Published online Sep 14, 2015. doi: 10.3748/wjg.v21.i34.10054
Peer-review started: April 7, 2015
First decision: May 18, 2015
Revised: June 3, 2015
Accepted: July 8, 2015
Article in press: July 8, 2015
Published online: September 14, 2015
De novo non-alcoholic fatty liver disease (NAFLD) is a common late complication for long-term survivors after liver transplantation. Genomic studies confirmed that PNPLA3 I148M and TM6SF2 E167K polymorphisms affected NAFLD susceptibility in the general population. However, this association was not validated in survivors after liver transplantation (LT). We performed a cross-sectional survey to investigate this relationship. A comprehensive survey, including anthropometric measurements, fasting venous blood sampling, ultrasound, and questionnaires was performed in the short-term. The clinical indications and patient’s steatosis status before LT were collected from inpatient medical records. Sixty-five long-term recipients with a survival exceeding 10 years were enrolled in the final analysis. De novo NAFLD was more frequent in PNPLA3 GG carriers (0.33 vs 0.10 for GG vs CC + CG carriers, P = 0.018), while the genetic impact on NAFLD susceptibility was insignificant when categorized by the TM6SF2 polymorphism (0.19 in CC vs 0.14 in CT + TT carriers, P = 0.883). Multi-covariate analysis revealed that PNPLA3 exerted a significant genetic effect on de novo NAFLD following a recessive model (GG vs CC + CG, OR = 14.2, 95%CI: 1.78-113, P = 0.012). Compared to recipients with only the PNPLA3 GG allele or obesity (defined as body mass index > 25 kg/m2), steatosis was highly prevalent (71.4%) in PNPLA3 GG carriers with obesity. In conclusion, PNPLA3 I148M, but not TM6SF2 E167K, affects de novo NAFLD occurrence with a prominent interaction with obesity. Weight control might be a meaningful method to reduce the genetic susceptibility to NAFLD exerted by PNPLA3 variants.
Core tip: Previous genomic studies identified PNPLA3 I148M and TM6SF2 E167K polymorphisms as the most prominent genetic variations associated with non-alcoholic fatty liver disease (NAFLD) susceptibility in general populations. However, these impacts have never been evaluated in long-term liver transplant recipients. In a collection of survivors 10 years after liver transplantation, we found that the PNPLA3 I148M, but not TM6SF2 E167K polymorphism, affected de novo NAFLD predisposition and interacted with obesity. Our results revealed that liver transplant recipients might benefit from weight control to limit the deleterious effect exerted by genetic factors.
- Citation: Liu ZT, Chen TC, Lu XX, Cheng J, Xie HY, Zhou L, Zheng SS. PNPLA3 I148M variant affects non-alcoholic fatty liver disease in liver transplant recipients. World J Gastroenterol 2015; 21(34): 10054-10056
- URL: https://www.wjgnet.com/1007-9327/full/v21/i34/10054.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i34.10054
De novo non-alcoholic fatty liver disease (NAFLD) is a common late complication for long-term survivors after liver transplantation (LT)[1]. De novo NAFLD affects allograft survival indirectly by increasing cardiovascular and infectious disease occurrence[2]. Previous genomic studies identified the PNPLA3 I148M and TM6SF2 E167K polymorphisms as the most likely single nucleotide polymorphisms to influence NAFLD susceptibility in the general population[3]. However, this relationship was not confirmed in long-term survivors after LT as a specific population. Therefore, we performed a cross-sectional survey to investigate the impact of genetic and environmental risk factors for de novo NAFLD in adult long-term survivors after receiving LT.
After obtaining written informed consent, a comprehensive survey, including anthropometric measurements (for body weight and height), fasting venous blood sampling (for liver function, lipid, glucose, viral biomarker testing, genotyping, etc.), ultrasound examination, and questionnaires (for alcohol intake, smoking, exercise, and immunosuppression) were performed over the short-term (December 13th-14th, 2014). Indications for LT and patients’ steatosis status before LT were collected from inpatient medical records. The study was approved by the Institutional Review Board of our hospital.
NAFLD and metabolic syndrome were defined according to previous criteria[4]. Participants with recurrent liver steatosis were excluded. Accordingly, 65 subjects (57 males and 8 females) receiving LT (from September, 1999 to November, 2004) in our hospital with a survival exceeding 10 years were enrolled into the final analysis.
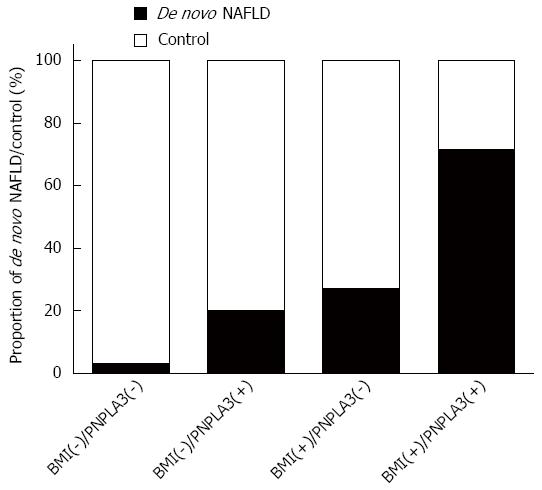
All survivors were Han Chinese and negative for hepatitis B virus DNA/hepatitis C virus RNA assay. As shown in Table 1, twelve of the patients were diagnosed with de novo NAFLD. By univariate analysis, the NAFLD subgroup hada significantly higher body mass index (BMI), triglyceride (TG) levels, and fasting blood glucose (FBG) levels. De novo NAFLD was more frequent in PNPLA3 GG carriers than in CC + CG carriers (0.33 vs 0.10, P = 0.018), while the genetic impact on NAFLD susceptibility was insignificant when categorized by the TM6SF2 polymorphism (0.19 in CC vs 0.14 in CT + TT carriers, P = 0.883). Multi-covariate analysis revealed that PNPLA3 exerted a significant genetic effect on de novo NAFLD following a recessive model (GG vs CC + CG, OR = 14.2, 95%CI: 1.78-113, P = 0.012). Compared to recipients only carrying the PNPLA3 GG allele or being obese (defined as BMI > 25 kg/m2), the prevalence of steatosis was disproportionally higher (71.4%) in PNPLA3 GG carriers who were obese (Figure 1).
| Univariate | Multivariate | ||||
| NAFLD (n = 12) | Control (n = 53) | P value | OR | P value | |
| Age (yr) | 56.5 ± 8.4 | 53.6 ± 10.1 | 0.356 | 1.04 (0.92-1.18) | 0.528 |
| Gender (M/F) | 10/2 | 47/6 | 0.611 | 1.40 (0.14-14.2) | 0.427 |
| Indication for LT | |||||
| Hepatitis/cirrhosis/ cancer/others | 1/8/2/1 | 7/35/9/2 | 0.889 | ||
| Survival time (yr) | 11.2 ± 0.9 | 11.5 ± 1.4 | 0.541 | ||
| BMI (kg/m2) | 25.1 ± 3.0 | 22.5 ± 2.6 | 0.003 | 1.47 (1.03-2.08) | 0.032 |
| TG (mmol/L) | 1.6 ± 1.1 | 1.1 ± 0.6 | 0.038 | 1.34 (0.38-4.71) | 0.652 |
| HDL-C (mmol/L) | 1.2 (1.0-1.4) | 1.3 (1.0-1.7) | 0.267 | ||
| FBG (mmol/L) | 7.6 ± 3.4 | 5.7 ± 1.9 | 0.013 | 1.49 (0.93-2.37) | 0.095 |
| Hypertension (Yes/no) | 3/9 | 21/32 | 0.343 | ||
| SUA (μmol/L) | 381.6 ± 75.6 | 342.6 ± 76.4 | 0.116 | ||
| MetS (Yes/no) | 4/8 | 9/44 | 0.201 | ||
| ALT (U/L) | 36.7 ± 7.0 | 38.8 ± 6.7 | 0.882 | ||
| Alcohol intake (g/wk) | 11.6 ± 7.3 | 21.0 ± 8.2 | 0.766 | ||
| Smoking (cigar/d) | 4.2 ± 3.4 | 4.3 ± 1.2 | 0.969 | ||
| Exercise (min/d) | 18.5 ± 6.0 | 22.9 ± 2.9 | 0.513 | ||
| Immunosuppression | |||||
| Tacrolimus/ cyclosporine/ MMF/sirolimus/ none | 11/1/0/0/0 | 36/12/1/2/2 | 0.575 | ||
| PNPLA3 (CC/CG/GG) | 1/3/8 | 16/21/16 | 0.018 | 14.2 (1.78-113) | 0.012 |
| TM6SF2 (CC/CT/TT) | 11/1/0 | 47/5/1 | 0.883 | 2.68 (0.25-28.5) | 0.413 |
This is the first report on the risk factors associated with de novo steatosis in Chinese long-term survivors after LT. PNPLA3, but TM6SF2, affects de novo NAFLD occurrence and has a prominent interaction with obesity. Weight control in recipients might be a potential method to reduce the genetic susceptibility of NAFLD exerted by the PNPLA3 variant.
P- Reviewer: Yu DY S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Hübscher SG. What is the long-term outcome of the liver allograft? J Hepatol. 2011;55:702-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Zezos P, Renner EL. Liver transplantation and non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:15532-15538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 118] [Cited by in F6Publishing: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Rinella ME, Sanyal AJ. NAFLD in 2014: Genetics, diagnostics and therapeutic advances in NAFLD. Nat Rev Gastroenterol Hepatol. 2015;12:65-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;14:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 213] [Cited by in F6Publishing: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |