Published online Apr 14, 2016. doi: 10.3748/wjg.v22.i14.3829
Peer-review started: November 6, 2015
First decision: December 11, 2015
Revised: January 6, 2016
Accepted: January 17, 2016
Article in press: January 18, 2016
Published online: April 14, 2016
AIM: To investigate member 3a of Wingless-type MMTV integration site family (Wnt3a) expression in cancerous and surrounding tissues and the relationship between clinicopathologic features of hepatocellular carcinoma (HCC) and Wnt3a expression.
METHODS: Wnt3a expression and cellular distribution and clinicopathologic characteristics in cancerous tissue and matched surrounding tissues were analyzed in 80 HCC patients from January 2006 to August 2008 by tissue microarrays and immunohistochemistry. The overall and disease-free survival rates were estimated using the Kaplan-Meier method and compared with the log-rank test. The prognostic analysis was carried out with univariate and multivariate Cox regressions models.
RESULTS: The incidence of oncogenic Wnt3a expression in the cancerous group was up to 96.25% (77 of 80), which was significantly higher (χ2 = 48.818, P < 0.001) than that in the surrounding group (46.25%, 37 of 80). Brown Wnt3a staining gradually increased with clinical staging that showed very strong staining in advanced HCC. The clinicopathologic features of high Wnt3a expression in HCC were related to poorly-differentiated grade (χ2 = 20.211, P < 0.001), liver cirrhosis (χ2 = 8.467, P < 0.004), hepatitis B virus (HBV) infection (χ2 = 12.957, P < 0.001), higher tumor-node-metastasis stage (χ2 = 22.960, P < 0.001), and 5-year survival rate (χ2 = 15.469, P < 0.001).
CONCLUSION: Oncogenic Wnt3a expression associated with HBV infection and cirrhotic liver might be an independent prognostic factor for HCC.
Core tip: Protein expression of member 3a of Wingless-type MMTV integration site family (Wnt3a) was shown to be increased progressively in hepatocarcinogenesis. Oncogenic Wnt3a expression associated with HBV infection and cirrhotic liver might be an independent prognostic factor for hepatocellular carcinoma (HCC). Survival analysis showed that HCC patients with high Wnt3a expression had a significantly shorter survival time compared with those low or no Wnt3a expression.
- Citation: Pan LH, Yao M, Cai Y, Gu JJ, Yang XL, Wang L, Yao DF. Oncogenic Wnt3a expression as an estimable prognostic marker for hepatocellular carcinoma. World J Gastroenterol 2016; 22(14): 3829-3836
- URL: https://www.wjgnet.com/1007-9327/full/v22/i14/3829.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i14.3829
Hepatocellular carcinoma (HCC) is one of the most common and rapidly fatal malignancies worldwide[1,2]. It develops mostly in cirrhotic livers, and risk factors include chronic persistent infection by the hepatitis B viruses (HBV) and C viruses (HCV) as well as non-viral liver diseases[3,4]. HCC is a highly chemo-resistant cancer with no effective systemic or well-established adjuvant therapies, and prognosis remains poor because of its high recurrence[5,6]. Hepatic resection or transplantation is the only potential curative treatment for HCC patients[7,8]. Unfortunately, the molecular mechanisms of hepatocarcinogenesis remain poorly understood. Recently, abnormal expression of some key molecules in the Wingless (Wnt)/β-Catenin pathway was associated with the development and progression of HCC[9,10]. The Wnt proteins are secreted glycoproteins that regulate multiple cellular events, such as cell proliferation, differentiation, and apoptosis through β-catenin- dependent canonical and β-catenin-independent non-canonical pathways, and play an important role in HCC prognosis[11,12].
The member 3a of Wnt-type MMTV integration site family (Wnt3a) is a canonical Wnt ligand[13,14]. Its gene is located on human 17 chromosome (17q21) and has been regarded as an activator of the canonical Wnt signaling pathway, inducing β-catenin accumulation and activating the canonical Wnt signaling pathway[13]. Studies on human Wnt3a have focused primarily on its key role in human malignancy, and its high expression in many kinds of tumors was correlated with a worse outcome[15-17]. However, limited data are available on the relationship between Wnt3a and HCC, and its prognostic value remains to be explored. The objective of the current study, therefore, was to investigate alterations in the expression of oncogenic Wnt3a in cancerous tissues and to explore its clinical utility as a novel molecular marker for HCC prognosis.
Human liver cancer and surrounding tissues in this study were obtained from 80 HCC patients who underwent operations between January 2006 and August 2008 at the Affiliated Hospital of Nantong University, Jiangsu Province, China. The tissues were immediately frozen in liquid nitrogen and kept at -80 °C until required. The patient cohort inclusion and exclusion criteria included: (1) accurate pathologic diagnosis of primary HCC; (2) complete clinicopathologic and follow-up data; and (3) previously untreated, with surgery as the first treatment. Thus, analysis of the data would reflect the actual impact of tumor biology on the clinical outcome. This study was approved by the Ethics Committee permission (No. TDFY2013008) of the Affiliated Hospital of Nantong University, and all patients signed informed consent. The related important clinical information of each patient was collected from their medical records.
The patient characteristics are listed in Table 1. The patient population consisted of 62 males and 18 females, with a median age of 51 years (range: 35-74). The sizes of tumors were 44 cases with > 5 cm and 36 with < 5 cm. α-fetoprotein (AFP) levels were 28 cases with ≥ 400 ng/mL and 52 with < 400 ng/mL. The tumor number was 64 cases with single and 16 with multiple. Tumor differentiation was graded using the Edmondson grading system[18] with 5 well-, 41 middle-, and 34 poor- differentiation. Tumor staging was 36 cases (45%, 36 of 80) at I and II and 44 cases (55%, 44 of 80) at III and IV based on the 6th edition of the tumor-node-metastasis (TNM) classification of the International Union against Cancer (IUAC). The incidence of hepatitis B surface antigen (HBsAg) was 81.25% (65 of 80) in these HCC tissues, and 63.75% (51 of 80) had a history of liver cirrhosis. All patients had regular follow-up. The follow-up period was defined as the duration from the date of the operation to the date of either death or the last follow-up. The last follow-up was October 2013. Overall survival (OS) was evaluated by the duration between the dates of surgery and death. During the follow-up period, a total of 48 patients died.
| Parameter | n |
| Age (yr) | |
| median (range) | 51 (35-74) |
| Gender | |
| Male | 62 |
| Female | 18 |
| Differentiation | |
| Poor | 34 |
| Moderate | 41 |
| Well | 5 |
| Tumor number | |
| Single | 64 |
| Multiple | 16 |
| Liver cirrhosis | |
| Absent | 29 |
| Present | 51 |
| HBV infection | |
| Absent | 15 |
| Present | 65 |
| Periportal embolus | |
| Absent | 9 |
| Present | 71 |
| AFP (ng/mL) | |
| < 400 | 52 |
| ≥ 400 | 28 |
| TNM staging | |
| I-II | 36 |
| III-IV | 44 |
| Five-year survival | |
| Yes | 32 |
| No | 48 |
Histopathologic examination of the livers with hematoxylin and eosin (HE) staining showed that all cancerous tissues were accurate pathologic diagnosis of HCC according to the World Health Organization criteria. HCC diagnosis was confirmed according to the criteria set by the Chinese National Collaborative Cancer Research Group[18].
All HCC tissues were stained by HE staining and reviewed by two histopathologists. Representative areas free from necrotic and hemorrhagic materials were marked in paraffin blocks. Tissue cores (2.0 mm) were taken from each representative tumor tissue and peritumoral tissue to construct the tissue microarray (TMA) slides.
The TMA slides used for immunohistochemistry (IHC) were deparaffinized, and peroxidase was quenched with methanol and 3% H2O2 for 15 min. For antigen retrieval, the sections were boiled under pressure in citrate buffer (pH 6.0) for 3 min. After that, the tissues were incubated for 120 min with primary mouse anti-Wnt3a (ab81614; Abcam, United Kingdom) at 1:50 dilution. Following washing with phosphate buffered saline (PBS), the sections were incubated with horseradish peroxidase conjugated goat anti-mouse IgG (A21010; Abcam, Cambridge, United Kingdom) for 15 min at 1:5000 dilution and washed again with PBS. The sections were incubated in diaminobenzidine solution, counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene, and cover-slipped. For the negative control reactions, PBS was used instead of the primary antibody.
The results of IHC staining were assessed by two independent pathologists without knowledge of the clinicopathologic features. The percentages for Wnt3a-positive cells were scored as follows: 0 (0%), 1 (1%-33%), 2 (34%-66%), and 3 (67%-100%). Staining intensity was stratified into four categories: 0 (negative), 1 (weakly), 2 (moderate), and 3 (strongly). The sum of the percentage and intensity score was defined as the IHC staining score. According to above criterion, the HCC tissues with Wnt3a expression were divided into two groups: low with 0-2 scores and high 3-6 scores. The higher score for positive percentages and staining intensity was taken as the final score when there was a difference between duplicate tissue scores.
Statistical analysis was carried out by using SPSS software (version 20.0, Armonk, NY, United States). The difference between HCC and para-cancerous groups was analyzed by χ2 test. Quantitative variables were analyzed by Mann-Whitney U test (Z). The relationship between Wnt3a expression and clinicopathological factors was analyzed by χ2 test. Survival curves were calculated using the Kaplan-Meier method and compared by the log-rank test. Factors shown to be of prognostic significance in the univariate models were evaluated in a multivariable Cox regression model. For all tests, the significance level for statistical analysis was set at P < 0.05.
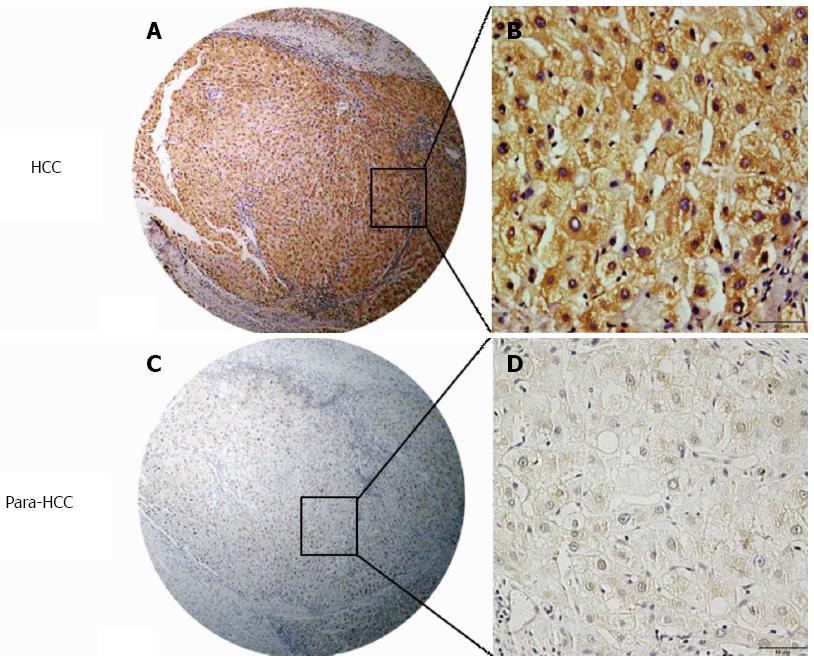
The over-expression of hepatic Wnt3a and its cellular distribution in cancerous or surrounding tissues analyzed by immunohistochemistry with human anti-Wnt3a antibodies are shown in Figure 1. Positive Wnt3a staining, identified as brown particles, was distributed in the cytosol and the membrane of hepatocytes or only a few cell nuclei. The incidence of hepatic Wnt3a expression in cancerous tissues (96.25%, 77 of 80) was significantly higher (χ2 = 48.818, P < 0.001) than that in surrounding tissues (46.25%, 37 of 80). Liver Wnt3a staining and its expression frequency in cancerous or surrounding tissues are shown in Table 2. The liver Wnt3a staining scores were significantly different (Z = 8.144, P < 0.001) between cancerous tissues and surrounding tissues, with strong expression (3-6 scores) in 71.25% (57 of 80) of cancerous tissues and low or no expression (0-2 scores) in 86.25% (69 of 80) of surrounding tissues.
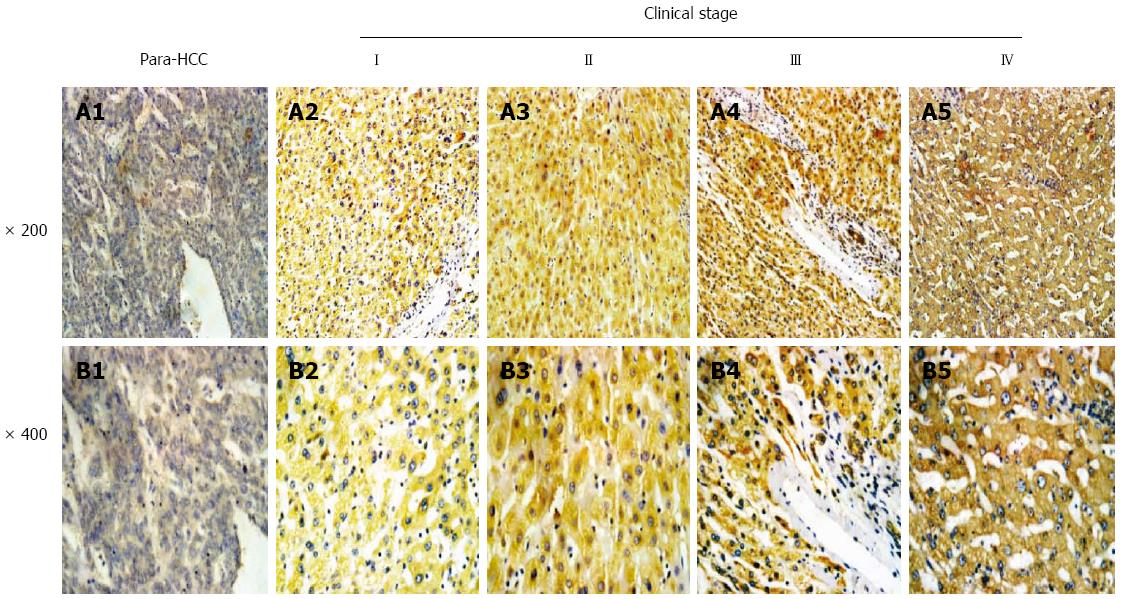
The relationship between hepatic Wnt3a expression and clinical staging of HCC is shown in Figure 2. According to the IUAC clinical staging criteria of HCC patients, there were nine cases at staging I (11.25%, 9 of 80), 27 at II (33.75%, 27 of 80), 38 at III (47.5%, 38 of 80), and six at IV (7.5%, 6 of 80) among 80 cancerous tissues. The incidences of high or low Wnt3a expression in HCC tissues were 22.2% (two of nine) or 77.8% (seven of nine) at I staging, 51.9% (14 of 27) or 48.1% (13 of 27) at II staging, 94.7% (36 of 38) or 5.3% (two of 38) at III staging, and 83.3% (five of six) or 16.7% (one of six) at IV staging, respectively. Brown Wnt3a expression was gradually increased in different clinical stages, with very strong staining at the advanced stage.
The correlation between Wnt3a levels and the clinicopathological parameters of 80 HCC patients is shown in Table 3. High Wnt3a expression was associated with poorly-differentiated grade (P < 0.001), liver cirrhosis (P = 0.004), HBV infection (P < 0.001), and higher TNM stage (P < 0.001). By contrast, there were no significant associations between hepatic Wnt3a expression and other clinicopathological features, such as HCC patients’ gender or age, tumor number, circulating AFP level, and periportal cancer embolus.
| Groups | Patients, n | % of total | Wnt3a high expression, n | % of total | χ2 | P value |
| Differentiation | 20.211 | < 0.001 | ||||
| Poor | 34 | 42.50 | 31 | 91.18 | ||
| Moderate | 41 | 51.25 | 26 | 63.41 | ||
| Well | 5 | 6.25 | 0 | 0.00 | ||
| Tumor number | 0.976 | 0.323 | ||||
| Single | 64 | 80.00 | 44 | 68.75 | ||
| Multiple | 16 | 20.00 | 13 | 81.25 | ||
| Liver cirrhosis | 8.467 | 0.004 | ||||
| Absent | 29 | 36.25 | 15 | 51.72 | ||
| Present | 51 | 63.75 | 42 | 82.35 | ||
| HBV infection | 12.957 | < 0.001 | ||||
| Absent | 15 | 18.75 | 5 | 33.33 | ||
| Present | 65 | 81.25 | 52 | 80.00 | ||
| Periportal embolus | 3.557 | 0.059 | ||||
| Absent | 9 | 11.25 | 4 | 44.44 | ||
| Present | 71 | 88.75 | 53 | 74.65 | ||
| AFP (ng/mL) | 1.020 | 0.313 | ||||
| < 400 | 52 | 65.00 | 39 | 75.00 | ||
| ≥ 400 | 28 | 35.00 | 18 | 64.28 | ||
| TNM staging | 22.960 | < 0.001 | ||||
| I-II | 36 | 45.00 | 16 | 44.44 | ||
| III-IV | 44 | 55.00 | 41 | 93.18 | ||
| Five-year survival | 15.469 | < 0.001 | ||||
| Yes | 32 | 40.00 | 15 | 46.88 | ||
| No | 48 | 60.00 | 42 | 87.50 |
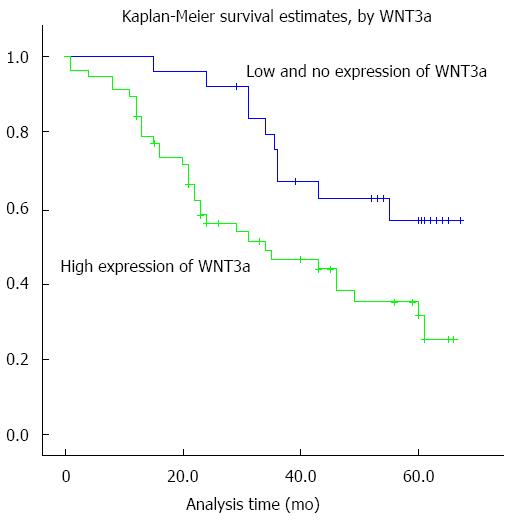
As expected, the overexpression of hepatic Wnt3a showed a significant relationship with 5-year survival of the 80 HCC patients (P < 0.001). Meanwhile, certain HCC clinical prognostic factors, such as liver cirrhosis (P = 0.035) and HBV infection (P = 0.029), also showed a statistically significant correlation with 5-year survival rate based on Cox regression univariate analysis (Table 4). All these factors were enrolled in a multivariable analysis. High Wnt3a expression (P < 0.001), liver cirrhosis (P = 0.033), and HBV infection (P = 0.010) were all identified as independent predictive factors for poor HCC outcome. The Kaplan-Meier survival curves (Figure 3) demonstrated that HCC patients with high Wnt3a expression had a significantly shorter survival time compared to those with low or no Wnt3a expression.
| Variable | Univariate analysis | Multivariable analysis | ||||
| HR | P >|z| | 95%CI | HR | P > |z| | 95%CI | |
| Age (yr) | ||||||
| ≤ 60 vs > 60 | 0.828 | 0.662 | 0.355-1.930 | |||
| Gender | ||||||
| Male vs female | 1.005 | 0.990 | 0.440-2.296 | |||
| Differentiation | ||||||
| Low vs high | 1.037 | 0.914 | 0.539-1.993 | |||
| Tumor number | ||||||
| Single vs multiple | 0.867 | 0.720 | 0.397-1.893 | |||
| Liver cirrhosis | ||||||
| Absent vs present | 2.101 | 0.035 | 1.053-4.195 | 2.021 | 0.033 | 1.057-3.864 |
| HBV infection | ||||||
| Absent vs present | 0.327 | 0.029 | 0.120-0.892 | 0.325 | 0.010 | 0.138-0.765 |
| Periportal embolus | ||||||
| Absent vs present | 0.499 | 0.360 | 0.113-2.210 | |||
| AFP (ng/mL) | ||||||
| < 400 vs≥ 400 | 0.938 | 0.725 | 0.655-1.342 | |||
| TNM staging | ||||||
| I-II vs III-IV | 0.686 | 0.318 | 0.328-1.436 | |||
| Wnt3a expression | ||||||
| Low vs high | 5.656 | < 0.001 | 2.682-11.926 | 3.651 | < 0.001 | 1.973-6.757 |
HCC is one of the most frequently occurring tumors worldwide [19]. Accumulating data have indicated that the Wnt/β-catenin pathway is associated with tumor progression by interacting with microenvironment and oncogenesis[20,21], and high expression of Wnt3a in different kinds of tumors was related to worse outcome[12]. Wnt3a is an important regulator of HCC cell growth in vitro, and induction of Wnt3a activates the canonical Wnt pathway after binding with sulfatase 2 and glypican-3 and increases cell proliferation in nude mouse xenografts in vivo[22,23]. However, limited data was available until now on the relationship between Wnt3a expression and HCC prognosis. In this present study, therefore, the alterations of oncogenic Wnt3a expression in cancerous tissues were investigated, and it was confirmed that Wnt3a may be a useful biomarker for HCC prognosis
Recent advances have shown that, apart from autocrine stimulation by growth factors and the dysregulation of different growth regulatory pathways, the Wnt/β-catenin pathway is frequently involved in hepatocarcinogenesis[24,25]. Growing evidence has highlighted the key molecules of the Wnt/β-catenin pathway in the control of HCC suppressors, growth, invasiveness, and curability[26-28]. Wnt3a expression in a large array of HCC tissues demonstrated that HCC tissues exhibited unique immunostaining patterns and that the staining was positively correlated with the clinical stage. The incidence of oncogenic Wnt3a expression in the cancerous group was significantly higher than that in the surrounding tissue group. Brown Wnt3a staining was gradually increased with clinical staging and showed very strong staining in advanced HCC, indicating that hepatic Wnt3a expression might be related to HCC progression.
The management of advanced HCC is entering a new era of molecular targeting therapy, which is of particular significance for HCC because of the lack of effective systemic therapies for HCC. Although the clinical significance of Wnt3a expression in tissue has been demonstrated in different human malignancies[13,14,16], little data until now was available on the relationship between Wnt3a expression and HCC. In this study, the clinicopathologic features of high Wnt3a expression in HCC were reported and associated with the poorly-differentiated grade, liver cirrhosis, HBV infection, higher TNM stage, and a relatively shorter survival time. Furthermore, univariate and multivariate analyses suggested that high Wnt3a expression in HCC patients might be an independent prognostic indicator, like the factors liver cirrhosis and HBV infection[29,30].
There are some possible limitations associated with this study. We evaluated a relatively small number of patients, and the study design was retrospective. In addition, this study is limited by the inclusion of only patients with resectable tumors. It is possible that these findings pertain only to those patients who have undergone resection. A larger prospective study examining Wnt3a expression is necessary to validate further the usefulness of this system. Inhibiting Wnt3a overexpression may prevent invasive progression and metastatic relapse, which would improve the prognosis and quality of life for HCC patients after hepatic resection. Targeting the Wnt3a pathway may be a therapeutic strategy for HCC that could be used in combination with conventional chemotherapy or radiotherapy. Wnt3a may play an important role in promoting HCC progression and metastasis.
In conclusion, to the best of our knowledge, this is the first report to investigate cancerous Wnt3a expression and to indicate that it may be a novel prognostic marker for HCC. Multiple key factors along the Wnt/β-catenin signaling cascade likely represent potential diagnosis, prognosis, or therapeutic targets as well[31,32]. Here, the findings are promising, and the initial evidence confirmed that Wnt3a is one of the key molecules in the Wnt/β-catenin pathway. Future studies should clarify the molecular mechanisms of the upregulation of oncogenic Wnt3a expression and its important role in hepatocarcinogenesis[33,34].
The authors thank Dr. T. FitzGibbon for comments on earlier drafts of the manuscript.
Hepatocellular carcinoma (HCC) is one of the most common and rapidly fatal malignancies worldwide. The oncogene member 3a of Wingless-type MMTV integration site family (Wnt3a) is located on chromosome 17q21 and has been regarded as an activator of the canonical Wnt signaling pathway. Some studies on human Wnt3a have focused primarily on its key role in human malignancy and its high expression in many kinds of tumors. However, little data is available regarding HCC, and the prognostic value of Wnt3a remains to be explored.
Recently, Pan et al found that higher levels of circulating Wnt3a expression (over 800 ng/L) were present in 92.5% HCC patients, and this elevation was significantly related to α-fetoprotein (AFP) level, liver cirrhosis, hepatitis B virus (HBV) infection, poor differentiation, tumor node metastasis, and extra-hepatic metastasis. Wnt3a level in HCC was obviously higher than that in any other group of benign liver diseases, except for 5.67% cirrhosis cases, suggesting that Wnt3a expression was associated with tumor progression and that it may be a novel specific biomarker for diagnosis and differentiation of HCC.
Some studies on human Wnt3a have focused primarily on its key role in human malignancy, and its high expression in many kinds of tumors was correlated with a worse outcome. The present data showed that the clinicopathologic features of high Wnt3a expression in HCC were related to poorly-differentiated grade, liver cirrhosis, HBV infection, higher tumor-node-metastasis (TNM) stage, and 5-year survival rate, indicating that oncogenic Wnt3a expression associated with HBV infection and cirrhotic liver might be an independent prognostic factor for HCC.
The authors report for the first time that Wnt3a expression is a novel prognostic marker for HCC. Multiple key factors along the Wnt/β-catenin signaling cascade may also represent potential diagnosis, prognosis, or therapeutic targets. The present findings are promising, and the initial evidence confirmed that Wnt3a is a key molecule in the Wnt/β-catenin pathway and further studies should clarify the molecular mechanisms of the upregulation of oncogenic Wnt3a expression and its important role in hepatocarcinogenesis.
Key molecules in the Wnt/β-catenin signaling pathway have been recognized to play an important role in HCC. These molecules regulate multiple cellular events through the β-catenin-dependent canonical pathway, such as Wnt1, Wnt2, Wnt3, Wnt3a, Wnt8a, Wnt8b, Wnt10a, and Wnt10b, characterized by the stabilization and subsequent nuclear transport of β-catenin, which result in the activation of transcriptional responses; and the β-catenin-independent non-canonical pathway, including Wnt4, Wnt5a, Wnt5b, Wnt6, ont7a, Wnt7b, and Wnt11, with more diverse and several different signaling modes that regulate cell behavior.
This research study is well presented, and the reviewers enjoyed reading the article, despite its limitations as a retrospective study. The main discovery by the authors will likely generate further interest in the use of Wnt3a as a molecular biomarker in HCC.
P- Reviewer: Koh PS S- Editor: Yu J L- Editor: Filipodia E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 2. | Ringelhan M, O’Connor T, Protzer U, Heikenwalder M. The direct and indirect roles of HBV in liver cancer: prospective markers for HCC screening and potential therapeutic targets. J Pathol. 2015;235:355-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [PubMed] [Cited in This Article: ] |
| 4. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2881] [Cited by in F6Publishing: 2983] [Article Influence: 229.5] [Reference Citation Analysis (0)] |
| 5. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 666] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 6. | Wang L, Yao M, Dong Z, Zhang Y, Yao D. Circulating specific biomarkers in diagnosis of hepatocellular carcinoma and its metastasis monitoring. Tumour Biol. 2014;35:9-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Yao M, Wang L, Qiu L, Qian Q, Yao D. Encouraging microRNA-based Therapeutic Strategies for Hepatocellular Carcinoma. Anticancer Agents Med Chem. 2015;15:453-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 9. | Rogacki K, Kasprzak A, Stępiński A. Alterations of Wnt/β-catenin signaling pathway in hepatocellular carcinomas associated with hepatitis C virus. Pol J Pathol. 2015;66:9-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Gao C, Xiao G, Hu J. Regulation of Wnt/β-catenin signaling by posttranslational modifications. Cell Biosci. 2014;4:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 11. | Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4:a007864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 275] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 12. | Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M, Ries C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Nalesso G, Sherwood J, Bertrand J, Pap T, Ramachandran M, De Bari C, Pitzalis C, Dell’accio F. WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J Cell Biol. 2011;193:551-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Kaur N, Chettiar S, Rathod S, Rath P, Muzumdar D, Shaikh ML, Shiras A. Wnt3a mediated activation of Wnt/β-catenin signaling promotes tumor progression in glioblastoma. Mol Cell Neurosci. 2013;54:44-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Lamb R, Ablett MP, Spence K, Landberg G, Sims AH, Clarke RB. Wnt pathway activity in breast cancer sub-types and stem-like cells. PLoS One. 2013;8:e67811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Verras M, Brown J, Li X, Nusse R, Sun Z. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 2004;64:8860-8866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in F6Publishing: 845] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 18. | Ministry of Health of the People's Republic of China. [Updated standards for the diagnosis and treatment of primary liver cancer]. Zhonghua Ganzangbing Zazhi. 2012;20:419-426. [PubMed] [Cited in This Article: ] |
| 19. | Yao M, Wang L, Yao Y, Gu HB, Yao DF. Biomarker-based MicroRNA Therapeutic Strategies for Hepatocellular Carcinoma. J Clin Transl Hepatol. 2014;2:253-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Wei Y, Shen N, Wang Z, Yang G, Yi B, Yang N, Qiu Y, Lu J. Sorafenib sensitizes hepatocellular carcinoma cell to cisplatin via suppression of Wnt/β-catenin signaling. Mol Cell Biochem. 2013;381:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, Xie S, Chen C, Hu L, Xu S. Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. 2013;34:962-973. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Lai JP, Oseini AM, Moser CD, Yu C, Elsawa SF, Hu C, Nakamura I, Han T, Aderca I, Isomoto H. The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology. 2010;52:1680-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 23. | Yang T, Cai SY, Zhang J, Lu JH, Lin C, Zhai J, Wu MC, Shen F. Krüppel-like factor 8 is a new Wnt/beta-catenin signaling target gene and regulator in hepatocellular carcinoma. PLoS One. 2012;7:e39668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Zhang S, Li J, He F, Wang XM. Abnormal nuclear expression of Pygopus-2 in human primary hepatocellular carcinoma correlates with a poor prognosis. Histopathology. 2015;67:176-184. [PubMed] [Cited in This Article: ] |
| 25. | Song K, Wu J, Jiang C. Dysregulation of signaling pathways and putative biomarkers in liver cancer stem cells (Review). Oncol Rep. 2013;29:3-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Jiang L, Yang YD, Fu L, Xu W, Liu D, Liang Q, Zhang X, Xu L, Guan XY, Wu B. CLDN3 inhibits cancer aggressiveness via Wnt-EMT signaling and is a potential prognostic biomarker for hepatocellular carcinoma. Oncotarget. 2014;5:7663-7676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 27. | Lu LC, Shao YY, Lee YH, Hsieh MS, Hsiao CH, Lin HH, Kao HF, Ma YY, Yen FC, Cheng AL. β-catenin (CTNNB1) mutations are not associated with prognosis in advanced hepatocellular carcinoma. Oncology. 2014;87:159-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Yao M, Pan LH, Yao DF. Glypican-3 as a specific biomarker for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14:122-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Chen J, Liu J, Jin R, Shen J, Liang Y, Ma R, Lin H, Liang X, Yu H, Cai X. Cytoplasmic and/or nuclear expression of β-catenin correlate with poor prognosis and unfavorable clinicopathological factors in hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9:e111885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Guan CN, Chen XM, Lou HQ, Liao XH, Chen BY, Zhang PW. Clinical significance of axin and β-catenin protein expression in primary hepatocellular carcinomas. Asian Pac J Cancer Prev. 2012;13:677-681. [PubMed] [Cited in This Article: ] |
| 31. | Ma L, Ji L, Yu Y, Wang J. Novel molecular targets for diagnosis and treatment of hepatocellular carcinoma. Discov Med. 2015;19:7-14. [PubMed] [Cited in This Article: ] |
| 32. | Lee MA, Park JH, Rhyu SY, Oh ST, Kang WK, Kim HN. Wnt3a expression is associated with MMP-9 expression in primary tumor and metastatic site in recurrent or stage IV colorectal cancer. BMC Cancer. 2014;14:125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Pinyol R, Nault JC, Quetglas IM, Zucman-Rossi J, Llovet JM. Molecular profiling of liver tumors: classification and clinical translation for decision making. Semin Liver Dis. 2014;34:363-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Guerrieri F, Belloni L, Pediconi N, Levrero M. Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin Liver Dis. 2013;33:147-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |