Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1554
Peer-review started: May 18, 2014
First decision: June 27, 2014
Revised: July 29, 2014
Accepted: September 19, 2014
Article in press: September 19, 2014
Published online: February 7, 2015
AIM: To investigate the treatment strategies and long-term outcomes of radiofrequency ablation (RFA) of hepatocellular carcinoma (HCC) in difficult locations and to compare the results with non-difficult HCC.
METHODS: From 2004 to 2012, a total of 470 HCC patients underwent ultrasound-guided percutaneous RFA. Among these HCC patients, 382 with tumors located ≤ 5 mm from a major vessel/bile duct (n = 87), from peripheral important structures (n = 232) or from the liver capsule (n = 63) were regarded as difficult cases. There were 331 male patients and 51 female patients, with an average age of 55.3 ± 10.1 years old. A total of 235 and 147 patients had Child-Pugh class A and class B liver function, respectively. The average tumor size was 3.4 ± 1.2 cm. Individual treatment strategies were developed to treat these difficult cases. During the same period, 88 HCC patients with tumors that were not in difficult locations served as the control group. In the control group, 74 patients were male, and 14 patients were female, with an average age of 57.4 ± 11.8 years old. Of these, 62 patients and 26 patients had Child-Pugh class A and class B liver function, respectively. Regular follow-up after RFA was performed to assess treatment efficacy. Survival results were generated from Kaplan-Meier estimates, and multivariate analysis was performed using the Cox regression model.
RESULTS: Early tumor necrosis rate in the difficult group was similar to that in the control group (97.6% vs 94.3%, P = 0.080). The complication rate in the difficult group was significantly higher than that in the control group (4.9% vs 0.8%, P = 0.041). The follow-up period ranged from 6 to 116 mo, with an average of 28 ± 22.4 mo. Local progression rate in the difficult group was significantly higher than that in the control group (12.7% vs 7.1%, P = 0.046). However, the 1-, 3-, 5-, and 7-year overall survival rates in the difficult group were not significantly different from those in the control group (84.3%, 54.4%, 41.2%, and 29.9% vs 92.5%, 60.3%, 43.2%, and 32.8%, respectively, P = 0.371). Additionally, a multivariate analysis revealed that tumor location was not a significant risk factor for survival.
CONCLUSION: There was no significant difference in long-term overall survival between the two groups even though the local progression rate was higher in the difficult group.
Core tip: Recently, many studies have reported the increasing treatment success rate and reduced frequency of complications following RFA treatment of tumors in difficult locations. However, the long-term outcomes of patients with tumors in difficult locations have been rarely reported. Our studies showed no difference in 5- or 7-year overall survival between the difficult location group and the control group even though the local progression rate was higher in the difficult group. These results highlight that optimized individual strategies could achieve acceptable efficacy and safety and could help to expand RFA indications and improve overall outcome.
- Citation: Yang W, Yan K, Wu GX, Wu W, Fu Y, Lee JC, Zhang ZY, Wang S, Chen MH. Radiofrequency ablation of hepatocellular carcinoma in difficult locations: Strategies and long-term outcomes. World J Gastroenterol 2015; 21(5): 1554-1566
- URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1554
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world, causing more than 500000 deaths every year. The incidence of HCC has been increasing worldwide due to the spread of hepatitis B and C virus infections[1-3]. Most patients with HCC confined to the liver are not candidates for resection because of the frequent association with cirrhosis and other contraindications. Furthermore, surgical resection is associated with a recurrence rate of 40%-60%[4,5]. Therefore, an effective locoregional therapy is required for many patients with HCC.
Clinical evidence has confirmed the efficacy of radiofrequency ablation (RFA) for the treatment of localized HCC[6-9], especially in small tumors (≤ 3 cm)[10-12]. The recent development of novel techniques and instruments has significantly improved the therapeutic effects of RFA for median to large tumors (3-6 cm)[13-15]. In addition, tumors are often found in difficult locations (e.g., abutting key structures such as the stomach, bowel, gallbladder, diaphragm and large vessels) that may be injured during RFA, resulting in subsequent complications. RFA with an insufficient safety margin might result in residual tumor and recurrence, restricting the extensive application of RFA. Recently, many studies have reported that new technologies are leading to increased tumor necrosis rates and reduced complications with RFA treatment in such tumors. However, the long-term outcomes (more than 5 years) after RFA have been rarely demonstrated in a large number of difficult tumors.
In the present study, we compared early tumor necrosis rates, local progression rates, long-term survival and complications between tumors in difficult locations and those in easily accessed locations treated by percutaneous RFA. Our objective is to confirm the safety and effectiveness of the RFA procedure in problematically located HCC.
This clinical study was approved by the Institutional Review Board of the Peking University Cancer Hospital. Written informed consent was obtained from all patients before their RFA treatment.
Indications for ultrasound-guided percutaneous RFA included: (1) accessibility of tumors via a percutaneous approach; (2) solitary tumor with a diameter ≤ 6 cm or multiple tumors (no more than 3) with the largest diameter ≤ 5 cm; (3) no invasion to nearby organs or distant metastasis; (4) absence of portal vein or inferior vena cava tumor thrombus; (5) international normalized ratio no greater than 1.6, and platelet count greater than 60000/L; and (6) lesions were surgically unresectable or the patient voluntarily chose nonsurgical treatment.
A diagnosis of HCC was confirmed by ultrasound-guided tumor biopsy using a 20/18-gauge needle in 351 cases (75%). Histological tumor differentiation grade was determined according to a modification of the Edmondson Grading System proposed by the Liver Cancer Study Group of Japan[16]. In the remaining 119 cases, diagnosis was based on typical imaging findings, i.e., arterial-phase hyperattenuation and late-phase contrast washout on at least two contrast imaging examinations. Tumor location was defined according to the Couinaud nomenclature after ultrasonography.
On the basis of previous literature[17] and our experience, we defined locations adjacent to large vessels, extrahepatic organs or the liver capsule as difficult locations. HCC nodules adjacent to large vessels were defined as those located ≤ 5 mm from a first or second branch of the portal vein, the base of hepatic veins, or the inferior vena cava, while nodules adjacent to extrahepatic organs were defined as those located ≤ 5 mm from the diaphragm, lung, gallbladder, right kidney, or gastrointestinal (GI) tract. The distance between the edge of the nodule and the large vessel, extrahepatic organ or liver capsule was measured by scanning multiple planes of ultrasound images.
From January 1, 2004 to November 1, 2012, 470 consecutive patients with HCC received ultrasound-guided percutaneous RFA at our institution. These patients underwent percutaneous RFA because they had unresectable tumors or preferred minimally invasive therapy. We retrospectively reviewed the database of these patients during this period. Among these patients, 382 (331 men, 51 women; average age 55.3 ± 10.1 years, range, 24-85 years) met the inclusion criteria (difficult group) and were enrolled in the study. The 382 patients in the difficult group were further divided into three subgroups according to tumor location: 87 had tumors abutting large vessels/intrahepatic major bile ducts, 232 had tumors adjacent to extrahepatic organs, and 63 had subcapsular tumors. Furthermore, 88 patients with HCC > 5 mm away from large vessels/bile ducts or adjacent organs/liver capsule who received conventional RFA served as the control group.
Pre-RFA examination: All patients underwent a baseline evaluation including an enhanced computed tomography (CT) or magnetic resonance imaging (MRI) scan of the abdomen and pelvis the month before RFA. Serum laboratory tests consisting of a complete blood count, coagulation profile, liver and kidney function tests and serum tumor markers [such as alpha-fetoprotein (AFP)] were performed at least two weeks before RFA. All patients underwent contrast-enhanced ultrasound to confirm tumor range and adjacent relations prior to RFA. The RFA scheme and needle placement were designed based on the imaging results.
Equipment for RFA and ultrasonography: Different RFA systems can be used depending on the tumor size, morphology and location. In the present study, three types of RFA systems were used: Model 1500X (RITA, United States), Valleylab (Tyco Healthcare, United States) and Celon Lab Power (Olympus, Germany).
The Model 1500X system consists of a 460-KHz generator unit that is capable of delivering a maximum power of 200 W through a 14-gauge, 15-cm-long electrode. The electrode contains nine hook-shaped needles that can be deployed from the applicator shaft. A sphere-like coagulation area of 2.0-5.0 cm in diameter can be produced by one circle in 20 min. Larger tumors (> 3.5 cm in diameter) were treated by multiple overlapping ablations depending on the tumor size and shape[18,19].
Standard mono-polar RFA was applied using a 480-kHz RFA generator (Model CC-1-220; Valleylab, Tyco Healthcare, United States). The 2-cm or 3-cm tip of a 17-gauge electrically insulated electrode (RFA2020/RFA2030 electrode; Valleylab, Tyco Healthcare) was used, and one circle lasted for 12 min.
The Celon Lab Power system provides a maximum power output of 250 W (rated frequency, 470 ± 10 kHz) and is capable of connecting one to three 15- to 20-cm-long electrodes with an exposed tip of 3-4 cm. In bipolar and multipolar modes, the size and shape of the coagulation depend on the length, number, distance between, power, and deploying time of the electrodes.
The Prosound α-10 (ALOKA) and Vivid E9 4D cardiovascular ultrasound systems (GE Healthcare) were used for ultrasound guidance and blood flow observation. Sonovue (Bracco, Italy) was used as a contrast medium in contrast-enhanced ultrasonography.
Treatment principle: All patients underwent conventional and contrast-enhanced ultrasonography for confirmation of the invasion range, and tumor size was determined in combination with enhanced CT/MRI. For tumors adjacent to extrahepatic organs, artificial ascites was used for tumor separation before and during RFA. A rational RFA scheme was designed according to the adjacent relations of the tumor, and the electrode was first deployed to its adjoining zone and then away to the center. The tumors ≥ 4 cm and supplied by rich vessels commonly received transarterial chemoembolization (TACE) once or twice before RFA. For tumors with visible feeding vessels by color Doppler flow imaging (CDFI), pre-ablation in the entrance zone of feeding vessels would provide temporary blood occlusion, and then increase the coagulation effect[20]. The needle track needed to avoid large vessels to prevent hemorrhage.
All percutaneous RFA treatments in this study were performed by two of four expert interventional radiologists (Chen MH, Yan K, Wu W, Yang W) with at least five years of interventional ultrasound operation experience, including RFA therapy for hepatic tumors.
Tumors adjacent to extrahepatic organs: Individual RFA protocols, i.e., protocols aimed at avoiding damage to different adjacent structures, were used for tumors adjacent to the GI, diaphragm and gallbladder because of the restricted safety margin.
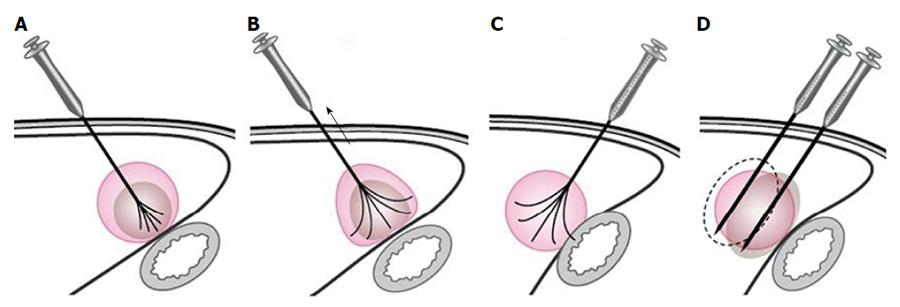
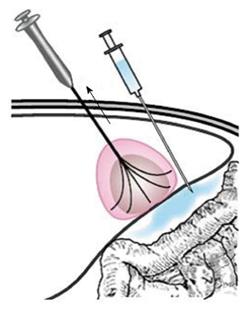
For tumors adjacent to the diaphragm or gallbladder, 100 to 500 mL of fluid was injected into the adjoining site, and this artificial ascites separated the tumor tissue from nearby organs effectively. RFA treatment in patients with tumors adjacent to the GI tract needed to be performed very carefully as follows: (1) patients were placed in a right anterior oblique position, allowing a left caudal downward shift of the GI tract, which was helpful for the separation of the HCC from the transverse colon, lesser curvature and other structures during RFA; (2) the multiple-tined electrode was inserted into the tumor in the direction perpendicular to the wall of the adjacent GI tract. When expanding the RF ablation prongs from 3 cm to 4-5 cm to ablate a tumor area near the GI tract, the RFA electrode was first lifted, and the tumor was slightly drawn away from the adjacent structure. Then, the position of the electrode was fixed while the movable hub was pushed to expand the prongs. With mono-polar electrodes, it is better to insert the electrode in the direction parallel to the wall of the adjacent GI tract (Figure 1); (3) artificial ascites was used between the tumor and nearby bowels before and during the ablation (Figures 2 and 3); (4) after the electrode was placed, the patients were asked to take deep abdominal breaths in an attempt to move the bowel relative to the tumor. Limited movement of the nearby bowel was assumed to suggest that the prong tips might have penetrated into the bowel wall. The generator was not turned on to start the treatment until we were confident that the prongs had not penetrated the adjacent structure; and (5) the patients fasted for 24-48 h and were given intravenous nutrition to allow observation for bowel perforation, and then a semifluid diet was given for 2 d before a full diet was resumed[21].
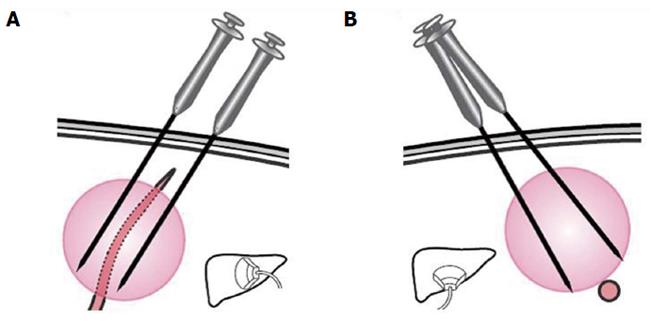
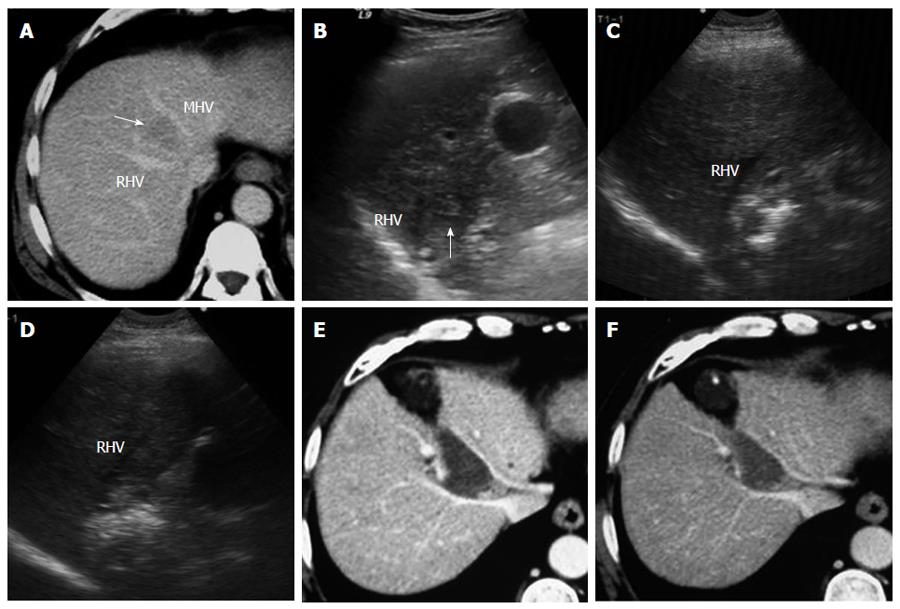
Tumors abutting large vessels or bile ducts: (1) a mono-polar RFA electrode was usually used for these patients because satisfactory visualization of the needle tip could be ensured. Mono-polar RFA was applied at bilateral sides of the large vessel (Figures 4 and 5); (2) the multiple-tined electrode was usually deployed parallel to vessels to avoid damage to large vessels; (3) effusion or hemorrhage in front of liver area and the needle track needed to be closely monitored during RFA; (4) all patients underwent ultrasound examination after the withdrawal of the electrode, with special emphasis on the detection of potentially new or increasing effusion or hemorrhage in front of liver area, in the space between the right lobe and the kidney, in the gallbladder fossa and in the lesser omental bursa; and (5) patients with suspicious bleeding after RFA usually underwent CDFI or contrast enhanced ultrasound examination to check for any active bleeding along the needle track and should be discharged only after confirmation of the absence of bleeding.
Tumors close to the liver surface: (1) an appropriate type of RFA electrode with a suitable length must first be selected, and the coagulation sphere should be estimated; (2) to prevent extrahepatic charring, artificial ascites was produced by injecting more than 100-500 mL of fluid into the anterior hepatic space or between the liver surface and the peritoneum before or during RFA; (3) the hyperechogenicity area induced by ablation was monitored by real-time ultrasonography, and the needling depth was adjusted when the region extended beyond the liver capsule; (4) when a large amount of fluid needed to be infused, a cannula paracentetic needle was introduced into the anterior hepatic space, and a cannula was placed for fluid infusion; and (5) repeated ablation at the same puncture point should be avoided to minimize needling track seeding or biliary fistula. Additionally, it is better not to directly puncture the superficial tumor, and a needle track passing through the normal liver parenchyma is preferred.
To evaluate early tumor necrosis resulting from RFA therapy, contrast-enhanced CT/MRI was performed 1 mo after the treatment. The tumor was considered early necrosis on the basis of all of the following findings at the one-month CT/MRI: (1) no contrast enhancement was detected within or around the tumor; (2) the margins of the ablation zone were clear and smooth; and (3) the ablation zone extended beyond the tumor borders. Subsequently, patients were followed by repeat CT/MRI every 2-3 mo during the first year and then every 4-6 mo after the first year. Contrast enhancement that was detected in the ablation zone on follow-up CT/MRI scans was considered to represent local tumor progression. If the imaging scan showed no contrast enhancement but abnormal tumor markers (e.g., AFP) were detectable and if CDFI detected an abnormal vascular area, a multiple-core-needle biopsy specimen was obtained to assess for possible tumor progression in the highly suspicious area. However, only a positive biopsy result was useful for diagnosis. The definition of a major complication was a complication that might threaten the patient’s life, lead to substantial morbidity and disability, or result in a lengthened hospital stay if left untreated. All other complications were considered minor[22,23].
Significant differences in baseline characteristics and treatment results were assessed by the χ2 test, Fisher exact test and t-test. Kaplan-Meier models and log-rank tests were used in overall survival and local progression-free survival analyses. Overall survival duration was counted in months from the date of RFA to death or last follow-up. Local progression-free survival duration was counted in months from the date of first RFA to local progression of tumor, death, or last follow-up. For the patients who were lost to follow-up, survival was counted in months from the date of RFA to the last follow-up, and the status was considered as censored data. Ten potential prognostic factors were considered in this study, including age, gender, tumor size, tumor number, tumor location, serum AFP, serum liver function enzyme, portal hypertension, previous treatment (such as TACE) and RFA device. Cox proportional hazards model was used for multivariate analysis. Portal hypertension was diagnosed when the dilatation of main branches of the portal system and the presence of collaterals were detected. Liver function enzyme [alanine transaminase (ALT) and aspartate transaminase (AST)] values were assessed, and the normal range was 0-40 IU/L for ALT and 0-45 IU/L for AST. SPSS 16.0 software (SPSS, Chicago, IL) was used to perform statistical analyses, and P < 0.05 was considered statistically significant.
In the difficult group, the average tumor size was 3.4 ± 1.2 cm (range, 1.0-6.0 cm). The maximum tumor size was ≥ 3 cm in 204 (53.4%) patients. In the control group, the average tumor size was 3.1 ± 1.1 cm (range, 1.1-6.0 cm). The maximum tumor size was ≥ 3 cm in 43 (48.9%) patients. There were no statistically significant differences in baseline characteristics between the two groups. The baseline characteristics of the patients are listed in Table 1.
| Characteristic | Difficult group(n = 382) | Control group(n = 88) | P value |
| Sex | |||
| Male | 331 (86.6) | 74 (84.1) | 0.531 |
| Female | 51 (13.4) | 14 (15.9) | |
| Age (yr) | 55.3 ± 10.1 | 57.4 ± 11.8 | 0.652 |
| Liver cirrhosis | 369 (96.6) | 84 (95.5) | 0.605 |
| Child-Pugh class | |||
| Class A | 235 (61.5) | 62 (70.5) | 0.117 |
| Class B | 147 (38.5) | 26 (29.5) | |
| Maximum diameter | 3.4 ± 1.2 | 3.1 ± 1.1 | 0.071 |
| > 3 cm | 204 (53.4) | 43 (48.9) | |
| > 5 cm | 40 (10.5) | 5 (5.7) | |
| Tumor number | 1.4 ± 0.6 | 1.3 ± 0.9 | 0.128 |
| Elevated AFP | 172 (45.0) | 38 (43.2) | 0.754 |
| Previous TACE | 95 (24.9) | 18 (20.5) | 0.382 |
| Previous hepatectomy | 58 (15.2) | 11 (12.5) | 0.521 |
According to one-month CT/MRI results, the early tumor necrosis rate in the difficult group was similar to that in the control group (97.6% vs 94.3%, P = 0.080). Additionally, there was no significant difference in the early tumor necrosis rates among tumors near a large vessel (93.7%), near peripheral structures (94.2%) or under the liver capsule (95.4%). In total, 31 tumors had residual tissue after initial RFA treatment. Three patients with residual un-ablated tumors gave up additional RFA session due to tumor adherence to the diaphragm or bowel; four patients received TACE due to the occurrence of new lesions in the liver, and the remaining 24 tumors in 24 patients were successfully ablated after the second or third RF ablation.
The follow-up period was 6 to 116 mo, with an average of 28 ± 22.4 mo. The local tumor progression after RFA was 2 to 26 mo, with an average of 10.3 ± 8.5 mo. There was a significant difference in local progression rate between the difficult location and control groups (12.7% vs 7.1%, P = 0.046). Additionally, local progression rate in the subgroup with tumors near peripheral structures was significantly higher than that in the control group (14.4% vs 7.1%, P = 0.018) (Table 2). Local progression and new lesions in the liver were re-treated if the patient’s physical condition was strong enough to tolerate another RF ablation session. In the difficult group, 114 HCC patients received 2-11 RFA sessions, while 20 HCC patients received 2-5 RFA sessions in the control group. The mean number of RFA sessions was significantly higher in the difficult group than in the control group (1.5 ± 1.0 vs 1.3 ± 0.7 sessions, P = 0.038).
| Group | Number of patients | Number of tumors | Tumor diameter (cm) | Early necrosis | Local progression |
| Control | 88 | 170 | 3.1 ± 1.1 | 166 (97.6) | 12 (7.1)1 |
| Difficult | 382 | 473 | 3.4 ± 1.2 | 446 (94.3) | 60 (12.7)1 |
| Near large vessels or bile ducts | 87 | 95 | 3.5 ± 1.5 | 89 (93.7) | 10 (10.5) |
| Near peripheral structures | 232 | 291 | 3.4 ± 1.2 | 274 (94.2) | 42 (14.4)1 |
| Under liver capsule | 63 | 87 | 3.1 ± 1.4 | 83 (95.4) | 8 (9.2) |
| Total | 470 | 643 | 3.3 ± 1.3 | 612 (95.2) | 72 (11.2) |
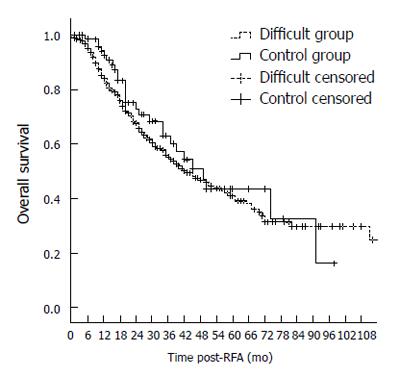
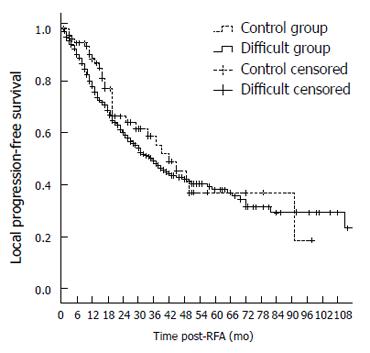
The 1-, 3-, 5-, and 7-year overall survival rates were 84.3%, 54.4%, 41.2%, and 29.9%, respectively, in the difficult location group and 92.5%, 60.3%, 43.2%, and 32.8% in the control group, respectively. There was no significant difference in overall survival between the two groups (P = 0.371). (Table 3, Figure 6). The 1-, 3-, 5-, and 7-year local progression-free survival rates were 77.9%,49.0%, 38.0%, and 29.1% in the difficult location group, respectively, and 88.2%,55.6%, 36.3%, and 36.3% in the control group, respectively. There was no significant difference in local progression-free survival between the two groups (P = 0.584) (Table 4 and Figure 7).
| Group | Number of patients | Overall survival (%)1 | |||
| 1-yr | 3-yr | 5-yr | 7-yr | ||
| Control | 88 | 92.5 | 60.3 | 43.2 | 32.8 |
| Difficult | 382 | 84.3 | 54.4 | 41.2 | 29.9 |
| Near large vessels or bile ducts | 87 | 86.3 | 41.4 | 38.0 | 33.8 |
| Near peripheral structures | 232 | 82.8 | 56.9 | 42.9 | 28.8 |
| Under liver capsule | 63 | 90.2 | 69.2 | 40.1 | 30.1 |
| Total | 470 | 85.8 | 56.8 | 41.6 | 30.4 |
| Group | Number of patients | Local progression-free survival (%)1 | |||
| 1-yr | 3-yr | 5-yr | 7-yr | ||
| Control | 88 | 88.2 | 55.6 | 36.3 | 36.3 |
| Difficult | 382 | 77.9 | 49.0 | 38.0 | 29.1 |
| Near large vessels or bile ducts | 87 | 81.7 | 35.6 | 33.4 | 30.7 |
| Near peripheral structures | 232 | 77.3 | 48.7 | 38.6 | 27.2 |
| Under liver capsule | 63 | 80.5 | 53.1 | 37.8 | 28.1 |
| Total | 470 | 79.8 | 50.3 | 37.5 | 29.8 |
Ten potential prognostic factors were included in the multivariate analysis using a Cox proportional hazards model. Among these factors, Child-Pugh classification (HR = 2.024, P < 0.001), tumor number (HR = 1.599, P = 0.003), serum liver function enzyme (HR = 1.426, P = 0.029) and tumor size (HR = 1.262, P = 0.047) were well related to the HCC patient prognosis. Child-Pugh classification was the highest risk factor (Table 5), whereas tumor location was not a significant risk factor.
| Variable | Wald value | P value | HR | 95%CI for HR | |
| Lower | Upper | ||||
| Child-Pugh classification | 28.046 | 0.000 | 2.024 | 1.559 | 2.628 |
| Tumor number | 8.555 | 0.003 | 1.599 | 1.167 | 2.189 |
| Liver function enzyme | 4.760 | 0.029 | 1.426 | 1.037 | 1.963 |
| Tumor size | 3.951 | 0.047 | 1.262 | 1.003 | 1.589 |
In the present study, 29 (4.2%, 29/689) patients had severe complications, including hemoperitoneum in 6 patients, biliary injury in 5 patients, hemothorax in 3 patients, pyothorax in 1 patient, liver abscess in 3 patients, intestinal perforation in 3 patients, cholecystitis in 2 patients, needle-track seeding in 6 patients and intestinal perforation-related death in 1 patient. The rate of major complications for the difficult group was 4.9%, and that for the control group was 0.8%; this difference was statistically significant (P = 0.041) (Table 6). Minor complications included self-limited capsule hematoma, small pleural effusions, slight cholangiectasis, transient jaundice and moderate thermal injury of the abdominal wall.
| Group | Number of patients | Number of RFA | Complication (%) | Common complication name |
| Control | 88 | 121 | 1 (0.8)1 | Needle seeding (1/1) |
| Difficult | 382 | 568 | 28 (4.9)1 | |
| Near large vessels or bile ducts | 87 | 151 | 6 (4.0) | Bile duct injury (4/6) |
| Near peripheral structures | 232 | 309 | 18 (5.8) | GI perforation (3/18) Bloody pleural (3/18) Needle seeding (3/18) |
| Under liver capsule | 63 | 108 | 4 (3.7) | Hemoperitoneum (2/4) Needle seeding (2/4) |
| Total | 470 | 689 | 29 (4.2) |
Adverse reactions included abdominal pain, shoulder pain, pain in the right upper abdomen associated with breathing, postoperative fever, and transient abnormal liver function. These complications were resolved with symptomatic treatment or without any special treatment.
In 1996, Rossi et al[24] first reported the therapeutic effect of percutaneous RFA in primary or metastatic malignancies confined to the liver. Thereafter, numerous studies have demonstrated the advantages of RFA over percutaneous ethanol injection (PEI) for treating small HCC[25-27]. However, the high recurrence and complication rates associated with RFA greatly restricted its application, especially for the treatment of large tumors, multifocal tumors and tumors in high-risk locations[28,29]. Early reports suggested that the major complications of RFA were closely related with tumor site. For example, RFA of tumors located centrally abutting the portal vein is liable to cause biliary injury, and in tumors located in the periphery of the liver, RFA can cause damage to adjacent structures, such as intestinal perforation. Therefore, these types of liver tumors are regarded as difficult cases for RFA protocols. In the past decade, significant advances in RF devices and technology have enabled the treatment of patients with unresectable liver tumors who were previously not eligible for RFA treatment with novel RFA strategies. These new RFA methods are based on individualized protocols and new technologies aimed at increasing the tumor necrosis rate while considering potential complications and overall safety. These advances enabled patients with HCC who were receiving palliative care to undergo radical therapy.
RFA treatment for tumors abutting key structures has attracted increasing attention. Chopra et al[30] reported a complete necrosis rate of 87% (7/8) in HCC adjacent to the gallbladder treated by RFA with no gallbladder injury. In another study, Choi et al[31] performed RFA of HCC adjacent to the GI tract, which resulted in a tumor necrosis rate of 93% (38/41). Ng et al[32] used an internally cooled electrode in 52 patients with perivascular HCC. The complete necrosis rate for small HCC was 92%, the local recurrence rate was 11%, and the 2-year overall survival rate was 75%. Koda et al[33] successfully treated 25 lesions under the diaphragm using RFA with artificial pleural effusion. Complete necrosis was achieved in 22 lesions (88%), and local recurrence was diagnosed in one patient (4.5%). Teratani et al[34] reported a clinical study that included 207 patients with 231 nodules in high-risk locations treated by percutaneous RFA and showed no significant differences in the 3-year local progression rate between nodules in high-risk locations and those located elsewhere. However, the outcomes of RFA in difficult locations still need to be verified in a large series of patients with long-term follow-up.
In the present study, we compared the 5- and 7-year survival rates between patients with tumors in difficult locations and those with tumors located elsewhere. In addition, the differences between tumors in different difficult locations, such as abutting major vessels or bile ducts, adjacent to extrahepatic organs, and/or located in the subcapsular space, were further analyzed. Our study involved large samples and analyzed the effect of RFA on the long-term survival of patients with tumors in different locations; the results provided important evidence for extending the indications of RFA and confirmed its efficacy for difficult cases of HCC.
In the present study, tumors adjacent to key extrahepatic organs, such as the gallbladder, intestines and diaphragm, tumors abutting large vessels and subcapsular tumors were included in the difficult group. With optimized RFA strategies, early tumor necrosis rates in different locations all yielded above 90%. The tumor necrosis rates for subgroups near vessels, near peripheral structures and under the liver capsule were 93.7%, 94.2%, and 95.4%, respectively, with no statistically significant differences (P = 0.872).
The local progression rate was lower in the difficult group than in the control group (12.7% vs 7.1%, P = 0.046) during follow-up. The comparatively higher progression rate in the difficult group may be attributed to its limited safety margin. In particular, the local progression rate for the subgroup with HCC tumors near peripheral structures was 14.4%, which was significantly higher than that in the control group (7.1%, P = 0.018). For tumors abutting large vessels or bile ducts, a larger safety margin was possible because of the heat-sink effect of blood flow and the protective effect of smooth muscle for the bile duct. In subcapsular tumors, fluid injection is an effective method to widen the extrahepatic space, which facilitates the complete destruction of a tumor by the ablation needle. However, in tumors adjacent to perihepatic organs, even artificial ascites injection could not achieve an effective separation, which resulted in a limited safety margin, and the residual tumor advanced toward local progression during follow-up.
A previous analysis of prognostic factors based on the results from 266 patients with HCC treated by RFA in our center suggested that the degree of liver function, tumor stage and standard therapy application were independent prognostic factors, while tumor location was excluded[14]. The results of the present study also showed that the 1-, 3-, 5-, and 7-year overall survival rates in the difficult group were similar to those in the control group (84.3%, 54.4%, 41.2%, and 29.9% vs 92.5%, 60.3%, 43.2%, and 32.8%, respectively, P = 0.371). In the multivariate analysis using a Cox proportional hazards model, Child-Pugh classification, tumor number, serum liver function enzyme and tumor size were independent risk factors for HCC patients, whereas tumor location was not a significant risk factor. This result reinforces the idea that RFA is also an effective treatment for HCC in difficult locations.
Additionally, the 1-, 3-, 5-, and 7-year local progression-free survival rates in the difficult group were not significantly different from those in the control group (88.2%, 55.6%, 36.3%, and 36.3% vs 77.9%, 49.0%, 38.0%, and 29.1%, respectively, P = 0.584). These results suggested that although difficult tumor location can result in a high rate of progression in tumors treated by RFA, location has little influence on the long-term outcomes. In should be noted that in our study, the number of RFA sessions in the difficult group was significantly higher than that in the control group (P = 0.038). This result indicated that the patients in the difficult group received more re-treatments by RFA for local progression than the control group. The frequent RFA retreatments might greatly contribute to overall survival.
The overall rate of complications of RFA is low compared with that of other localized treatments, such as surgery and cryoablation. However, RFA-related death and severe complications should not be underestimated. In a multicenter study in Italy, the mortality rate was 0.3%, the rate of additional major complications was 2.2%, and the rate of minor complications was less than 5%[23]. In the present study, complications occurred in 28 patients (4.9%) in the difficult group; only one patient (0.8%) in the control group experienced needle-track seeding. Biliary injury, which was the most common complication of RFA in tumors abutting large vessels or bile ducts, is related to thermal or mechanical damage as well as alterations in the blood supply of the biliary track. In the present study, four patients who developed cholangiectasis with jaundice (direct bilirubin/total bilirubin, > 50%) underwent percutaneous transhepatic cholangial drainage (PTCD) and/or percutaneous transhepatic cholangiography (PCT) for biliary drainage, and one patient was treated against infection. Jaundice resolved in these four patients after treatment.
The most severe complication in superficially located liver tumors treated by RFA is gastrointestinal perforation, which occurs most frequently in the colon (0.06%-0.30%)[23,34-36]. In the present study, delayed colon perforation occurred one week after RFA treatment in 3 patients with liver tumors adjacent to the colon. These patients were treated by surgical repair and drainage. One of the 3 patients had a history of biliary surgery, and postoperative adhesion made it difficult to separate the liver from the intestine. These data indicated that intestinal perforation mostly occurred in patients with intestinal adhesions as a result of previous abdominal surgery. Perforation always occurred 2-3 d after RFA because of the 24 h of fasting, and in some cases, perforation was latent, suggesting that patients at a high risk of perforation should be carefully observed for at least one week. All 3 patients with GI perforation were treated by multiple-tined electrode, indicating that the mono-polar electrode is preferred in such cases. Hemorrhage, another major complication, occurred in 6 patients. Two of these patients had recurrent tumors located superficially, with a tumor size > 5 cm and a protruding section of 1/4 to 2/5 of the entire tumor. Cough occurred 2 h after RFA, and the increased abdominal pressure caused by position changes resulted in the rupture and bleeding of the tumor, suggesting that these cases were not suitable for RFA. In another patient with HCC combined with portal hypertension, the dilated collateral vessel branches that extended and adhered to the liver surface were punctured by the RFA electrode, causing moderate bleeding. This occurrence underscored the importance of imaging reading pre-RFA to avoid large vessels, arteriovenous fistulas, and phlebangioma. The other 3 patients developed minor or moderate hemoperitoneum as a consequence of improper operation. Thermal ablation in situ was applied, and the bleeding was controlled in all patients before discharge[37].
Three patients with HCC located under the diaphragm developed hemothorax and/or abscess in the right thoracic cavity; two were attributed to the large size and convex surface of the tumor, whereas the other one was induced by poor visualization of the needle tip, which punctured a vessel in the diaphragm. No severe complications, such as diaphragm perforation, were observed. The risk of needle-track seeding has been reported to vary widely, with rates ranging from 0.2%-12.5% in patients treated by percutaneous RFA[23,36,38,39]. In the present study, 0.9% of the patients in the difficult group showed needle-track seeding. To reduce the risk of needle-track seeding, the electrode should be retracted slowly, and the needle temperature should be controlled.
Twenty percent of patients included in this study had received TACE, indicating that the improvement of the long-term survival and recurrence may have been the result of the combination of TACE and RFA. However, the percent of patients who had previously received TACE in the difficult group was equal to that in the control group, and the combination of TACE and RFA was used as a routine method for the treatment of large HCC with rich blood supply in both groups. Thus, the use of TACE in some patients should not bias the analysis of the long-term outcomes between the two groups. In addition, the success of RFA for difficult HCC relied greatly on the experience and skill of the operator, and the outcome was expected to improve with an increasing number of cases treated by RFA. This single center study was a 10-year retrospective investigation, and the data obtained in the early years and in the later years were analyzed together. Therefore, the results obtained could have differed from those reported in other centers.
In conclusion, although the complication rate and local progression rate were notably higher in the difficult group (4.9% vs 0.8% and 12.7% vs 7.1%, respectively), these results were not substantial enough to prevent consideration of RFA in these cases (7-year overall survival rate: 29.9% vs 32.8%). With appropriate attention to technique and adjunctive measures and with judicious patient selection, ultrasound-guided percutaneous RFA of lesions close to vital structures remains a reasonable option in patients willing to accept the complication risk.
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world, causing more than 500000 deaths every year. Clinical evidence has confirmed the efficacy of radiofrequency ablation (RFA) for the treatment of localized HCC. However, tumors are often found in difficult locations (e.g., abutting key structures such as the stomach, bowel, gallbladder, diaphragm and large vessels) that may be injured during RFA with subsequent complications or recurrence. Recently, many studies have reported an increased tumor necrosis rate and a reduced rate of complications with RFA treatment in such tumors. However, the long-term outcomes, such as an analysis of the 5-year survival and beyond, in a large number of difficult tumors have been rarely demonstrated.
In the present study, tumors adjacent to key extrahepatic organs, such as the gallbladder, intestines and diaphragm, tumors abutting large vessels and subcapsular tumors were included in the difficult group. Individual treatment strategies were developed to treat these difficult cases, and early tumor necrosis rates in different locations all yielded above 90%.
Recently, many studies have reported an increased tumor necrosis rate and a reduced rate of complications with RFA treatment of HCC tumors in difficult locations. However, the outcomes in patients with difficult HCC treated by RFA still needs to be verified in a large population of patients with long-term follow-up. In the present study, we compared the 5- and 7-year survival rates between patients with tumors in difficult locations and those with tumors located elsewhere. In addition, the differences between tumors in different difficult locations, such as abutting major vessels/bile ducts, adjacent to extrahepatic organs, and subcapsular, were analyzed.
With appropriate attention to technique and adjunctive measures and with judicious patient selection, ultrasound-guided percutaneous RFA of lesions close to vital structures remains a reasonable option in patients willing to accept the complication risk.
RFA: a medical procedure in which part of the electrical conduction system of the heart, tumor or other dysfunctional tissue is ablated using the heat generated from high-frequency alternating current. The radiofrequency waves passing through the electrode increase the temperature within tumor tissue, which results in tumor destruction. HCC in difficult location: nodules adjacent to large vessels were defined as those located ≤ 5 mm from a first or second branch of the portal vein, the base of hepatic veins, or the inferior vena cava, while nodules adjacent to extrahepatic organs were defined as those located ≤ 5 mm from the diaphragm, lung, gallbladder, right kidney, or gastrointestinal tract.
This study evaluated HCC patients treated by RFA to investigate the long-term outcomes (tumor recurrence and survival) in normal or high risk HCC location groups. The manuscript is very interesting, presenting valuable evidence to support the practice of RFA treatment in the field of interventional management of HCC and showing excellent presentation and detailed description of the methods and techniques used. The strengths of this paper are the increased number of patients and the sufficient follow-up.
P- Reviewer: Bellanti F, El-Bendary M, Rodriguez-Peralvarez M, Silva LD S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 626] [Cited by in F6Publishing: 702] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 2. | Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, Bhoori S, Lee SG. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13286] [Cited by in F6Publishing: 13416] [Article Influence: 706.1] [Reference Citation Analysis (1)] |
| 4. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Makuuchi M. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 467] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-577; quiz 578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 537] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 7. | Chen MH, Yang W, Yan K, Gao W, Dai Y, Wang YB, Zhang XP, Yin SS. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol. 2005;11:6395-6401. [PubMed] [Cited in This Article: ] |
| 8. | N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, Vicaut E, Trinchet JC, Sellier N, Beaugrand M. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475-1483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 9. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 10. | Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 247] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Hayashi H, Nitta H, Ishiko T, Takamori H, Baba H. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res. 2013;43:853-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Wang KF, Pan W, Wang F, Wang GF, Madhava P, Pan HM, Kong DX, Liu XG. Geometric optimization of a mathematical model of radiofrequency ablation in hepatic carcinoma. Asian Pac J Cancer Prev. 2013;14:6151-6158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, Xing BC, Huang XF. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Park MJ, Kim YS, Rhim H, Lim HK, Lee MW, Choi D. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J Vasc Interv Radiol. 2011;22:771-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | The Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer (in Japanese). 3rd ed. Tokyo, Japan: Kanehara 1992; . [Cited in This Article: ] |
| 17. | Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267-1274. [PubMed] [Cited in This Article: ] |
| 18. | Chen MH, Yang W, Yan K, Zou MW, Solbiati L, Liu JB, Dai Y. Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients--mathematic model, overlapping mode, and electrode placement process. Radiology. 2004;232:260-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Chen MH, Wei Y, Yan K, Gao W, Dai Y, Huo L, Yin SS, Zhang H, Poon RT. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006;17:671-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Hou YB, Chen MH, Yan K, Wu JY, Yang W. Adjuvant percutaneous radiofrequency ablation of feeding artery of hepatocellular carcinoma before treatment. World J Gastroenterol. 2009;15:2638-2643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Chen MH, Yang W, Yan K, Hou YB, Dai Y, Gao W, Zhang H, Wu W. Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging. 2008;33:428-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Lewis CA, Allen TE, Burke DR, Cardella JF, Citron SJ, Cole PE, Drooz AT, Drucker EA, Haskal ZJ, Martin LG. Quality improvement guidelines for central venous access. The Standards of Practice Committee of the Society of Cardiovascular & amp; Interventional Radiology. J Vasc Interv Radiol. 1997;8:475-479. [PubMed] [Cited in This Article: ] |
| 23. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1003] [Cited by in F6Publishing: 912] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 24. | Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 566] [Cited by in F6Publishing: 508] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 25. | Oeda S, Mizuta T, Isoda H, Kuwashiro T, Iwane S, Takahashi H, Kawaguchi Y, Eguchi Y, Ozaki I, Tanaka K. Survival advantage of radiofrequency ablation for hepatocellular carcinoma: comparison with ethanol injection. Hepatogastroenterology. 2013;60:1399-1404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 26. | Shen A, Zhang H, Tang C, Chen Y, Wang Y, Zhang C, Wu Z. Systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J Gastroenterol Hepatol. 2013;28:793-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 28. | Inoue T, Minami Y, Chung H, Hayaishi S, Ueda T, Tatsumi C, Takita M, Kitai S, Hatanaka K, Ishikawa E. Radiofrequency ablation for hepatocellular carcinoma: assistant techniques for difficult cases. Oncology. 2010;78 Suppl 1:94-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 582] [Cited by in F6Publishing: 645] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 30. | Chopra S, Dodd GD, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: feasibility and safety. AJR Am J Roentgenol. 2003;180:697-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Choi D, Lim HK, Kim MJ, Kim SH, Lee WJ, Kim SH, Lim JH, Paik SW, Koh KC, Yoo BC. Therapeutic efficacy and safety of percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the gastrointestinal tract. AJR Am J Roentgenol. 2004;183:1417-1424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Ng KK, Poon RT, Lam CM, Yuen J, Tso WK, Fan ST. Efficacy and safety of radiofrequency ablation for perivascular hepatocellular carcinoma without hepatic inflow occlusion. Br J Surg. 2006;93:440-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004;183:583-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 487] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 36. | Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, Lee WJ, Lim HK, Nam GJ, Han SS. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123-134; discussion 134-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Chen MH, Dai Y, Yan K, Yang W, Gao W, Wu W, Liao SR, Hao CY. [Intraperitoneal hemorrhage during and after percutaneous radiofrequency ablation of hepatic tumors: reasons and management]. Chin Med J (Engl). 2005;118:1682-1687. [PubMed] [Cited in This Article: ] |
| 38. | Solbiati L, Ierace T, Goldberg SN, Sironi S, Livraghi T, Fiocca R, Servadio G, Rizzatto G, Mueller PR, Del Maschio A. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997;202:195-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 414] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [PubMed] [Cited in This Article: ] |