0.001 |
| NLR | 0.593 | 0.498-0.688 | 0.063 |
| PNI | 0.512 | 0.415-0.609 | 0.808 |
| PLR | 0.554 | 0.457-0.652 | 0.279 |
| TNM | 0.706 | 0.621-0.791 | < 0.001 |
| BCLC | 0.656 | 0.572-0.741 | 0.002 |
| CLIP | 0.721 | 0.644-0.797 | < 0.001 |
We proposed a combined score of the GPS and CLIP score (Table 5). The combined score had a higher AUC of 0.777 (95%CI: 0.692-0.862) when compared to the CLIP score alone or the GPS alone. The survival curve showed that the combined score divided patients into subgroups more accurately (Table 4, Figure 2).
| Variables | Score | ||
| 0 | 1 | 2 | |
| Child-Pugh stage | A | B | C |
| Tumor morphology | Uninodular and extension ≤ 50% | multinodular and extension ≤ 50% | multinodular or extension > 50% |
| AFP (ng/mL) | < 400 | ≥ 400 | |
| Portal vein thrombosis | No | Yes | |
| GPS | CRP ≤ 10 mg/L and albumin ≥ 35 g/L | (CRP ≤ 10 mg/L and albumin < 35 g/L) or (CRP > 10 mg/L and albumin ≥ 35 g/L) | CRP > 10 mg/L and albumin < 35 g/L |
We compared the prognostic power of six inflammation-based prognostic scores (GPS, mGPS, NLR, PLR, PI, and PNI) for patients undergoing TACE for HBV-related HCC, and proposed a combined score of the GPS and CLIP score. Our results demonstrate that the GPS is an independent predictor of OS for patients undergoing TACE, and superior to other inflammation-based prognostic scores. The combination of the GPS and CLIP score improves the prognostic power.
The pathogenesis of HCC is based on inflammation. Especially in China, most cases of HCC develop from underlying chronic hepatitis B. As the last and most redoubtable clinical consequence of cirrhosis, the onset of HCC is related to a myriad of proinflammatory stimuli, triggered by well-recognized noxae such as infection by hepatotropic viruses, iron or copper accumulation, or ethanol consumption[25]. The inflammatory markers are associated with the development and progression of HCC[16-20,26-28]. We demonstrate that these inflammation-based prognostic scores are associated with a number of clinicopathologic characteristics of HCC. In particular, elevated scores are associated with a larger tumor size, vascular invasion, and advanced clinical stages, suggesting the presence of a systemic inflammatory response is predictive of a more aggressive clinical phenotype.
Several studies had shown that the inflammation-based prognostic scores are associated with prognosis in patients with HCC. However, few studies compare among the inflammation scores, and which scores are more suitable for predicting outcome in patients undergoing TACE has not been evaluated. Although univariate analysis showed that GPS, mGPS, NLR, and PI were associated with OS, comparison between inflammation scores demonstrated that the GPS consistently had a higher AUC value at 6 mo, 12 mo, and 24 mo in comparison with other scores, and the multivariate analysis also showed that only the GPS is an independent predictor of OS. Although based on a different patient population, Kinoshita et al[15] compared the prognostic value of these inflammation scores in 150 patients with newly diagnosed HCC in various stages of disease and different liver functional status treated with various methods, and their results demonstrated that the GPS was an independent marker of poor prognosis in patients with HCC and was superior to the other inflammation-based prognostic scores in terms of prognostic ability. It suggested that the GPS is more suitable for predicting outcome in patients with HCC.
The ability of three widely utilized staging systems to predict survival in patients undergoing TACE was also compared. Our results show that the CLIP consistently had a higher AUC value at 6 mo, 12 mo, and 24 mo in comparison with TNM and BCLC staging systems. Similarly, Huitzil-Melendez et al[29] compared the prognostic value of seven available staging systems in 187 newly diagnosed HCC patients with advanced disease not amenable to curative or local therapies, and their results demonstrated that CLIP was the most informative staging system in predicting survival in patients with advanced HCC. Collette et al[30] compared Okuda, CLIP, and BCLC staging systems using different statistical tools in patients with advanced HCC included in two French clinical trials, and their results also indicate that CLIP seems to be the most adaptive staging system in the setting of advanced disease.
As sustained inflammation acts as one of the main factors thought to promote development of neoplastic foci within the chronically injured liver parenchyma[31], we hypothesize that the combination of clinical staging system and inflammation scores might improve their prognostic power for HCC. In the present study, GPS and CLIP were the most useful inflammation score and clinical staging system, respectively. Thus, we proposed a combined score of GPS and CLIP, which simply added the GPS score into CLIP score. Our results demonstrate that combined score has a higher AUC of 0.777 when compared to the CLIP alone (AUC = 0.722) or GPS alone (AUC = 0.706), and the Kaplan-Meier survival curves showed that the combined score divides patients into subgroups more accurately. We validated the concept that the combination of GPS with CLIP may increase the predictive ability of the latter, suggesting the additive value of systemic inflammation to an accurate prognostic assessment in HCC. In present study, there were 90 BCLC C patients and 75 patients with vascular invasion. However, Child B patients comprised a very small cohort in this study, indicating that the study cohort had more tumor-related sickness than the liver disease per se. The results show that the higher discriminatory power of combined inflammatory score and CLIP score may be related to this specific subset of patients. This was also supported by poor one-year survival in this study group of only 38.4%. It also should be pointed out that, according to our combined scores for patients with a combined score > 4, their prognoses after TACE were generally poor, which indicated that these patients could not benefit from TACE treatment and other treatment strategies or clinical trials should be suggested. Thus, our combined score can provide evidence for individualized treatment in clinical practice. Meanwhile, GPS, as a combination of CRP and albumin, is almost universally available and adds minimal additional cost to routine preoperative workup, which makes it a reasonable candidate for clinical application.
The presence of an elevated AST level was also revealed as one of prognostic factors for poor outcome, as it had been reported in previous studies[32]. Of note, in patients with chronic hepatitis and cirrhosis, an increase in AST/ALT ratio is associated with progressive liver functional impairment.
There were some limitations that should be noted in present study: (1) it was a retrospective analysis from a single institution; and (2) the patient population was based on HBV-related HCC. Whether these results can be applied to Western populations wherein HCV, nonalcoholic steatohepatitis, and other etiologies of liver disease predominate requires further study. Therefore, a large-scale prospective validation study is needed to confirm the results.
For patients undergoing TACE for HBV-related HCC, the prognostic ability of the GPS is superior to the other inflammation-based prognostic scores, and CLIP is superior to TNM and BCLC staging systems. The combination of clinical staging system (CLIP) and inflammation scores (GPS) might improve the prognostic power.
Hepatocellular carcinoma (HCC) is one of the most frequent cancers worldwide. When diagnosed, up to 70%-90% of HCCs are not suitable for curative treatment because of advanced tumor stage. Transarterial chemoembolization (TACE), as the main treatment for HCC, has demonstrated improved survival benefit for select patients, but is not suitable for all patients. The key point is stratifying and choosing the most suitable HCC patients. To this aim, the authors compared the prognostic ability of six inflammation scores, and then proposed a new score by combining the optimal inflammation score with the staging system to recruit patients who would most likely benefit from TACE in such a heterogeneous HCC population.
There is increasing interest in the role of systemic inflammation as a predictor of outcome in HCC. The current research hotspot is to compare six inflammation scores in HCC patients undergoing TACE.
Previous studies have found that the inflammation scores, including the Glasgow Prognostic Score (GPS), modified GPS, platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, Prognostic Nutritional Index, and Prognostic Index, are associated with patient survival, but which one is most suitable for HCC patients undergoing TACE is still controversial. Furthermore, the prognostic ability for only one score to predict survival is still weak. So, in the present study, the authors compared the prognostic ability of six inflammation scores and three clinical staging systems, and then combined the optimal score (GPS) and clinical staging system [Cancer of the Liver Italian Program (CLIP)] together. The results showed that the combined score can improve the prognostic power; thus it can help the interventional physician to recruit patients for TACE.
Those patients with combined score > 3 would not benefit from TACE, other treatments, for example sorafenib or clinical trials, should be considered.
The authors made an interesting and useful study to improve the estimation of the prognosis in patients with hepatitis B virus-related HCC after TACE. The result shows GPS is superior to other inflammation scores and combined use of GPS and CLIP can improve the prognostic power for overall survival. According to the combined scores, it can select patients who would most likely benefit from TACE, and predict survival benefit in these patients.
P- Reviewer: Grassi G, Mihaila RG, Miki K, Wakiyama S S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25175] [Article Influence: 1936.5] [Reference Citation Analysis (3)] |
| 2. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 599] [Cited by in F6Publishing: 630] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 3. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 630] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 4. | Cabibbo G, Maida M, Cammà C, Craxì A. Is the efficacy of sorafenib treatment in patients with hepatocellular carcinoma affected by age? Expert Rev Anticancer Ther. 2013;13:1355-1361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3241] [Cited by in F6Publishing: 3212] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 7. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2207] [Cited by in F6Publishing: 2190] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 8. | Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1461] [Cited by in F6Publishing: 1515] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2645] [Cited by in F6Publishing: 2715] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 10. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 908] [Cited by in F6Publishing: 932] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 11. | Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dagg K, Scott HR. A prospective longitudinal study of performance status, an inflammation-based score (GPS) and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;92:1834-1836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, Yamaoka T, Iwatani Y, Akazawa K, Takenaka K. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103:1856-1864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Usefulness of a modified inflammation-based prognostic system for predicting postoperative mortality of patients undergoing surgery for primary hepatocellular carcinoma. J Surg Oncol. 2011;103:801-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Fushiya N, Koike K, Nishino H, Tajiri H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107:988-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 323] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 17. | Halazun KJ, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 18. | Huang ZL, Luo J, Chen MS, Li JQ, Shi M. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol. 2011;22:702-709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27:553-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer. 2012;106:1439-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 21. | Kasymjanova G, MacDonald N, Agulnik JS, Cohen V, Pepe C, Kreisman H, Sharma R, Small D. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol. 2010;17:52-58. [PubMed] [Cited in This Article: ] |
| 22. | Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 23. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4333] [Cited by in F6Publishing: 4404] [Article Influence: 231.8] [Reference Citation Analysis (0)] |
| 24. | Luo J, Peng ZW, Guo RP, Zhang YQ, Li JQ, Chen MS, Shi M. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology. 2011;259:286-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Chalasani N, Horlander JC, Said A, Hoen H, Kopecky KK, Stockberger SM, Manam R, Kwo PY, Lumeng L. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94:2988-2993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, Kubota K, Sharma R. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol. 2012;57:1013-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Fu SJ, Shen SL, Li SQ, Hua YP, Hu WJ, Liang LJ, Peng BG. Prognostic value of preoperative peripheral neutrophil-to-lymphocyte ratio in patients with HBV-associated hepatocellular carcinoma after radical hepatectomy. Med Oncol. 2013;30:721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Lai Q, Castro Santa E, Rico Juri JM, Pinheiro RS, Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int. 2014;27:32-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Huitzil-Melendez FD, Capanu M, O’Reilly EM, Duffy A, Gansukh B, Saltz LL, Abou-Alfa GK. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28:2889-2895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 30. | Collette S, Bonnetain F, Paoletti X, Doffoel M, Bouché O, Raoul JL, Rougier P, Masskouri F, Bedenne L, Barbare JC. Prognosis of advanced hepatocellular carcinoma: comparison of three staging systems in two French clinical trials. Ann Oncol. 2008;19:1117-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46:48-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 32. | Kang IK, Kim SW, Hahn SH, Cho SC, Gham CW, Lee DH. [A comparison of patients with hepatocellular carcinoma between a short-term (less than 6 months) survival group and a long-term (over 24 months) survival group after treatment with transcatheter arterial chemoembolization]. Taehan Kan Hakhoe Chi. 2002;8:189-200. [PubMed] [Cited in This Article: ] |