Published online Mar 14, 2016. doi: 10.3748/wjg.v22.i10.3023
Peer-review started: September 21, 2015
First decision: October 14, 2015
Revised: November 5, 2015
Accepted: December 8, 2015
Article in press: December 8, 2015
Published online: March 14, 2016
AIM: To determine the discriminatory performance of fatty liver index (FLI) for non-alcoholic fatty liver disease (NAFLD).
METHODS: The data of 5052 subjects aged over 18 years were analyzed. FLI was calculated from body mass index, waist circumference (WC), triglyceride, and gamma glutamyl transferase data. Logistic regression analysis was conducted to determine the association between FLI and NAFLD. The discriminatory performance of FLI in the diagnosis of NAFLD was evaluated by receiver operating characteristic analysis. Area under the curves (AUCs) and related confidence intervals were estimated. Optimal cutoff points of FLI in the diagnosis of NAFLD were determined based on the maximum values of Youden’s index.
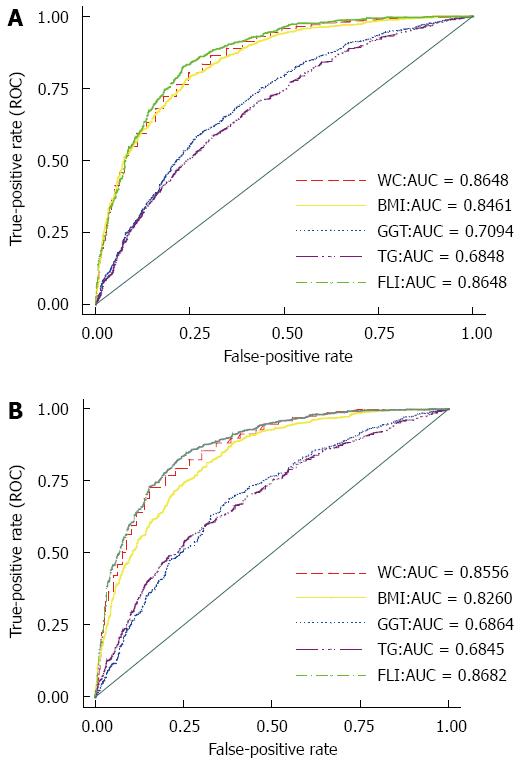
RESULTS: The mean age of men and women in the study population were 44.8 ± 16.8 and 43.78 ± 15.43, respectively (P = 0.0216). The prevalence of NAFLD was 40.1% in men and 44.2% in women (P < 0.0017). FLI was strongly associated with NAFLD, so that even a one unit increase in FLI increased the chance of developing NAFLD by 5.8% (OR = 1.058, 95%CI: 1.054-1.063, P < 0.0001). Although FLI showed good performance in the diagnosis of NAFLD (AUC = 0.8656 (95%CI: 0.8548-0.8764), there was no significant difference with regards to WC (AUC = 0.8533, 95%CI: 0.8419-0.8646). The performance of FLI was not significantly different between men (AUC = 0.8648, 95%CI: 0.8505-0.8791) and women (AUC = 0.8682, 95%CI: 0.8513-0.8851). The highest performance with regards to age was related to the 18-39 age group (AUC = 0.8930, 95%CI: 0.8766-0.9093). The optimal cutoff points of FLI were 46.9 in men (sensitivity = 0.8242, specificity = 0.7687, Youden’s index = 0.5929) and 53.8 in women (sensitivity = 0.8233, specificity = 0.7655, Youden’s index = 0.5888).
CONCLUSION: Although FLI had acceptable discriminatory power in the diagnosis of NAFLD, WC was a simpler and more accessible index with a similar performance.
Core tip: The present study was carried out to evaluate the discriminatory capability of fatty liver index in the diagnosis of non-alcoholic fatty liver disease (NAFLD) among the general population of northern Iran. Our results showed that the chance of NAFLD occurrence was increased by 5.8% after a one unit increase in fatty liver index (FLI). Although we found that FLI has good discriminatory power, its capability was not superior to that of waist circumference.
- Citation: Motamed N, Sohrabi M, Ajdarkosh H, Hemmasi G, Maadi M, Sayeedian FS, Pirzad R, Abedi K, Aghapour S, Fallahnezhad M, Zamani F. Fatty liver index vs waist circumference for predicting non-alcoholic fatty liver disease. World J Gastroenterol 2016; 22(10): 3023-3030
- URL: https://www.wjgnet.com/1007-9327/full/v22/i10/3023.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i10.3023
Non-alcoholic fatty liver disease (NAFLD) is a chronic condition characterized by the accumulation of fat in the liver in the absence of other causes of steatosis, including excess consumption of alcohol or drugs[1]. The prevalence of this condition in western countries varies from 20% to 30% depending on applied diagnostic tools, the population under study, and related definitions[2]. Although still lower than that found in western countries, there is an increasing trend in NAFLD prevalence in Asian countries, due to the recent increase in the incidence of obesity, metabolic syndrome, and diabetes type 2[3]. Additionally, while the prevalence of NAFLD is estimated to be 15%-20% in the general Asian population, one population-based study on adults in northern Iran resulted in an incidence of almost 44%[3,4]. NAFLD can lead to a wide range of clinical conditions, from simple steatosis to cirrhosis, or even hepatocellular carcinoma[5-9].
Although the two most common methods used in the diagnosis of fatty liver are histologic methods and imaging procedures, no single diagnostic procedure has been shown to be reliable enough in the diagnosis of fatty liver[10-12]. Despite liver biopsy being the gold standard procedure for the diagnosis of NAFLD, it is an invasive and expensive tool that has some health risks and economic costs[11-14].
Recently, a number of indices were introduced to diagnose NAFLD that consist of simple measures[15-18]. Fatty liver index (FLI) is one of these indices developed as a convenient tool based on body mass index (BMI), waist circumference (WC), triglyceride (TG), and gamma glutamyl transferase (GGT) levels[18]. In one previous study, this index showed good predictive performance in the diagnosis of NAFLD, with an AUC of 0.813[19]. When a diagnostic tool displays accurate predictive capability, it is important that an optimal cutoff point is determined. To the best knowledge of the authors, no such study has been conducted among the Iranian population. Therefore, this study was carried out to assess the discriminatory ability of FLI in the diagnosis of NAFLD among a population in northern Iran, as well as to propose an optimal cutoff point for FLI.
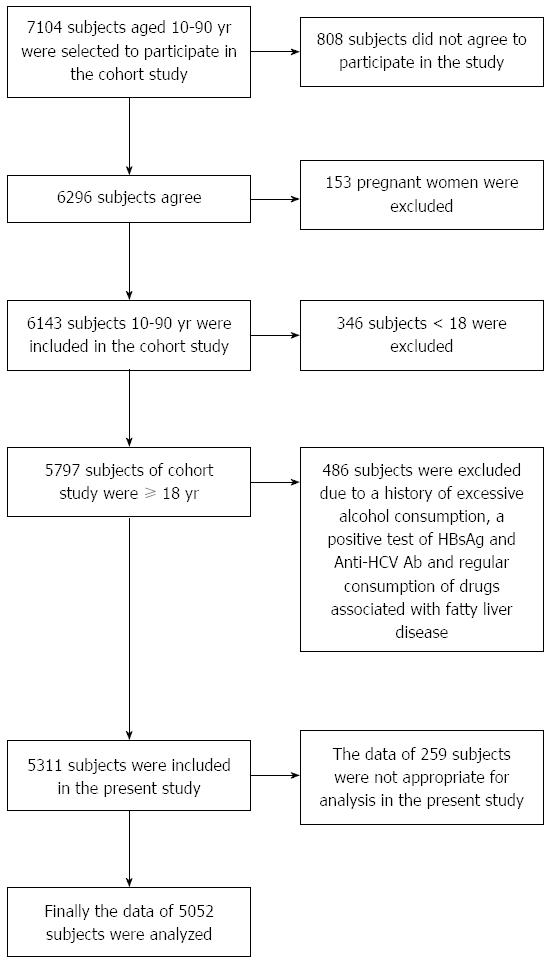
Of 6140 participants in a baseline cohort study conducted among individuals aged between 10 and 90 years-of-age, data from 5052 participants aged 18 or older were analyzed in the present study. The baseline study was carried out in Amol, a densely-populated city in northern Iran. Local health centers, where almost all study participants had health record files, were used to collect the data. Sampling for the cohort study is explained elsewhere[20]. A schematic diagram of study participants and exclusion criteria is shown in Figure 1.
Weight, height, waist circumference, hip circumference, and blood pressure were measured in health centers, where trained healthcare staff members were responsible for data collection. Height was measured while the participants were standing with their heels and buttocks pressed up against a wall. Waist circumference was determined at the midpoint between the lowest costal ridge and the upper border of the iliac crest. The largest circumference between waist and knee was determined as hip circumference. Measurements were performed with a non-stretchable and accurately calibrated scale with 0.5 cm precision.
Blood pressure was measured, after a minimum 5-min rest period in a quiet room, with the use of a fitted cuff while participants were in the sitting position with their back supported and legs uncrossed. Systolic and diastolic blood pressures were determined and recorded as the first appearance and disappearance of Korotkoff sounds, respectively.
A venous blood sample was drawn from each participant following 12-h fasting to assess fasting blood sugar (FBS) and lipid profiles. All tests, including FBS, triglycerides (TGs), high-density lipoprotein (HDL), low-density lipoprotein, and cholesterol were assessed enzymatically using the BS200 Auto analyzer (Mindray, China). Screening tests for hepatitis B and C viral markers, as well as for autoimmune hepatitis, were performed.
Ten percent of the blood samples were evaluated by the Iranian National Reference Laboratory, with the coefficients of variation being between 1.7% and 3.8% of all laboratory values.
NAFLD was determined via evidence of hepatic steatosis in the sonogram and a lack of evidence of other causes of acute or chronic hepatitis. Evidence of secondary hepatic fat accumulation, such as significant alcohol consumption, use of steatogenic medication, or hereditary disorders, was also used to determine NAFLD.
All ultrasound examinations were carried out by a single sonographer who was an expert in the field of radiology. A 3-5 MHz transducer was used to examine the liver parenchyma and thereby provide sagittal, longitudinal, lateral, and intercostal views. Steatosis was confirmed if a marked increase of hepatic echogenicity was diagnosed or if the hepatic vessels and diaphragm appeared abnormal.
Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated based on the following formula:
HOMA-IR = [insulin (mU/mL) × Glucose (mg/dL)]/405
FLI was calculated based on laboratory and anthropometric measures, including TG, GGT, BMI, and WC, by using the following formula:
FLI = [e0.953 × ln (TG) + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC - 15.745/(1 + e0.953 × ln (TG) + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC - 15.745)] × 100
The capability of FLI to discriminate between subjects with and without NAFLD was evaluated using receiver operating characteristic (ROC) curve, for which the sensitivity of infinite decision thresholds of FLI was plotted against their false positive rates and thus the related areas under the curves (AUCs) were calculated. The lower boundary line for AUC was considered to be 0.5, with a significantly greater area than 0.5 showing some ability of FLI to discriminate between patients with and without NAFLD. The optimal cutoff point of FLI was also determined using maximal values of Youden’s J statistics [max (J = sensitivity + specificity - 1)]. The value of FLI corresponding to a maximum value of the Youden’s index was considered the optimal cutoff point for FLI.
Multivariable logistic regression analysis was conducted on NAFLD as an outcome variable alongside additional relevant predictor variables. Five potential predictor variables, including age, gender, MAP, HDL, and the HOMA-IR test were entered into the model in addition to FLI. In the multivariable model, Cox and Snell’s R2, as well as Nagelkerke’s R2, were calculated to determine how much NAFLD variance could be explained by the model. The Hosmer-Lemeshow test was used to evaluate the adequacy or suitability of the model. The odds ratio and related confidence intervals were reported along with P values. The significance level for all analyses was considered to be 0.05. All statistical analyses were conducted using version 21 of SPSS Inc., Chicago statistical software and STATA software, version 12 (StataCorp, Texas, United States).
Table 1 shows the mean age, anthropometric characteristics, and laboratory values of the study participants. Significant differences were reported between the two sexes for all variables except TG.
| Characteristics | Men (n = 2860) | Women (n = 2192) | P value |
| Age (yr) | 44.77 ± 16.77 | 43.78 ± 15.43 | 0.0216 |
| Weight (kg) | 76.70 ± 15.05 | 72.60 ± 14.36 | < 0.0001 |
| Height (cm) | 169.94 ± 8.02 | 156.40 ± 7.05 | < 0.0001 |
| WC (cm) | 90.76 ± 12.32 | 91.55 ± 13.89 | 0.0199 |
| DBP (mmHg) | 76.61 ± 12.69 | 75.82 ± 13.05 | 0.0191 |
| SBP (mmHg) | 117.42 ± 15.76 | 115.42 ± 17.60 | < 0.0001 |
| MAP (mmHg) | 90.22 ± 12.90 | 89.01 ± 13.62 | |
| BMI (kg/m2) | < 0.0001 | ||
| mean ± SD | 26.46 ± 4.60 | 29.65 ± 5.67 | |
| Prevalence of BMI ≥ 30 (%) | 21.6 (20.2-23.0) | 47.3 (45.4-49.3) | |
| TG (mg/dL) | 145.55 ± 98.25 | 141.12 ± 95.99 | 0.0966 |
| HDL (mg/dL) | 43.37 ± 11.48 | 46.42 ± 12.08 | < 0.0001 |
| Cholesterol | 178.49 ± 41.95 | 189.47 ± 43.20 | < 0.0001 |
| ALT (U/L) | 26.46 ± 19.04 | 19.59 ± 14.03 | < 0.0001 |
| AST (U/L) | 23.98 ± 12.65 | 19.98 ± 9.71 | < 0.0001 |
| GGT (U/L) | 29.90 ± 28.61 | 25.62 ± 21.64 | < 0.0001 |
| FBS | 98.62 ± 29.86 | 103.98 ± 41.30 | < 0.0001 |
| Insulin (mU/L) | 8.87 ± 6.74 | 10.36 ± 6.88 | < 0.0001 |
| HOMA-IR | 2.19 ± 1.90 | 2.72 ± 2.45 | < 0.0001 |
| FLI | 45.76 ± 28.93 | 52.24 ± 29.87 | < 0.0001 |
In the total population, the AUC of FLI in the diagnosis of NAFLD was 0.8656 (95%CI: 0.8548-0.8764), in which no significant difference was detected between men (AUC = 0.8648, 95%CI: 0.8504-0.8791) and women (AUC = 0.8682, 95%CI: 0.8513-0.8851). Analysis by age group showed that the greatest capability was related to the 18-40 age group (AUC = 0.8930, 95%CI: 0.8766-0.9093), while the lowest accuracy was related to the 40-60 age group (AUC = 0.8293, 95%CI: 0.8095-0.8492). The AUC for the ≥ 60 age group was 0.8403 (95%CI: 0.8124- 0.8683). Although the predictive performance of FLI was significantly higher than BMI (AUC of BMI = 0.8258, 95%CI: 0.8139-0.8378), TG (AUC of TG = 0.6840, 95%CI: 0.6676-0.7004), and gamma glutamyl transferase (GGT) (AUC of GGT = 0.6927, 95%CI: 0.6772-0.7081), with P < 0.0001, no significant difference was detected between the performance of FLI and WC (AUC of WC = 0.8533, 95%CI: 0.8419-0.8646). Figure 2 shows the related ROC curves for FLI, WC, BMI, TG, and GGT in both men and women.
Gender-based optimal cutoff points of FLI were also obtained for FLI in the diagnosis of NAFLD. The optimal cutoff points of FLI were 46.9 in men (sensitivity = 0.8242, specificity = 0.7687, Youden’s index = 0.5929) and 53.8 in women (sensitivity = 0.8233, specificity = 0.7655, Youden’s index = 0.5888).
According to these cutoff points, the prevalence of a high FLI was 0.4809 (0.4610-0.5007) in men and 0.5021 (0.4782-0.5260) in women. No significant difference was detected between the two sexes.
Univariate logistic regression analysis was performed on NAFLD by entering predictor variables, including age, sex, systolic blood pressure, diastolic blood pressure, HOMA-IR test, HDL, and FLI. The results are reported in Table 2.
| Variables | Univariable logistic regression | Multivariable logistic regression | ||||
| Wald test | P value | Odds ratio and CI | Wald test | P value | Odds ratio | |
| Age | 408.6 | < 0.001 | 1.037 (1.033-1.040) | 56.700 | < 0.001 | 1.022 (1.016-1.028) |
| Gender | 9.828 | 0.002 | 1.183 (1.065-1.315) | 9.020 | 0.003 | 0.764 (0.641-0.911) |
| MAP | 476.4 | < 0.001 | 1.052 (1.047-1.057) | 9.225 | 0.002 | 1.011 (1.004-1.018) |
| HOMA-IR | 364.3 | < 0.001 | 1.408 (1.359-1.4457) | 2.402 | 0.121 | 1.036 (0.991-1.084) |
| HDL | 273.7 | 0.001 | 0.959 (0.954-0.964) | 1.381 | 0.240 | 1.005 (0.997-1.013) |
| FLI | 1148.9 | < 0.001 | 1.061 (1.058-1.065) | 722.5 | < 0.001 | 1.058 (1.054-1.063) |
Before conducting the multivariate model, its suitability and adequacy were evaluated using relevant specific tests. In the multi-collinearity diagnostic test, SBP and DBP were located in a single common dimension, while a variance proportion of 0.97 was related to SBP. As a result, we replaced SBP and DBP with mean arterial pressure (MAP) [MAP = DRP + 1/3 (SBP|DBP)] multivariate model. Collinearity tests were rechecked before conducting multivariable logistic regression, with no variance proportion ≥ 0.9 related to predictor variables. Tolerances varied from 0.599 to 0.951, with each predictor variable being located separately in a related independent dimension. In the multivariate model, Cox & Snell’s R2 and Nagelkerke’s R2 that can be explained by the model. On the other hand, Hosmer-Lemeshow test (χ2 = 14.476, df = 8 and P = 0.07) indicated that a significant difference could not be established between observed and expected frequencies, thereby confirming the adequacy and suitability of our proposed model. After removing the effects of other predictors in the multivariable model, age (P < 0.001), gender (P = 0.002), MAP (P = 0.002), and FLI (P < 0.001) were significantly associated with NAFLD. FLI was highly associated with NAFLD, to the point that even a one-unit increase in FLI increased the chance of NAFLD occurrence by 5.8%. The odds ratios of the other predictor variables are shown in Table 2.
Table 3 shows the prevalence of NAFLD and high FLI, with the latter being calculated based on our study cutoff points; the second and third columns of the table are related to the prevalence of NAFLD and high FLI. Although an increasing trend of NAFLD prevalence among women was detected according to age, among men the prevalence was highest in the 40-59 age group. We observed a similar pattern for high FLI.
| Population | Prevalence of NAFLD (%) | Prevalence of NAFLD High FLI1 (%) |
| Men | ||
| Total men (n = 2860) | 40.1 (38.4-41.8) | 48.1 (46.1-50.1) |
| 18-39 (n = 1136) | 27.3 (24.9-29.7) | 37.6 (34.6-40.7) |
| 40-59 (n =1124) | 50.6 (47.8-53.3) | 58.3 (55.2-61.5) |
| ≥ 60 (600) | 44.6 (40.9-48.3) | 48.9 (44.6-53.2) |
| Women | ||
| Total women(n =2192) | 44.2 (42.3-46.1) | 50.2 (47.8-52.6) |
| 18-39 (n = 902) | 20.4 (17.9-22.9) | 28.4 (24.9-31.8) |
| 40-59 (n = 900) | 59.4 (56.4-62.4) | 63.3 (59.7-66.9) |
| ≥ 60 (390) | 64.1 (59.6-68.5) | 66.4 (61.3-71.5) |
The present study revealed that FLI has a high discriminatory power in the diagnosis of NAFLD. Analyses based on sex and age groups showed that this index has an appropriate performance in both sex and all age groups of 18-39, 40-59, and ≥ 60. A significantly strong association between NAFLD and FLI was also confirmed by binary regression, to the point that a one-unit increase in FLI led to a 5.8% increase in the chance of developing NAFLD. These results were in line with the findings of a previous study in which FLI showed good predictive performance in the diagnosis of NAFLD, with an AUC of 0.813[19].
This result could be somewhat anticipated due to the fact that FLI is composed of four quantities related to NAFLD, including BMI, WC, GGT, and TG[18]. A high BMI or WC, the main obesity indices, is considered an essential risk factor for NAFLD, and the prevalence of NAFLD substantially increases in obese individuals[21]. GGT can be considered an independent predictor for NAFLD, since this enzyme increases in NAFLD to protect against the adverse effects of insulin resistance due to its antioxidant activity[22,23]. On the other hand, among the markers of dyslipidemia, TG is strongly associated with NAFLD[24]. However, we found no significant difference between the performance of FLI and WC. An almost equal performance between obesity indices and FLI could be somewhat expected, as although obesity is strongly associated with NAFLD, it is also associated with other components involved in the calculation of FLI, including TG and liver enzymes[25,26]. Insulin resistance plays a key role in the pathogenesis of NAFLD and there is a strong association between this condition and the abnormal components of metabolic syndrome (MetS), where NAFLD is considered the hepatic manifestation of MetS[27]. As a result, the high discriminatory capability of WC for NAFLD is a logical expectation due to the undeniable role of visceral adiposity in MetS. However, Koehler et al[19] obtained a slightly significantly higher performance for FLI than obesity indices in elderly inhabitants of a district of Rotterdam, the Netherlands.
This study also suggests separate optimal cutoff points of FLI for men and women, with values of 46.9 and 53.8, respectively. A higher cutoff point for women is perhaps the result of a lower vulnerability of females to this condition due to the protective effect of estrogen, although its underlying mechanism is not fully understood[28,29]. Furthermore, metabolic risk factors alone play different roles in the development of NAFLD between males and females[30].
Although NAFLD has previously been discussed as a predominantly male condition, our study obtained a significantly higher prevalence of it among women compared to men[31]. This disagreement may be partly attributed to the markedly higher prevalence of obesity among women (47.3%) compared to men (21.6%). On the other hand, despite a higher estimation of NAFLD among women, our results appear to confirm that the female sex has a protective mechanism against this disease. In univariable analysis, women seemed to show greater odds than men for developing NAFLD, but by removing the effects of other predictors in the multivariable model, an inverse result was obtained which indicated that a higher chance of NAFLD was related to the male sex. On the other hand, men had a higher prevalence of NAFLD in the under-40 age group than women, while prevalence was higher in women than men in the over-60 age group, perhaps as a result of an attenuated protective effect of estrogen against NAFLD in menopausal women. Moreover, the prevalence of fatty liver among men was reduced in the over-60 age group compared with the 40-60-year-old group, which could be due to the declining effects of male sexual hormones with age[32].
Our study had a population-based design and a large sample size in which a reliable non-invasive approach was applied to diagnose NAFLD. Although liver biopsy is the gold standard for diagnosis of fatty liver disease, this approach is not only invasive, but also has a relatively high false negative rate in the diagnosis of this condition[10-12,33]. As a result, it may be better that a multifaceted non-invasive approach is implemented in order to diagnose NAFLD in population-based studies until a more reliable evaluation can be obtained from the performance of FLI.
In conclusion, FLI has a promising predictive power in the diagnosis of NAFLD. However, according to our findings, FLI was not more effective than WC in the discrimination of NAFLD. While the performance of FLI was not different between the two sexes, a higher cutoff point of FLI was obtained for women than men.
The authors would like to express their gratitude to Iran University Medical Sciences and Tehran University of Medical Sciences for their support of this project; to the staff and management of Hefdah Shahrivar Hospital, Amol, Iran, for their cooperation; and to the members of the Amol Cohort Study Center.
Non-alcoholic fatty liver disease (NAFLD) is a chronic condition. The prevalence of this condition varied from 20% to 30% in different countries. The most common methods used in the diagnosis of fatty liver are histological and imaging procedures, but these have their own limitations. Nevertheless, this disease can lead to a wide range of clinical conditions and, as a result, make its diagnosis difficult. In this context, some indices were recently introduced to diagnose NAFLD, with fatty liver index (FLI) being one example that has had a good prediction value in previous reports.
It is estimated that the prevalence of NAFLD among the Asian and Middle Eastern population is more than 20%. However, some cohort studies in Iran have shown a rate of up to 40%. Therefore, usage of a simple diagnostic modality with high predictive value for the detection of NAFLD is an important issue in this region.
As is the case with other countries, the prevalence of NAFLD in Iran is undergoing an increasing trend that is predicted to cause major health problem in the near future. Therefore, early diagnosis of NAFLD has become more important. In this study, we revealed that FLI has a high discriminatory power in the diagnosis of NAFLD in our population. The performance of FLI was not found to be different between two genders. Furthermore, the highest performance of FLI was seen in the 18-39 age group. The optimal cutoff points of FLI were 46.9 in men and 53.8 in women.
Based on the present study, we can suggest that WC has almost the same value as FLI in practice. In fact, WC could be considered an easy and economic modality with a high discrimination value for the detection of NAFLD.
NAFLD is a chronic condition with vast clinical presentation which is characterized by the accumulation of fat in the liver in the absence of other causes of steatosis, including excess consumption of alcohol or drugs. FLI was calculated based on laboratory and anthropometric measures, including TG, GGT, BMI, and WC by using a specific formula.
There is not enough data regarding diagnosis and discrimination of NAFLD, particularly in the Middle Eastern region. This study presents the discriminatory power of FLI and its cutoff point for NAFLD. The results are notable, with FLI showing no superiority over WC in the discrimination of NAFLD. Furthermore, although the performance of FLI was not different between the two sexes, a higher cutoff point of FLI was obtained for women than for men.
P- Reviewer: He JY, Tarantino G, Zhu X S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Ma S
| 1. | Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 Suppl:S186-S190. [PubMed] [Cited in This Article: ] |
| 2. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [PubMed] [Cited in This Article: ] |
| 3. | Ashtari S, Pourhoseingholi MA, Zali MR. Non-alcohol fatty liver disease in Asia: Prevention and planning. World J Hepatol. 2015;7:1788-1796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Amirkalali B, Poustchi H, Keyvani H, Khansari MR, Ajdarkosh H, Maadi M, Sohrabi MR, Zamani F. Prevalence of Non-Alcoholic Fatty Liver Disease and Its Predictors in North of Iran. Iran J Public Health. 2014;43:1275-1283. [PubMed] [Cited in This Article: ] |
| 5. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2143] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 6. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] [Cited in This Article: ] |
| 7. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [PubMed] [Cited in This Article: ] |
| 8. | Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000-3004. [PubMed] [Cited in This Article: ] |
| 9. | Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042-2047. [PubMed] [Cited in This Article: ] |
| 10. | Otgonsuren M, Estep MJ, Hossain N, Younossi E, Frost S, Henry L, Hunt S, Fang Y, Goodman Z, Younossi ZM. Single non-invasive model to diagnose non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). J Gastroenterol Hepatol. 2014;29:2006-2013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [PubMed] [Cited in This Article: ] |
| 12. | Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2413] [Cited by in F6Publishing: 2453] [Article Influence: 204.4] [Reference Citation Analysis (0)] |
| 14. | Conlon BA, Beasley JM, Aebersold K, Jhangiani SS, Wylie-Rosett J. Nutritional management of insulin resistance in nonalcoholic fatty liver disease (NAFLD). Nutrients. 2013;5:4093-4114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 15. | Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, Capron D, Abella A, Massard J, Ngo Y. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. [PubMed] [Cited in This Article: ] |
| 16. | Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, Massard J, Bonyhay L, Tahiri M, Thabut D. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. [PubMed] [Cited in This Article: ] |
| 17. | Poynard T, Imbert-Bismut F, Munteanu M, Ratziu V. FibroTest-FibroSURE: towards a universal biomarker of liver fibrosis? Expert Rev Mol Diagn. 2005;5:15-21. [PubMed] [Cited in This Article: ] |
| 18. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [PubMed] [Cited in This Article: ] |
| 19. | Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11:1201-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Zamani F, Sohrabi M, Alipour A, Motamed N, Saeedian FS, Pirzad R, Abedi K, Maadi M, Ajdarkosh H, Hemmasi G. Prevalence and risk factors of cholelithiasis in Amol city, northern Iran: a population based study. Arch Iran Med. 2014;17:750-754. [PubMed] [Cited in This Article: ] |
| 21. | Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330-9337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 198] [Reference Citation Analysis (1)] |
| 22. | Bi WR, Yang CQ, Shi Q, Xu Y, Cao CP, Ling J, Wang XY. Large-scale analysis of factors influencing nonalcoholic fatty liver disease and its relationship with liver enzymes. Genet Mol Res. 2014;13:5880-5891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Ma H, Xu C, Xu L, Yu C, Miao M, Li Y. Independent association of HbA1c and nonalcoholic fatty liver disease in an elderly Chinese population. BMC Gastroenterol. 2013;13:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Tomizawa M, Kawanabe Y, Shinozaki F, Sato S, Motoyoshi Y, Sugiyama T, Yamamoto S, Sueishi M. Triglyceride is strongly associated with nonalcoholic fatty liver disease among markers of hyperlipidemia and diabetes. Biomed Rep. 2014;2:633-636. [PubMed] [Cited in This Article: ] |
| 25. | Lam GM, Mobarhan S. Central obesity and elevated liver enzymes. Nutr Rev. 2004;62:394-399. [PubMed] [Cited in This Article: ] |
| 26. | Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, Taylor HA. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419-5426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 27. | Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol. 2013;19:3375-3384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 100] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 28. | Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402-409. [PubMed] [Cited in This Article: ] |
| 29. | McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: a randomized placebo-controlled trial. Clin Endocrinol (Oxf). 2006;65:40-44. [PubMed] [Cited in This Article: ] |
| 30. | North KE, Graff M, Franceschini N, Reiner AP, Feitosa MF, Carr JJ, Gordon-Larsen P, Wojczynski MK, Borecki IB. Sex and race differences in the prevalence of fatty liver disease as measured by computed tomography liver attenuation in European American and African American participants of the NHLBI family heart study. Eur J Gastroenterol Hepatol. 2012;24:9-16. [PubMed] [Cited in This Article: ] |
| 31. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [PubMed] [Cited in This Article: ] |
| 32. | Zhang H, Liu Y, Wang L, Li Z, Zhang H, Wu J, Rahman N, Guo Y, Li D, Li N. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013;54:345-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1449] [Cited by in F6Publishing: 1419] [Article Influence: 94.6] [Reference Citation Analysis (0)] |