Published online Apr 7, 2016. doi: 10.3748/wjg.v22.i13.3621
Peer-review started: September 23, 2015
First decision: October 15, 2015
Revised: October 30, 2015
Accepted: December 30, 2015
Article in press: December 30, 2015
Published online: April 7, 2016
AIM: To establish a computed tomography (CT)-morphological classification for hepatic alveolar echinococcosis was the aim of the study.
METHODS: The CT morphology of hepatic lesions in 228 patients with confirmed alveolar echinococcosis (AE) drawn from the Echinococcus Databank of the University Hospital of Ulm was reviewed retrospectively. For this reason, CT datasets of combined positron emission tomography (PET)-CT examinations were evaluated. The diagnosis of AE was made in patients with unequivocal seropositivity; positive histological findings following diagnostic puncture or partial resection of the liver; and/or findings typical for AE at either ultrasonography, CT, magnetic resonance imaging or PET-CT. The CT-morphological findings were grouped into the new classification scheme.
RESULTS: Within the classification a lesion was dedicated to one out of five “primary morphologies” as well as to one out of six “patterns of calcification”. “primary morphology” and “pattern of calcification” are primarily focussed on separately from each other and combined, whereas the “primary morphology” V is not further characterized by a “pattern of calcification”. Based on the five primary morphologies, further descriptive sub-criteria were appended to types I-III. An analysis of the calcification pattern in relation to the primary morphology revealed the exclusive association of the central calcification with type IV primary morphology. Similarly, certain calcification patterns exhibited a clear predominance for other primary morphologies, which underscores the delimitation of the individual primary morphological types from each other. These relationships in terms of calcification patterns extend into the primary morphological sub-criteria, demonstrating the clear subordination of those criteria.
CONCLUSION: The proposed CT-morphological classification (EMUC-CT) is intended to facilitate the recognition and interpretation of lesions in hepatic alveolar echinococcosis. This could help to interpret different clinical courses better and shall assist in the context of scientific studies to improve the comparability of CT findings.
Core tip: Computed tomography (CT), mostly combined with positron emission tomography, provides one of the most important diagnostic tools in suspected alveolar echinococcosis. Aim of the study was to establish a new CT-classification based on a large patient collective with confirmed hepatic alveolar echinococcosis. The Echinococcosis Multilocularis Ulm Classification-CT presented in this paper is intended to facilitate the recognition and interpretation of hepatic lesions in alveolar echinococcosis based on CT-morphological criteria. It can also be used to more objectively interpret different clinical courses and enhance the comparability of CT findings in the context of scientific studies.
- Citation: Graeter T, Kratzer W, Oeztuerk S, Haenle MM, Mason RA, Hillenbrand A, Kull T, Barth TF, Kern P, Gruener B. Proposal of a computed tomography classification for hepatic alveolar echinococcosis. World J Gastroenterol 2016; 22(13): 3621-3631
- URL: https://www.wjgnet.com/1007-9327/full/v22/i13/3621.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i13.3621
Alveolar echinococcosis (AE) is caused by the larval (metacestode) stage of the cyclophyllid tapeworm, Echinococcus multilocularis. It is considered the most dangerous parasitic disease of Europe and has to be distinguished from the worldwide more common cystic echinococcosis (CE) that is caused by a related tapeworm species, E. granulosus[1]. The species’ very different patterns of growth in humans result in two distinct disease entities[2]. Thus, in about 98% of cases, AE presents as a malignant-appearing hepatic lesion with a tendency to infiltrative growth and the potential for metastasis; by comparison, CE most often presents as a smooth, clearly demarcated cyst and affects the liver in only about 60% of cases[2]. Humans are infected through the accidental ingestion of the helminth ova. Formation of protoscolices from the larval germinal epithelium occurs only rarely in humans, hence their designation as a dead-end or incidental host of E. multilocularis[1].
As a parasitic disease of humans, AE is rare; left untreated, however, it is associated with a high mortality. Southwestern Germany, together with other regions, mostly in neighboring countries, represents one of the largest endemic areas for this parasitosis in Europe[3,4]. Further endemic areas in North America and Asia lie exclusively in temperate climatic zones of the Northern Hemisphere[5,6]. As a result of this regional accumulation of cases, numerous patients with AE have been treated at the University Hospital of Ulm, which has for many years maintained a databank of AE cases.
In addition to serodiagnostics[1,7-10], diagnostic imaging plays a crucial role in the work-up of suspected AE. Ultrasonography (US) is often the initial imaging method[7,11]. Computed tomography (CT), usually in combination with 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET), represents one of the most important imaging modalities, both for initial diagnosis and for monitoring patients’ subsequent disease course[12-14]. Calcifications are especially well-visualized at CT while magnetic resonance imaging (MRI) most clearly shows the small vesicular structures that are typical for the disease[15].
Because AE mimics the biological behavior of malignant tumors, the World Health Organization (WHO) Informal Working Group in Echinococcosis developed a classification for staging this disease that is analogous to the TNM system for malignant diseases. The PNM classification (P = parasitic liver lesion; N = infiltration of neighboring organs; M = metastases, yes/no) uses the findings of diagnostic imaging and provides a standardized assessment of disease stage and stage-adapted therapy recommendations[16].
Whereas a classification of hepatic AE based on its morphological characteristics at MRI had been proposed as early as 2003[17], no systematic characterization of the CT morphology of this disease has been published. Thus, the objective of the present study was to propose a new CT classification for alveolar echinococcosis based on a large patient collective with confirmed hepatic AE. The CT morphological classification proposed in the present study seeks to facilitate the recognition and interpretation of lesions in hepatic AE and to aid in the frequently challenging differential diagnosis that includes neoplasms of the liver such as cholangiocellular carcinoma, biliary cystadenoma and cystadenocarcinoma, or metastases of other tumors[17,18]. The new classification should also assist in the interpretation of patients’ clinical course and improve the comparability of CT findings in the context of scientific studies.
As an acronym for the new CT classification, we propose EMUC-CT (Echinococcosis Multilocularis Ulm Classification - CT).
The CT morphology of hepatic lesions in 228 patients (n = 106 males; 122 females; mean age at diagnosis: 50.8 ± 17.1 years; mean age at time of PET-CT examination: 55.6 ± 17.3 years) with confirmed AE drawn from the Echinococcus Databank of the University Hospital of Ulm was reviewed retrospectively. The diagnosis of AE was made in patients with unequivocal seropositivity; positive histological findings following diagnostic puncture or partial resection of the liver; and/or findings typical for AE at either US, CT, MRI or PET-CT. According to the modified WHO criteria of Brunetti et al[1] 116 cases (50.9%) were confirmed by positive histopathology and proven specific enzyme linked immunosorbent assay (ELISA) from tissue samples. An additional 97 patients (42.5%) were considered probable cases with positive serology in two different methods and positive imaging for AE with two respective imaging techniques, while 15 patients (6.6%) were considered possible cases with a positive medical history and a positive result for imaging and serology in one test each.
Based on the reviewer’s many years’ experience together with reports in the literature of CT findings in patients with hepatic AE, individual CT-morphological findings were grouped according to a new classification scheme. For this reason, CT datasets of combined PET-CT examinations were evaluated: these were, in most cases, contrast-enhanced images acquired during the venous phase, though some were native images. All examinations were performed at the University Hospital of Ulm between March 2006 and December 2014 using the following CT scanners: Siemens, Biograph mCT-S(40) (CT: Somatom Definition AS 40; collimation 16 mm × 1.2 mm; - images viewed at 1.5 to 5.0 mm slice thickness with reconstruction intervals equal to or less to slice thickness). General Electrics, Discovery LS (CT: Lightspeed plus; - collimation 4 mm × 5.0 mm; reconstruction slice thickness is 5 mm and the slice interval is 4.25 mm). Prior imaging from other centers was not included. Subsequent assessment of patients’ clinical course (data not shown) was based on both the initial and final (as of the date of the study) CT examinations. The initial dataset, which was used for creation of the classification and the present analyses, was not necessarily the first of the entire examination series but the first to be electronically documented in the PACS system.
Review of CT scans revealed broad variability in the morphological appearance of lesions in patients with hepatic AE. Lesions were grouped into five primary morphologies. In cases with multiple hepatic lesions, the largest lesion was generally selected and described according to the classification system. In these cases, it was generally possible to recognize a single primary morphological pattern. Less frequently, lesions in the same liver exhibited different primary morphologies: these must be studied separately during the clinical course.
Assessment of a lesion’s calcification pattern separate from its primary morphology was found to be useful since the extent and morphology of calcifications may vary widely over the course of the disease or as a result of therapy and may, in some cases, contribute to a markedly changed appearance, both of the lesions and their primary morphology. Thus, in the context of the classification, lesions were assigned to both a “primary morphology” and a “calcification pattern”.
Based on the five primary morphologies, further descriptive sub-criteria were appended to types I-III. Thus, the presence of cystic components in types I and II and the presence of more solid portions at the edges in type IIIa and IIIb, lead to further characterization of the lesion.
The study design complies with the requirements of the Helsinki Declaration and was approved by the Ethics Commission of Ulm University.
Statistical analyses were performed using the SAS statistical software package (version 9.2; SAS Institute Inc., Cary, NC, United States). Data were analyzed descriptively with regard to absolute and relative frequencies, means and standard deviation.
The primary morphologies and their respective sub-criteria established for the present study were as follows: I. Diffuse infiltrating (with/without cystoid portion); II. Primarily circumscribed tumor-like (with/without cystoid portion); IIIa. Primarily cystoid - intermediate (with/without more solid portions at the edge) and IIIb. Primarily cystoid - widespread (with/without more solid portions at the edge); IV. Small-cystoid/metastatic*; and V. Mainly calcified (Tables 1 and 2).
| Primary morphology |
| I Diffuse infiltrating |
| With cystoid portion |
| Without cystoid portion |
| II Primarily circumscribed tumor-like |
| With cystoid portion |
| Without cystoid portion |
| III (a) Primarily cystoid - intermediate (approximately 3-8 cm) |
| With more solid portions at the edge |
| Without more solid portions at the edge |
| (b) Primarily cystoid - widespread (approximately > 8 cm) |
| With more solid portions at the edge |
| Without more solid portions at the edge |
| IV Small-cystoid/metastatic* (approximately < 3 cm) |
| V Mainly calcified |
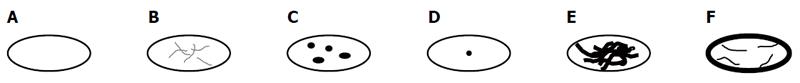
| Pattern of calcification |
| Without calcifications (Figure 1A) |
| With feathery calcifications (Figure 1B) |
| With focal calcifications (Figure 1C) |
| With a central calcification* (Figure 1D) |
| With diffuse calcifications (Figure 1E) |
| With calcifications primarily at the edge (Figure 1F) |
With the exception of primary morphology type V, the following six calcification patterns were assigned: without calcifications; with feathery calcifications; with focal calcifications; with a central calcification* (possible only with type IV *); with diffuse calcifications; with calcifications primarily at the edge (Tables 1 and 2; Figure 1).
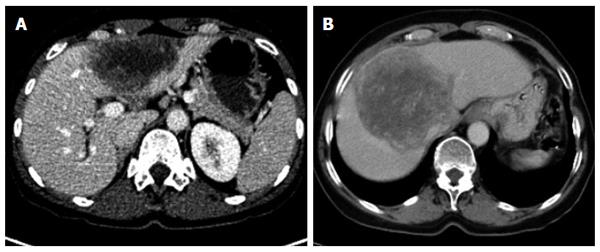
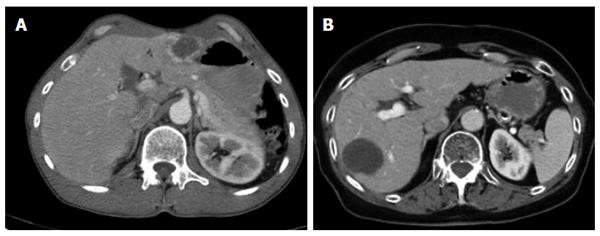
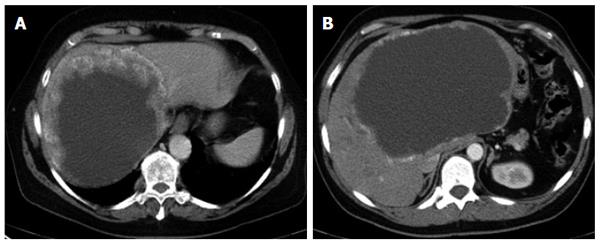
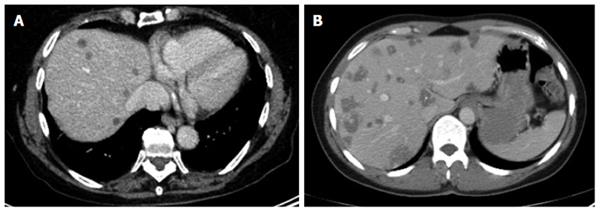
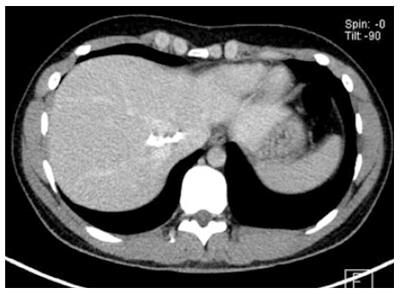
The different primary morphologies I-V (together with their respective sub-criteria) are demonstrated from corresponding CT images (Figures 2-7). For primary morphologies I-IV, preferably non- or rather less-calcified lesions were selected in order to illustrate the relevant morphological features unobscured by significant calcification overlay.
Notwithstanding the separate initial consideration of primary morphology and calcification pattern, certain associations between the two classification criteria were observed, as will be discussed below. For example, the specific calcification pattern of a central calcification was noted to be a characteristic occurrence with type IV small-cystoid/metastatic disease and can serve in these cases as a crucial diagnostic clue for assignment of unclear CT findings of this manifestation type. Based on this observation, this primary morphology was established as a separate type IV, distinct from the primary cystoid intermediate or widespread disease described by types IIIa and IIIb, respectively. By contrast, these latter types are often associated with a primarily marginal calcification at the edges. The diffusely infiltrating type I often exhibits initially feathery, less often focal, calcifications that tend to become more diffuse as the disease progresses. With primarily circumscribed, tumor-like type II lesions, there may often be either focal or diffuse calcification. However, in the present classification scheme, the various calcification patterns can, in principle, be associated with any of the primary morphologies (with the exception of type V, “mainly calcified”). Furthermore the special case of a central calcification can be observed, which is, when it occurs, exclusively linked to type IV disease (shown below, Figure 8). The primay morphologies type I-V are defined as follows:
The diffuse infiltrating type does not show any defined central focus of growth but instead is characterized primarily by a diffuse, at times fan-shaped, sometimes longitudinally extending spread of the lesion into surrounding tissue. Frequently, tiny vesicular structures may be recognized as the fundamental structure of the lesion. These small vesicular elements, which are well-known from MRI imaging and often better visualized with that imaging modality, represent a characteristic feature of the development of this disease. Distinct from these vesicular elements are the intrahepatic bile ducts which in many cases are observed peripheral to the lesion: these may be focally congested and appear to converge on the lesion. With larger type I lesions, or over the course of the disease or therapy, larger cystoid structures may develop, either centrally or decentralized in the lesion: these are distinct from the small vesicular elements described above. Unlike the small vesicular structures, these may represent necrotic areas of the lesion and may be significant as a parameter indicative of the course of the disease. In some cases, these large cystoid areas may also represent a conglomerate of multiple small vesicular elements.
Of the five primary morphological types, the primarily circumscribed tumor-like type presents with the broadest variety of morphological manifestations and its identification represents the greatest challenge. Not only is it difficult to distinguish from type I but also from type III, especially when the latter, over the course of the disease or therapy, changes the shape of its solid and cystoid components. Presumably, type II lesions showing an affinity with disease type I would more frequently exhibit the small vesicular elements that are better visualized at MRI.
While it could be argued that, based on this high morphological variability, type II represents a mixed type that comprises special cases of type I and type III disease, we nevertheless consider this primary morphological pattern to represent a distinct disease type: its primarily circumscribed appearance could, for example, provide information about the patient’s immunological response, especially in cases in which an affinity to type I is suspected. In other type II cases, as already noted above, conclusions may be drawn regarding therapy response or regarding the activity of the lesion or course of the disease in the association of an type III lesion. Corresponding to the morphological variability of type II, assignment of a given lesion to this group depends, to a greater degree than with the other groups, on the examiner’s subjective judgment. In general, the lesions subsumed under type II include those in which a primarily circumscribed, central and predominantly solid lesion could be defined. Some type II lesions may exhibit short offshoots into the surrounding hepatic tissue. In contrast to type II disease, the related type I is not characterized by a central, circumscribed tumor body and, in general, presents with a larger number of diffuse, often longitudinally extending offshoots (see above). As with type I, type II lesions may also contain larger cystoid portions: these may be located centrally or, again distinct from type III a/b “primary cystoid” disease, may be decentralized. Cystoid portions in type II lesions can in many cases be interpreted as necrotic regions but sometimes may as well represent a conglomerate of multiple small vesicular elements (see also above/description of type I lesions).
Primarily cystoid manifestations define the third group of primary morphologies. Lesions in this group may reach intermediate size or spread to such an extent that they occupy large areas of a hepatic lobe. An exact size boundary between the two subgroups (IIIa - intermediate; IIIb - widespread) was not drawn and, as will be explained below, is not useful. However, intermediate-sized lesions tend to fall within a range of 3-8 cm in diameter, while widespread lesions generally exhibit larger diameters. Although the present classification does not establish an exact cut-off, the two subgroups were established based on the observation that the intermediate cystoid lesions mostly occur as multiple, disseminated lesions, whereas widespread cystoid lesions often consist of one single lesion. An exact analysis of this phenomenon remains to be performed. There appears to be a correlation between uni-/multifocality and lesion size, which may depend on differences in the behavior of the parasite or, more likely, on differences in the host’s immune response. The phenomenon may also be a reflection of the number of larvae reaching the liver, which then, in turn, could exert an influence on the growth of the individual lesions. Further studies to elucidate this question are planned.
Primarily cystoid lesions in group III may manifest as purely cystoid in form or exhibit a more or less thick, solid marginal rim, while the cystoid portion remains relatively central. It is possible that, during the course of the disease, a centrifugal decrease of the more solid portions of the lesion might give the tumor a more and more cystoid appearance (this fact might represent an initial overlap with type II). It remains for future studies to assess whether the observation of such changes in those lesions can be confirmed and whether an decreasing solid component corresponds with progressing inactivity of the lesion or with patients’ response to therapy. In this case, as has already been mentioned above, the cystoid portion in type III lesions should be interpreted as a necrotizing area in the sense of a potentially increasing inactivation, as presumably occurs in type I and in some type II lesions.
For several reasons, the small-cystoid/metastatic* morphology of type IV was not designated as a subgroup of the cystoid types in group III. Although the observation made with type III lesions of an inverse relationship between the size and number of lesions would appear to apply to type IV and extend to a typical pattern of often very disseminated disease involvement, type IV lesions exhibit a number of characteristics that are not observed in type III disease. For example, a very specific calcification pattern, characterized by a tiny, centrally located calcification, may occur in type IV lesions. As with other disease types, these calcifications may become more pronounced as the disease progresses. In addition, type IV lesions do not exhibit the more or less extensive solid portions at their margins that are frequently encountered in type III lesions, which typically calcify at their edges. In light of these significant morphological differences, we deferred a strict delineation of lesion diameters between type IV and type III disease. Most type IV lesions are less than 3 cm in diameter. In the absence, at least initially, of a central calcification* in type IV disease, the correlation of liver pathology to the diagnosis of AE may be very difficult, as the lesions tend to resemble protein-rich cysts or hypodense metastases. Knowledge of this manifestation form should prompt the clinician to consider a diagnosis of AE in patients with corresponding clinical suspicion.
Subsumed under primary morphology type V - “mainly calcified” are those lesions consisting predominantly of a calcified component. Although smaller in extent, parenchymal/solid or cystoid portions are occasionally observed: they are usually marginal and quite insignificant within the overall extent of the lesion. Type V is the sole primary morphological group in which the calcification is an obligatory part of the main description and thus the only group among the primary morphologies with no further separate calcification pattern associated with the lesion description. Type V lesions are, for the most part, rather small. They often show a longitudinal or oval configuration, and may be solitary or multiple in number. Whether these represent older, inactive foci that have evolved from other primary morphologies, or belong to a distinct, fully or partially active disease form characterized by rapid and pronounced ossification, remains to be determined in further studies. The former hypothesis is supported by the relatively small size of the lesions, their frequently longitudinal configuration, and the fact that there is little or no dissemination; all of these being important criteria for differentiating these lesions from other disease types. It is possible that a specific immune response by the host is responsible for this rapid, pronounced calcification.
In addition, predominantly calcified, longitudinally configured lesions observed in the context of recurrent disease at resection margins, representing either active lesions or post-operative cicatricial changes, are in part subsumed under type V. Other lesions, especially belonging to type I, may also occur at resection margins and may also be calcified. Under these considerations, “predominantly calcified” lesions at resection margins likely differ in terms of their behavior from “original” type V lesions that occur centrally in the hepatic parenchyma in patients without prior resection.
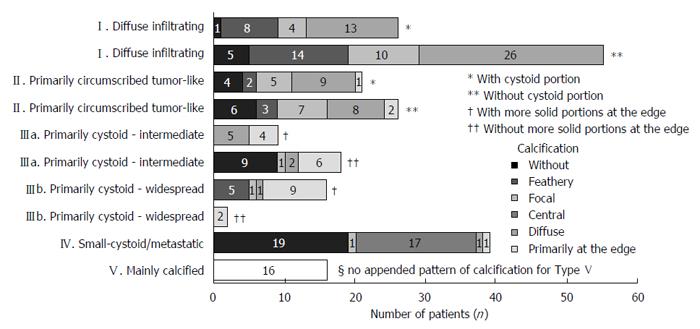
The cumulative occurrence of the different primary morphologies and their appended sub-criteria, respectively, as well as the occurrence of the different calcification patterns in association with the different primary morphologies, are shown in Figure 8.
The most frequently encountered CT-morphological pattern among the 228 patients was the diffuse infiltrating pattern type I: 35.5% (n = 81), followed, in 20.6% (n = 47), by the primarily circumscribed tumor-like appearance (type II), in 19.7% (n = 45) by the primarily cystoid type III (type IIIa - intermediate, n = 27; type IIIb - widespread, n = 18), and in 17.1% (n = 39) by the small-cystoid/metastatic* morphology (type IV). Much less frequently observed was the mainly calcified appearance of type V: 7% (n = 16), (Figure 8).
An analysis of the calcification pattern in relation to the primary morphology revealed the exclusive association of the central calcification with type IV primary morphology (Figure 8). Similarly, certain calcification patterns exhibited a clear predominance for other primary morphologies, which again underscores the delimitation of the individual primary morphological types from each other. These relationships in terms of calcification patterns extend into the primary morphological sub-criteria, demonstrating the clear subordination of the sub-criteria to their respective main primary morphological types.
AE presents with a wide variety of CT-morphological manifestations. The present paper, based on a large patient collective, proposes a new classification of hepatic AE lesions, which assigns them to five groups of primary morphologies (types I-V) and correlates these with a primary separate assignment of calcification patterns.
The CT morphological classification proposed in the present study seeks to facilitate the recognition and interpretation of lesions in hepatic AE and to aid in the frequently challenging differential diagnosis that includes neoplasms of the liver[17,18] such as cholangiocellular carcinoma (similar to type I), biliary cystadenoma and cystadenocarcinoma (resemblance to type III), or metastases of other tumors (similar to type II or IV).
The most frequently encountered CT-morphological pattern among the 228 patients was the diffuse infiltrating pattern (type I), followed by the primarily circumscribed tumor-like appearance (type II), the primarily cystoid type (type III) and by the small-cystoid/metastatic* morphology (type IV). Much less frequently observed was the mainly calcified appearance (type V).
As a rule, the presence of multiple AE lesions within a single liver is associated with only one primary morphological pattern. In rare cases, multiple lesions in a given liver may be characterized by more than one primary morphology; these must be assessed separately over the course of the disease.
Based on the five primary morphologies, further descriptive sub-criteria were appended to types I-III. Thus, the presence of cystic components in types I and II and the presence of more solid portions at the edges in type IIIa and IIIb, lead to further characterization of the lesion.
Calcification patterns were first assessed independently of the primary morphology. This was shown to be useful as the extent and morphology of calcifications may change over the course of the disease or in response to therapy and thus give the respective primary morphology a quite different appearance.
An analysis of the calcification pattern in relation to the primary morphology revealed the exclusive association of the central calcification with type IV primary morphology. Similarly, certain calcification patterns exhibited a clear predominance for other primary morphologies, which again underscores the delimitation of the individual primary morphological types from each other. These relationships in terms of calcification patterns extend into the primary morphological sub-criteria, demonstrating the clear subordination of the sub-criteria to their respective main primary morphological types.
In the present classification scheme, the different calcification patterns can, in principle, be associated with any of the primary morphologies (with the exception of type V, “mainly calcified”). Furthermore the special case of a central calcification can be observed, which is, when it occurs, exclusively linked to type IV disease.
Potential morphological changes occurring during the course of the disease or as a result of therapy, especially as they relate to sub-criteria and calcification patterns, will be addressed in targeted studies.
In summary, the EMUC-CT classification allows for a very comprehensive description of hepatic AE lesions based on their CT-morphology. Assignment to one of five primary morphologies modified by an exact characterization of variable sub-criteria (insofar as sub-criteria are available for a given primary morphology) and in correlation to a respective calcification pattern should also assist in the interpretation of patients’ clinical course and improve the comparability of CT findings in the context of scientific studies.
In order to describe the lesion completely according to the present classification, the criteria should be followed in order. Following is an example for the descriptions of individual types: I. Diffuse infiltrating, without cystoid portion, with feathery calcifications; II. Primarily circumscribed tumor-like, with cystoid portion, with focal calcifications; IIIa. Primarily cystoid - intermediate - with more solid portions at the edge, with calcifications primarily at the edge; IV. Small-cystoid/metastatic*, with a central calcification*; V. Mainly calcified.
The knowledge of the primary morphologies of hepatic AE lesions according to these criteria may be of great assistance in the primary diagnostic work-up of this rare and morphologically variable disease entity. If changes in individual criteria are observed at follow-up monitoring, this can be clearly and reproducibly noted in the report of findings. Changes are more likely to relate to subcriteria of the primary morphologies and to calcification patterns; the primary morphological type often remains.
An exhaustive description of the findings of diagnostic imaging will naturally also enumerate the size and number of lesions, as well as their exact location within the liver (in addition to describing any extrahepatic manifestations). The precise characterization of the liver lesion based on the present classification will contribute to a much more comprehensive description of the disease manifestation. Unlike malignant diseases, the size of hepatic AE lesions often does not change significantly as a response to therapy; hence changings in size by themselves do not allow any unequivocal conclusions regarding the course of the disease. Changes in lesion morphology, however, could provide a number of additional criteria for assessing patients’ clinical course. 18F-FDG-PET, which is often used as an adjunct to CT, can yield some important data, though their value for assessing actual disease activity remains controversial[12-14,19,20]. Use of the morphological EMUC-CT classification could provide additional aspects regarding therapy planning as part of staging according to the PNM classification[16].
Unlike the WHO ultrasonographic classification of cystic echinococcosis (CE WHO-IWGE standardized classification)[1], the present CT classification of AE does not focus primarily on different stages of the disease: instead, it defines AE’s very different morphological manifestations in the liver independent of the disease course. In the case of CE, a classification according to disease stages is supported by the fact that the form of individual lesions does not differ fundamentally between different individual hosts and, in comparison with one another, remains relatively constant within their stages (CE1-5). With AE, however, it would appear, as noted above, that there may be overlap between different morphological types (type I/II) and probably also transition in the disease course and response to therapy (type II/III), as well as alterations, for example, in the sense of a terminal phase (type V) or recurrent lesions (type V; type I). It remains for further studies to determine whether and, if so, which individual primary morphology types are in fact to be interpreted as sequentially different disease stages or whether the alterations in lesions are due primarily to the modifiable criteria related to clinical course (subcriteria and calcification patterns).
A primary objective of the present classification is to facilitate the diagnosis of this rare disease entity in routine clinical practice. In addition, many provocative questions are raised as a by-product of this systematization:
Are the different morphologies the product of variable characteristics of the parasite or dependent on differences in host immunology? Does the parasite load at the time of initial infection play a role in the development of lesion morphology? How do the lesions develop over time, e.g., with regard to their calcification pattern? In this regard, are transitions between different morphologies truly observable over the course of the disease and, if so, which? How are cystoid vs. solid components of different lesion types to be interpreted in the clinical course? Do the larger cystoid areas in type I and possibly also in type II lesions always represent necrotic zones or sometimes conglomerates of multiple small vesicular elements? Is there a correlation between different morphologies and (primary) behavior at 18F-FDG-PET, or can conclusions be drawn regarding their activity based on PET imaging in cases of morphological changes in the lesions? Does the classification provide initial prognostic hints regarding patients’ future disease course? Is there in this regard a correlation between different morphology types and the presence of extrahepatic disease or a tendency to intrahepatic infiltration of biliary or vascular structures (MRI is superior in answering this question)[17,21]? Can the classification, if, for example, correlations with specific immunohistochemical markers can be identified[22], serve as a building block for management decisions regarding surgical options with respect to resectability or for defining safe distances and resection margins for hepatic lesions?
While the small vascular elements within some lesions that are typical for AE are better visualized with MRI, CT offers a more nuanced view of other morphological properties and especially the calcifications[15,17]. Sound wave attenuation secondary to the calcifications can, in turn, hinder ultrasonographic diagnostics. If corresponding parallel imaging data is available, a further goal could be a correlation of CT morphology as defined by the present classification with MRI findings or comparison with corresponding ultrasonographic lesion descriptions.
AE’s rarity makes it difficult to assess inter-rater reliability. The lack of inter-rater reliability remains a limitation of the proposed classification. It was the objective of the present study to establish the crucial CT-morphological criteria for hepatic AE lesions and, for the first time, to systematically describe them.
In conclusions, the EMUC-CT classification presented in this paper is intended to facilitate the recognition and interpretation of hepatic lesions in AE based on CT-morphological criteria. It can also be used to more objectively interpret different clinical courses and enhance the comparability of CT findings in the context of scientific studies.
Members of the Echinococcus Multilocularis Study Group in alphabetical order: Sarina Ansari-Bitzenberger, Max G Bachem, Thomas F Barth, Ambros J Beer, Meinrad Beer, Bernhard O Boehm, Franziska Ehing, Martina Furitsch, Martin Gottstein, Tilmann Graeter, Beate Gruener, Mark M Haenle, Doris Henne-Bruns, Andreas Hillenbrand, Tanja EM Kaltenbach, Peter Kern, Wolfgang Kratzer, Max Kurlbaum, Suemeyra Oeztuerk, Thomas Seufferlein.
Human alveolar echinococcosis (AE) is the most lethal human helminthic infection and is one of the 17 neglected tropical diseases prioritized by the World Health Organization. Its incidence is low in endemic regions of Central and Western Europe (0.03-0.05/100000) and high in central Asia. Current studies suggest that the occurrence of alveolar echinococcosis is increasing worldwide and is spreading to previously unaffected regions. Morbidity and treatment costs of the disease are high.
Despite the importance of computed tomography (CT), mostly combined with positron emission tomography, as an image modality in the work-up of hepatic AE, there is no CT-morphological classification of hepatic AE lesions.
Objective of the present study was to establish a CT-classification based on a large sample of patients with confirmed hepatic AE as a way of facilitating the diagnosis of the disease entity.
The CT-morphological classification proposed in the present study shall facilitate the diagnosis, interpretation, classification and comparison of CT-morphological findings in patients with alveolar echinococcosis of the liver, both in routine clinical practice and in the context of scientific studies.
The manuscript aims to provide a new CT-classification based on a large patient collective with confirmed hepatic AE. This draft provides a possibility for diagnostic tool of rare but sometimes fatal hepatic AE, and is valuable for sharing data in concern of determination of morphological status of hepatic AE, as well.
P- Reviewer: Lee YS S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Brunetti E, Kern P, Vuitton DA, Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1108] [Cited by in F6Publishing: 1152] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 2. | Stojkovic M, Junghanss T. Cystic and alveolar echinococcosis. Handb Clin Neurol. 2013;114:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. 2009;13:125-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 594] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 4. | Romig T, Dinkel A, Mackenstedt U. The present situation of echinococcosis in Europe. Parasitol Int. 2006;55 Suppl:S187-S191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 6. | Craig PS, Echinococcosis Working Group in China. Epidemiology of human alveolar echinococcosis in China. Parasitol Int. 2006;55 Suppl:S221-S225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Kern P, Kratzer W, Reuter S. Alveolar echinococcosis: diagnosis. Dtsch Med Wochenschr. 2000;125:59-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Frosch M. Labordiagnose der zystischen und alveolären Echinokokkose. J Lab Med. 2003;27:389-392. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Ito A, Craig PS. Immunodiagnostic and molecular approaches for the detection of taeniid cestode infections. Trends Parasitol. 2003;19:377-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Kern P. Clinical features and treatment of alveolar echinococcosis. Curr Opin Infect Dis. 2010;23:505-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Kratzer W, Reuter S, Hirschbuehl K, Ehrhardt AR, Mason RA, Haenle MM, Kern P, Gabelmann A. Comparison of contrast-enhanced power Doppler ultrasound (Levovist) and computed tomography in alveolar echinococcosis. Abdom Imaging. 2005;30:286-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Reuter S, Schirrmeister H, Kratzer W, Dreweck C, Reske SN, Kern P. Pericystic metabolic activity in alveolar echinococcosis: assessment and follow-up by positron emission tomography. Clin Infect Dis. 1999;29:1157-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Reuter S, Buck A, Manfras B, Kratzer W, Seitz HM, Darge K, Reske SN, Kern P. Structured treatment interruption in patients with alveolar echinococcosis. Hepatology. 2004;39:509-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Reuter S, Grüner B, Buck AK, Blumstein N, Kern P, Reske SN. Long-term follow-up of metabolic activity in human alveolar echinococcosis using FDG-PET. Nuklearmedizin. 2008;47:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Reuter S, Nüssle K, Kolokythas O, Haug U, Rieber A, Kern P, Kratzer W. Alveolar liver echinococcosis: a comparative study of three imaging techniques. Infection. 2001;29:119-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, Delabrousse E, Kratzer W, Bresson-Hadni S. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int. 2006;55 Suppl:S283-S287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 205] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 17. | Kodama Y, Fujita N, Shimizu T, Endo H, Nambu T, Sato N, Todo S, Miyasaka K. Alveolar echinococcosis: MR findings in the liver. Radiology. 2003;228:172-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Karçaaltincaba M, Sirlin CB. CT and MRI of diffuse lobar involvement pattern in liver pathology. Diagn Interv Radiol. 2011;17:334-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Crouzet J, Grenouillet F, Delabrousse E, Blagosklonov O, Thevenot T, Di Martino V, Piarroux R, Mantion GA, Bresson-Hadni S. Personalized management of patients with inoperable alveolar echinococcosis undergoing treatment with albendazole: usefulness of positron-emission-tomography combined with serological and computed tomography follow-up. Clin Microbiol Infect. 2010;16:788-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Stumpe KD, Renner-Schneiter EC, Kuenzle AK, Grimm F, Kadry Z, Clavien PA, Deplazes P, von Schulthess GK, Muellhaupt B, Ammann RW. F-18-fluorodeoxyglucose (FDG) positron-emission tomography of Echinococcus multilocularis liver lesions: prospective evaluation of its value for diagnosis and follow-up during benzimidazole therapy. Infection. 2007;35:11-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Bresson-Hadni S, Delabrousse E, Blagosklonov O, Bartholomot B, Koch S, Miguet JP, Mantion GA, Vuitton DA. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol Int. 2006;55 Suppl:S267-S272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Barth TF, Herrmann TS, Tappe D, Stark L, Grüner B, Buttenschoen K, Hillenbrand A, Juchems M, Henne-Bruns D, Kern P. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl Trop Dis. 2012;6:e1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |