Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1845
Peer-review started: May 31, 2014
First decision: July 9, 2014
Revised: September 4, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: February 14, 2015
AIM: To investigate the clinicopathologic features of patients with extra-gastrointestinal stromal tumors (EGISTs) in South Korea.
METHODS: A total of 51 patients with an EGIST were identified. The clinicopathologic features, including sex, age, location, tumor size, histology, mitotic rate, immunohistochemical features, genetic status and survival data, were analyzed.
RESULTS: The median age was 55 years (range: 29-80 years), and male:female ratio was 1:1.04. The most common site was in the mesentery (n = 15) followed by the retroperitoneum (n = 13) and omentum (n = 8). The median tumor size was 9.0 cm (range: 2.6-30.0 cm) and the median mitotic rate was 5.0/50HPF. (1/50 - 185/50). KIT was analyzed in 16, which revealed 10 cases with wild-type KIT and 6 cases with an exon 11 mutation. Among 51 patients, 31 patients had undergone surgery, and 10 had unresectable disease and had taken palliative imatinib, which resulted in 22.7 mo of progression-free survival. Of the patients who had undergone surgery, 18 did not take adjuvant imatinib, and 8 of these were categorized as “high risk” according to the risk criteria. However, the relapse-free survival was not different (P = 0.157) between two groups.
CONCLUSION: Because the biologic behaviors of GISTs differ according to the location of the tumor, a more stratified strategy is required for managing EGISTs including incorporation of molecular features.
Core tip: A gastrointestinal stromal tumor arising outside the gastrointestinal tract is called an extra-gastrointestinal stromal tumor (EGIST). In this study, we analyzed 51 patients with an EGIST and found that, patients with an EGIST have unique clinicopathologic features and distinct disease courses. Therefore, the risk stratification of this disease should be distinguished from that of GISTs.
- Citation: Yi JH, Park BB, Kang JH, Hwang IG, Shin DB, Sym SJ, Ahn HK, Lee SI, Lim DH, Park KW, Won YW, Lim SH, Park SH. Retrospective analysis of extra-gastrointestinal stromal tumors. World J Gastroenterol 2015; 21(6): 1845-1850
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1845.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1845
A gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract (GIT) with approximately 10 new cases diagnosed per 1 million each year[1-3]. Surgery is the only treatment leading to a potential cure, but more than 40% of cases recur and metastasize[4].
A GIST is thought to originate from the interstitial cells of Cajal (ICC), the pacemaker of the peristaltic movement of the GIT[5]. More than 95% of GISTs express the KIT protein, and recently DOG1 (discovered on GIST-1) has also been suggested as a useful diagnostic marker. These two immunohistochemical markers are considered to be the most specific and sensitive markers for GIST[1,6,7]. As for the genetic aberrations, approximately 80% of GISTs have a KIT mutation, and 8% to 10% have mutations in the gene encoding the platelet-derived growth factor receptor alpha polypeptide (PDGFRa). The gain-of-function mutations of those genes are critical in the carcinogenesis of GIST[8]. Thus, inhibitors of KIT and PDGFRα, such as imatinib[9], sunitinib[10] and regorafenib[11] are reasonable options for treatment.
The most common primary site of a GIST is the stomach (60% to 70%) followed by the ileum to jejunum (25% to 30%), the colorectum (5% to 15%), the duodenum (5%), and the esophagus (< 2%)[12,13]. The prognosis and genetic features are distinguishable according to the anatomical location; a gastric GIST has a better prognosis and a higher incidence of an exon 11 mutation of KIT, which is a favorable predictive marker for imatinib treatment, than that of a small intestinal GIST[13,14]. Some GIST develops outside the GIT, such as in the omentum, mesentery, and retroperitoneum, and this type of tumor is called an extra-gastrointestinal stromal tumor (EGIST)[12,15]. Although the incidence of EGISTs is reported to be approximately 10% of all GIST cases[16,17], the clinicopathologic parameters and clinical implications of an EGIST have yet to be defined because of the rarity of these tumors. Moreover, the role of imatinib, the drug of choice for this disease, is still unclear.
In the current study, we analyzed the clinicopathologic features of patients with an EGIST from multiple institutes in South Korea.
Patients who were diagnosed with an EGIST from 2004 to 2012 were included in the analysis. The inclusion criteria were as follows; (1) a pathologically confirmed diagnosis of a GIST; (2) tumors that arose outside the GIT; and (3) a complete medical record, including demographics, site of primary tumor and pathologic reports. Patients with tumors that were attached to the serosa of the GIT, as determined by either radiologic or surgical field findings, were excluded.
We retrospectively collected clinicopathologic parameters from patients’ medical records, including age, sex, primary tumor site, tumor size, histology, mitotic rate (per 50 high-power fields, HPF, 400 × magnification level), histologic grade, immunohistochemical findings (KIT, CD34, DOG1), genetic analysis (KIT, PDGFRα), use of imatinib and the date of surgery, recurrence, progression and death. Mutations analysis was done in exons 9, 11, 13, and 17 of the KIT gene and those of exons 12 and 18 of the PDGFRα gene. The analysis of mutations via the polymerase chain reaction amplification of genomic DNA for the KIT gene (exons 9, 11, 13 and 17) and the PDGFRα gene (exons 12 and 18) was performed as previously described[18,19].
Overall survival (OS) was measured from the date of diagnosis of the GIST to the date of death or last follow-up. Relapse-free survival (RFS) was measured from the date of curative surgery to the date of recurrence or last follow-up. Progression-free survival (PFS) was measured from the date of diagnosis of a metastatic or unresectable GIST to the date of progressive disease, death or last follow-up. All of the survival parameters were calculated using the Kaplan-Meier method and were compared using a log-rank test. P values > 0.05 were considered statistically significant, and all the P-values corresponded to two-sided significance tests.
A total of 51 patients from 7 institutes were found to be eligible for the analysis. The median age of patients was 51 years (range: 29-80 years) and male:female ratio was 1:1.04. The most common primary site was in the mesentery (n = 15) followed by the retroperitoneum (n = 13) and omentum (n = 8). Other primary sites were the vagina (n = 3), liver (n = 3), ovary (n = 2), pancreas (n = 2), perianal area (n = 2), chest wall (n = 1), pleura (n = 1) and prostate (n = 1). The median size of the tumor was 9.0 cm (range 2.6-30.0 cm), and the median mitotic rate per 50 HPF was 5.0 (range: 1-185). Regarding the morphology, 15 (29.4%) were epithelioid, 27 (52.9%) were spindle cell; and 9 (17.7%) were a pleomorphic type. On immunohistochemical analysis, KIT was positive in 47 (92.2%) cases; CD34 was positive in 25 cases (80.6%, 31 cases examined), and DOG1 was positive in 13 cases (100.0%, 13 cases examined). The 4 cases not expressing KIT were with KIT gene analysis. Regarding the genetic status, KIT analysis was performed in 16 cases with 10 (62.5%) cases of wild-type and 6 (37.5%) cases of exon 11 mutation identified. PDGFRα analysis was carried out in 6 cases among cases with wild-type KIT, and 4 (66.7%) of those examined had an exon 18 mutation. These are summarized in Table 1.
| Age, median (range) | 51 (29-80) |
| Sex | |
| Male | 25 (49.0) |
| Female | 26 (51.0) |
| Primary site | |
| Mesentery | 15 (29.4) |
| Retroperitoneum | 13 (25.5) |
| Omentum | 8 (15.7) |
| Vagina | 3 (5.9) |
| Liver | 3 (5.9) |
| Ovary | 2 (3.9) |
| Pancreas | 2 (3.9) |
| Perianal area | 2 (3.9) |
| Chest wall | 1 (2.0) |
| Pleura | 1 (2.0) |
| Prostate | 1 (2.0) |
| Tumor size, median (range) | 9.0 cm (2.6-30.0) |
| Mitotic rate1, median (range) | 5.0 (1-185) |
| Histologic morphology | |
| Epithelioid | 15 (29.4) |
| Spindle | 27 (52.9) |
| Pleomorphic | 9 (17.7) |
| KIT IHC | |
| Positive | 47 (92.2) |
| Negative | 4 (7.8) |
| CD34 IHC (n = 31) | |
| Positive | 25 (80.6) |
| Negative | 6 (19.4) |
| DOG1 IHC (n = 13) | |
| Positive | 13 (100.0) |
| Negative | 0 (0.0) |
| KIT gene analysis (n = 16) | |
| Exon 11 mutation | 6 (37.5) |
| Wild-type | 10 (62.5) |
| PDGFRα gene analysis (n = 6) | |
| Exon 18 mutation | 4 (66.7) |
| Wild type | 2 (33.3) |
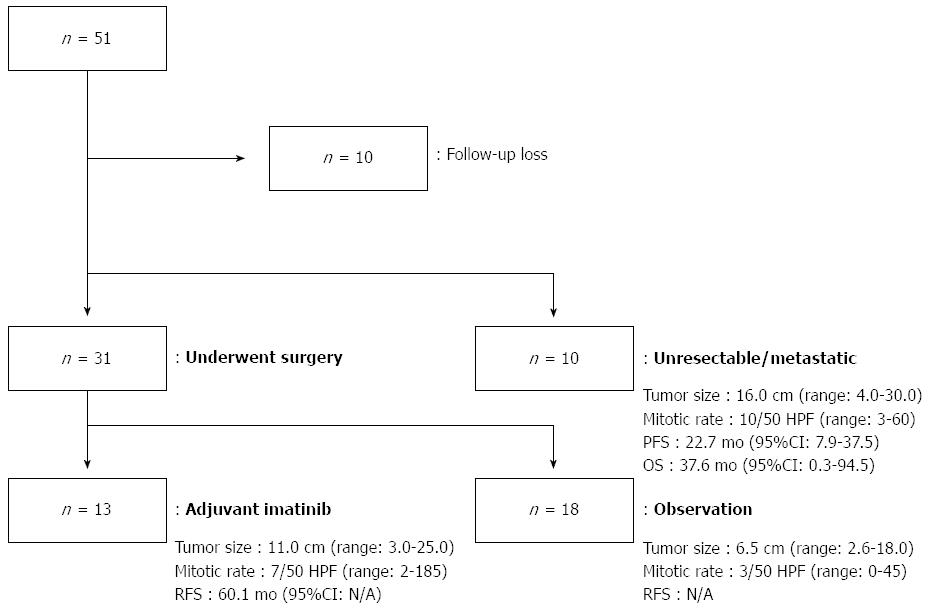
Among 51 patients, 10 patients did not receive any type of treatment, and they were lost to follow up after the initial diagnosis. Out of the 41 remaining patients, 31 patients underwent a curative resection, 13 of whom received imatinib as an adjuvant treatment and 18 of whom did not receive adjuvant treatment. Ten of the 41 were diagnosed with metastatic or unresectable disease at the time of diagnosis and received imatinib as palliative treatment.
Regarding the histologic features of the patients who had undergone curative resection, the physicians used somewhat different criteria for imatinib treatment compared to the NIH criteria[20]. The histologic features for the 13 patients who were treated with adjuvant imatinib were as follows: median tumor size was 11.0 cm (range: 3.0-25.0 cm); the median mitotic rate was 7.0 per 50 HPF (range: 2-185 per 50 HPF); and the median RFS was 60.1 mo. The histologic features for the 18 patients who did not receive adjuvant imatinib after surgery were as follows; the median tumor size was 6.5 cm (range: 2.6-18.0 cm); the median mitotic rate was 3.0 per 50 HPF (range: 0-45 per 50 HPF); and the median RFS could not be calculated because of the small number of event (n = 2). When we categorized these patients according to the NIH criteria for risk of recurrence, 10 patients were categorized as “low-intermediate risk”, whereas 8 patients fell into the “high risk” group. However, there was no difference in the RFS between two groups (P = 0.157). When separating the two prognostic factors, a mitotic rate higher than 5/50 HPF showed a trend to predict more recurrence (P = 0.061), but this result did not reach statistical significance, and a tumor size > 5.0 cm was not associated with the risk of recurrence (P = 0.866).
Regarding the 10 patients who were treated with imatinib as a palliative measure, the median tumor size was 16.0 cm (range: 4.0-30.0 cm), the median mitotic rate was 10.0 per 50 HPF (range: 3-60), and the median PFS was 22.7 mo (95%CI: 7.9-37.5). Median OS of these patients was 37.6 mo (95%CI: 0.3-94.5).
The hospital courses of the patients and their clinicopathologic features are described in Figure 1.
In the current study, we analyzed the clinicopathologic features of 51 patients with EGISTs across 7 institutes. The demographics such as age and the sex ratio were similar to those in patients with stromal tumors that had arisen inside the GIT. And with a GIST, the KIT-positive rate was over 90% (47/51), and DOG1-positive rate was 100% (13/13). With regard to the genetic status, however, rate of KIT wild-type was higher than expected (67.5%). Imatinib achieved median PFS of 22.7 mo which is comparable to that of advanced GIST patients who were treated with the drug[21,22].
Compared to a GIST, the prognosis of EGIST is known to be less favorable[15,17]. This is assumed because an EGIST harbors poor prognostic factors, including high proliferative indices, a large tumor size, lymph node involvement, and distant metastasis. Because development outside GIT may result in a delay of the presentation of clinical symptoms, a considerable portion of EGIST cases are diagnosed at a late stage, which can make it difficult to manage the case surgically and thereby results in worse clinical outcomes. In contrast, there are several reports that tumor size does not impact the prognosis of EGIST patients. Reith et al[15] found that a tumor size larger than 10.0 cm dose not influence the clinical outcomes of 48 patients with EGIST. Furthermore, in Yamamoto’s report, tumor size did not correlate with patient survival[23]. However, in these two studies, proliferation indices, such as the mitotic rate, cellularity and Ki-67 expression, were shown to be prognostic factors for survival.
In the current study, we observed similar results. Although tumor size was not associated with survival, the mitotic rate showed a tendency to be associated with survival. Because a substantial portion of EGISTs are diagnosed with a large tumor size, it is possible that tumor size itself may not reflect the biology of the EGIST. Because the tumor size has different clinical implications to the anatomical sites[13], the prognostic role of tumor size in EGISTs requires further analysis.
Approximately two-thirds of patients with a conventional GIST have a KIT mutation at exon 11[3]. Regarding an EGIST, the incidence of this type of mutation is reported to be approximately 40%-50%, which is somewhat lower[16,23]. In the present study, we found 6 cases with an exon 11 mutation out of 16 patients (37.5%). According to these results, it appears that EGIST patients less frequently harbor an exon 11 mutation. As this mutation is indicative of a good response to imatinib, further analysis with a greater number of cases is required.
Surgery has been the frontline treatment of an EGIST[24-30]. After surgery, the administration of imatinib usually follows according to the NIH criteria, which are determined by the tumor size, mitotic rate and anatomic location. In the current study, physicians did not strictly apply these criteria. Although the median tumor size (11.0 cm vs 6.5 cm) and the median mitotic rate (7.0/50 vs 3.0/50) were higher in patients who were administered imatinib (n = 13) than those of the patients who were only observed after surgery (n = 18), 8 out of 18 patients should have nevertheless been treated with imatinib according to the NIH criteria. However, as previously mentioned, RFS was not different (P = 0.157).
Several hypotheses have been suggested for the carcinogenesis of an EGIST. The tumor is identical to a GIST regarding the histologic, immunohistochemical and genetic features[12,15,23]. Because the presence of interstitial Cajal-like cells has been reported in many organs outside the GIT, it is rational to suppose that an EGIST originates from common precursor cells that differentiate into the ICC-derived neoplasm during their development outside of the GIT. Another hypothesis is that this tumor might come from pluripotential stem cells located outside the GIT. The extramural extension of a stromal tumor within the GIT is another hypothesis.
We reported an analysis of the clinicopathologic features and hospital courses of 51 patients with an EGIST. Considering the distinct features of EGISTs, a more precise strategy is required for managing this tumor.
Gastrointestinal stromal tumors (GISTs) constitute approximately 1% of tumors of the gastrointestinal tract. A curative surgical resection and the use of KIT inhibitors are the most important treatment modalities. This type of tumor sometimes develops outside the alimentary canal and is called an extra-gastrointestinal stromal tumor (EGIST).
There are several studies suggesting that the clinicopathologic and molecular features differ between a GIST and an EGIST. Because the anatomic location, histologic features and genetic status are well-known risk factors for a GIST, understanding these features of an EGIST may help us to treat this disease.
The current study is one of the largest analyses dealing with patients with EGIST, and as with other studies, the authors have found that the tumor size itself was not associated with survival. The authors are one of the first to show that the clinical outcomes of imatinib treatment in patients with an EGIST are comparable to those of patients with a GIST.
This study suggests that different risk criteria may be applied when making clinical decisions for patients with an EGIST.
Authors evaluate the efficacy of strategies including surgery and treatment with imatinib in patients suffering from extra-gastrointestinal stromal tumors. This work adds new data of interest to establish the more appropriate treatment of these tumors. This paper can be accepted after minor revision.
P- Reviewer: Caboclo JLF, Grizzi F, Mayol J, Plaza MA S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 425] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [PubMed] [Cited in This Article: ] |
| 3. | Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 560] [Cited by in F6Publishing: 574] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 4. | Joensuu H. Adjuvant treatment of GIST: patient selection and treatment strategies. Nat Rev Clin Oncol. 2012;9:351-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Wang X, Mori I, Tang W, Utsunomiya H, Nakamura M, Nakamura Y, Zhou G, Kakudo K. Gastrointestinal stromal tumors: are they of cajal cell origin? Exp Mol Pathol. 2002;72:172-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009;33:1401-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 342] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Lasota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs). Semin Diagn Pathol. 2006;23:91-102. [PubMed] [Cited in This Article: ] |
| 9. | Sleijfer S, Wiemer E, Verweij J. Drug Insight: gastrointestinal stromal tumors (GIST)--the solid tumor model for cancer-specific treatment. Nat Clin Pract Oncol. 2008;5:102-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1942] [Cited by in F6Publishing: 1830] [Article Influence: 101.7] [Reference Citation Analysis (0)] |
| 11. | Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 938] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 12. | Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, Sobin LH. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109-1118. [PubMed] [Cited in This Article: ] |
| 13. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [PubMed] [Cited in This Article: ] |
| 14. | Søreide K, Sandvik OM, Søreide JA, Gudlaugsson E, Mangseth K, Haugland HK. Tyrosine-kinase mutations in c-KIT and PDGFR-alpha genes of imatinib naïve adult patients with gastrointestinal stromal tumours (GISTs) of the stomach and small intestine: relation to tumour-biological risk-profile and long-term outcome. Clin Transl Oncol. 2012;14:619-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Reith JD, Goldblum JR, Lyles RH, Weiss SW. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol. 2000;13:577-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 362] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 16. | Du CY, Shi YQ, Zhou Y, Fu H, Zhao G. The analysis of status and clinical implication of KIT and PDGFRA mutations in gastrointestinal stromal tumor (GIST). J Surg Oncol. 2008;98:175-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Cho MY, Sohn JH, Kim JM, Kim KM, Park YS, Kim WH, Jung JS, Jung ES, Jin SY, Kang DY. Current trends in the epidemiological and pathological characteristics of gastrointestinal stromal tumors in Korea, 2003-2004. J Korean Med Sci. 2010;25:853-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160:1567-1572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 342] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 19. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1712] [Cited by in F6Publishing: 1660] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 20. | Goh BK, Chow PK, Yap WM, Kesavan SM, Song IC, Paul PG, Ooi BS, Chung YF, Wong WK. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified Armed Forces Institute of Pathology risk criteria. Ann Surg Oncol. 2008;15:2153-2163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1270] [Cited by in F6Publishing: 1166] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 22. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1782] [Cited by in F6Publishing: 1599] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 23. | Yamamoto H, Oda Y, Kawaguchi K, Nakamura N, Takahira T, Tamiya S, Saito T, Oshiro Y, Ohta M, Yao T. c-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue). Am J Surg Pathol. 2004;28:479-488. [PubMed] [Cited in This Article: ] |
| 24. | Hu X, Forster J, Damjanov I. Primary malignant gastrointestinal stromal tumor of the liver. Arch Pathol Lab Med. 2003;127:1606-1608. [PubMed] [Cited in This Article: ] |
| 25. | Lee JR, Anstadt MP, Khwaja S, Green LK. Gastrointestinal stromal tumor of the posterior mediastinum. Eur J Cardiothorac Surg. 2002;22:1014-1016. [PubMed] [Cited in This Article: ] |
| 26. | Park JK, Choi SH, Lee S, Min KO, Yun SS, Jeon HM. Malignant gastrointestinal stromal tumor of the gallbladder. J Korean Med Sci. 2004;19:763-767. [PubMed] [Cited in This Article: ] |
| 27. | Long KB, Butrynski JE, Blank SD, Ebrahim KS, Dressel DM, Heinrich MC, Corless CL, Hornick JL. Primary extragastrointestinal stromal tumor of the pleura: report of a unique case with genetic confirmation. Am J Surg Pathol. 2010;34:907-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Siddiq MA, East D, Hock YL, Warfield AT. Gastrointestinal stromal tumour of the pharynx. J Laryngol Otol. 2004;118:315-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Fagkrezos D, Touloumis Z, Giannila M, Penlidis C, Papaparaskeva K, Triantopoulou C. Extra-gastrointestinal stromal tumor of the omentum: a rare case report and review of the literature. Rare Tumors. 2012;4:e44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Franzini C, Alessandri L, Piscioli I, Donato S, Faraci R, Morelli L, Del Nonno F, Licci S. Extra-gastrointestinal stromal tumor of the greater omentum: report of a case and review of the literature. World J Surg Oncol. 2008;6:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |