Published online May 28, 2016. doi: 10.3748/wjg.v22.i20.4802
Peer-review started: March 21, 2016
First decision: March 31, 2016
Revised: April 11, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: May 28, 2016
Inflammatory bowel disease (IBD) could be associated with several extra-intestinal manifestations (EIMs) involving musculoskeletal, hepatopancreatobiliary, ocular, renal, and pulmonary systems, as well as the skin. In the last years, hidradenitis suppurativa (HS) is acquiring an increasing interest. IBD, especially Crohn’s disease (CD), is among the most reported associated diseases in HS patients. The aim of this paper is to give a brief overview of data showing a possible epidemiologic and pathogenetic association between IBD and HS. We performed a pooled-data analysis of four studies and pooled prevalence of HS in IBD patients was 12.8%, with a 95%CI of 11.7%-13.9%. HS was present in 17.3% of subjects with CD (95%CI: 15.5%-19.1%) and in 8.5% of UC patients (95%CI: 7.0%-9.9%). Some items, especially altered immune imbalance, are generally involved in IBD pathogenesis as well as invoked by HS. Smoking is one of the most relevant risk factors for both disorders, representing a predictor of their severity, despite, actually, there being a lack of studies analyzing a possible shared pathway. A role for inheritance in HS and CD pathogenesis has been supposed. Despite a genetic susceptibility having been demonstrated for both diseases, further studies are needed to investigate a genetic mutual route. Although the pathogenesis of IBD and HS is generally linked to alterations of the immune response, recent findings suggest a role for intestinal and skin microbiota, respectively. In detail, the frequent finding of Staphylococcus aureus and coagulase-negative staphylococci on HS cutaneous lesions suggests a bacterial involvement in disease pathogenesis. Moreover, microflora varies in the different cutaneous regions of the body and, consequently, two different profiles of HS patients have been identified on these bases. On the other hand, it is well-known that intestinal microbiota may be considered as “the explosive mixture” at the origin of IBD despite the exact relationship having not been completely clarified yet. A better comprehension of the role that some bacterial species play in the IBD pathogenesis may be essential to develop appropriate management strategies in the near future. A final point is represented by some similarities in the therapeutic management of HS and IBD, since they may be controlled by immunomodulatory drugs. In conclusion, an unregulated inflammation may cause the lesions typical of both HS and IBD, particularly when they coexist. However, this is still a largely unexplored field.
Core tip: The present topic outlines the main data regarding a possible association between hydradenitis suppurativa and inflammatory bowel disease with particular attention to epidemiology, etiopathogenetic factors, genetic susceptibility, intestinal/skin microbiota and therapeutic analogies. Finally, an unregulated inflammation leading to microscopic granulomatous wounds may cause the lesions typical of both diseases, particularly when they coexist. However, this is still a largely unexplored field, and further studies are required.
- Citation: Principi M, Cassano N, Contaldo A, Iannone A, Losurdo G, Barone M, Mastrolonardo M, Vena GA, Ierardi E, Di Leo A. Hydradenitis suppurativa and inflammatory bowel disease: An unusual, but existing association. World J Gastroenterol 2016; 22(20): 4802-4811
- URL: https://www.wjgnet.com/1007-9327/full/v22/i20/4802.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i20.4802
Inflammatory bowel disease (IBD) is a group of chronic inflammatory conditions of the alimentary tract, that are mainly represented by Crohn’s disease (CD) and ulcerative colitis (UC)[1]. These disorders could be associated with several extra-intestinal manifestations (EIMs) involving musculoskeletal, hepatopancreatobiliary, ocular, renal, and pulmonary systems, as well as the skin. In particular, joint, liver, eye, and skin EIMs are considered the most relevant and frequent manifestations[2].
Joint involvement is the most common EIM of IBD[2] and includes peripheral arthropathy, subclassified in pauciarticular, polyarticular forms, and axial arthropathy, such as sacroileitis and spondilitis. Primary sclerosing cholangitis represents the most common cause of hepatobiliary involvement in IBD patients, especially in UC[3,4]. Ocular complications, including episcleritis, scleritis and uveitis, occur more frequently in patients with isolated small intestinal CD[2].
Different dermatological manifestations may arise during the course of IBD. Indeed, pyoderma gangrenosum, psoriasis, Sweet’s syndrome, aphthous stomatitis can be observed, even if erythema nodosum represents the most common IBD-associated dermatological disease. Moreover, in recent years, hydradenitis suppurativa (HS) has been acquiring an increasing interest, even though it may be frequently misdiagnosed as a consequence of an inadequate expertise[5].
HS[2] is defined as “a chronic inflammatory, recurrent, debilitating follicular skin disease that usually presents after puberty with painful deep seated inflamed lesions in the apocrine gland-bearing areas of the body, most commonly the axillae, inguinal and anogenital regions”[3]. HS diagnosis is based on the following clinical criteria: (1) the presence of typical lesions, (2) their characteristic sites, and (3) the chronic course of disease, showing recurring flares[5]. Hurley classification identifies three progressive stages of disease severity: (1) abscess formation, single or multiple, without sinus tract and scarring; (2) recurrent abscesses, with tract formation and healing wound, as well as single or multiple widely separated lesions; and (3) diffuse or multiple interconnected tracts and abscesses across entire area[4].
IBD, especially CD, is among the most reported comorbid diseases in HS patients[5].
Patients with HS and CD have more often been found to be smokers, and more likely to develop perianal disease, and to show an increased need for immunosuppressants and surgical resections[6]. Moreover, on the basis of recent evidence supporting the role of immune imbalance in both conditions[1-3,5-8], a shared pathogenesis between IBD and HS may be presumed. Indeed, multiple predisposing factors could influence the onset and progression of both diseases, i.e., gut luminal agents, genetics and environmental factors[2].
The aim of this paper is to give a brief overview of data showing a possible epidemiologic and pathogenetic association between IBD and HS.
The first series of patients with both CD and HS was described by Church et al[9]. Twenty-four patients were recruited. The diagnosis of CD pre-dated that of HS by an average of 3.5 years. More recently, other 15 patients with CD and HS followed at Mount Sinai Medical Center in the period 2003-2013 have been reported[10]. Apart from these few cohort studies, only case reports about association of IBD-HS have been published. Such single cases are summarized in Table 1[11-22].
| Ref. | n | Localization of CD | Localization of HS | CD predates HS |
| Ostlere et al[11], 1991 | 3 | Colon | Anogenital | NR |
| Burrows et al[12], 1992 | 2 | Colon | Anogenital, axillae, groin | NR |
| Gower-Rousseau et al[13], 1992 | 1 | Ileo-colon | Perineum | NR |
| Attanoos et al[14], 1993 | 3 | Colon, ileo-colon, colon-jejunum | Anogenital, axillae, perineum | Yes |
| Tsianos et al[15], 1995 | 1 | Colon | Anogenital, axillae, sternum | Yes |
| Roy et al[16], 1997 | 1 | Ileo-colon | Axillae | NR |
| Martínez et al[17], 2001 | 1 | Ileo-colon | Axillae | Yes |
| Roussomoustakaki et al[18], 2003 | 1 | Ileo-colon | Anogenital, axillae, groin | No |
| Yazdanyar et al[19], 2010 | 2 | Colon | Axillae, groin, submammary | No |
| Goertz et al[20], 2008 | 1 | Colon | Perianal | Yes |
| dos Santos et al[21], 2012 | 1 | Rectum | Perianal | Yes |
| Hiraiwa et al[22], 2013 | 1 | Ulcerative colitis | Groin | Yes |
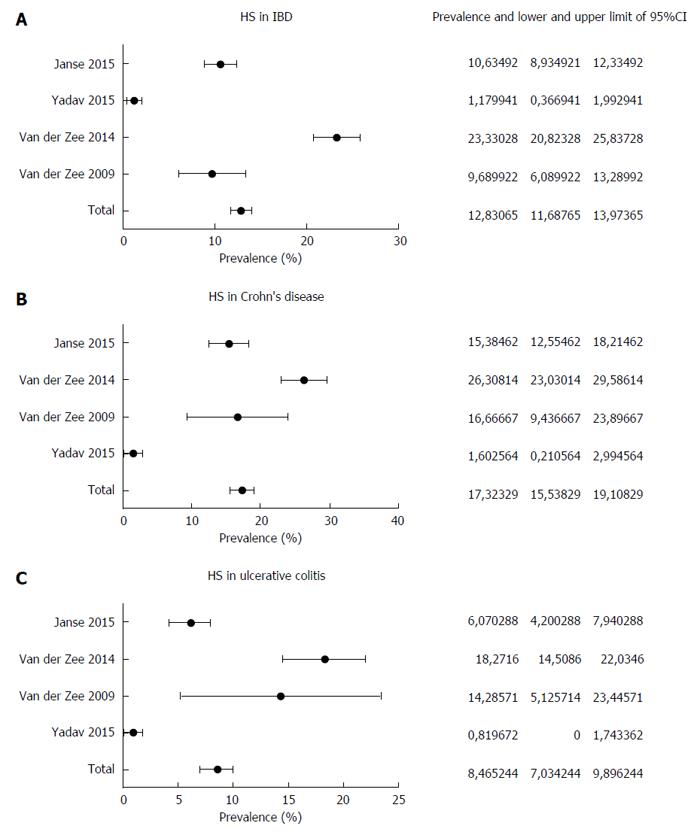
Currently, the prevalence of HS in IBD has been investigated in four studies[23-26]. In the pilot one[23], 158 patients with IBD were asked by a standardized questionnaire about the presence of symptoms suggestive of HS, such as recurrent painful boils in the axillae and/or groin[27]. Further, a picture representing a classical HS skin lesion was shown to the patients in order to have a visual comparison with the injury they were suffering from. On the basis of this method, HS prevalence of 16% in patients with IBD was detected (17% and 14% in CD and in UC patients, respectively). The same authors replicated this study in a larger sample (1093 IBD patients), with an overall prevalence of 23%, in detail 26.3% for CD and 18.3% for UC[24]. A female predominance and a correlation between smoking and severe HS course were recorded. More recently, two other epidemiological studies were carried out. In a cohort study performed in the Olmsted county in Minnesota[25], 679 IBD patients were followed up over a median period of 19.8 years. In such patients, the clinical diagnosis of HS was directly established by dermatologists. HS was found in 8 patients (1.8%), 5 with CD and 3 with UC. A significant association with obesity, female sex and perianal CD disease was found. Two out of 3 subjects with UC had undergone ileal pouch-anal anastomosis. Compared with the control group, the incidence rate ratio of HS in IBD was 8.9 [95% confidence interval (CI): 3.6-17.5]. The 10- and 30-year cumulative incidence of HS was 0.85% and 1.55%, respectively. Axillae, groin, and thighs were the most common sites of involvement. Finally, Janse et al[26] showed an HS prevalence of 10.6% (134 out of 1260) in their IBD cohort, with a higher association with CD (15.1%) than with UC (6.1%). In this study, the diagnosis was achieved using a questionnaire validated for HS[27].
We performed a pooled-data analysis of the four cited studies, as shown in Figure 1. The pooled prevalence of HS in IBD patients was 12.8%, with a 95%CI of 11.7%-13.9%. HS was present in 17.3% of subjects with CD (95%CI: 15.5%-19.1%) and in 8.5% of UC patients (95%CI: 7.0%-9.9%), thus confirming a stronger association with CD. In three out of four studies, the diagnosis of HS was established by means of a questionnaire, and these three studies showed the highest prevalence rates. This detail may lead to the conclusion that such diagnostic strategy, despite validated, could overestimate the prevalence of HS in comparison to the clinician direct evaluation.
The clinical pattern of the IBD-HS association appears to be characterized by female predominance, increased frequency of tobacco smoking and by the fact that intestinal disease foregoes skin involvement. Clinical and pathogenetic features of HS and IBD association are summarized in Table 2.
| CD | UC | HS | |
| Localization | Entire alimentary tract | Colon | Inverse areas of the skin |
| Layer of inflammation | Transmural | Mucosa | Deep derm |
| Confluency of lesions | No (skip lesions) | Yes | Yes |
| Fistulae | Yes | No | Yes |
| Influence of smoking | Aggravates | No (or improvement) | Aggravates |
| Disease chronicity | Yes | Yes | Yes |
| Genetic predisposition | Yes | Yes | Yes |
| Influence of microbiota | Yes | Yes | Yes |
| Female predominance | ↑ | ↑ | ↑↑ |
| Response to anti-TNFα therapy | Yes | Yes | Yes |
The pathogenesis of HS is still obscure. Ever-growing attention has been focused on the role of the immune system, and recent findings suggest the involvement of the interleukin (IL)-23/Th17 pathway in HS-related inflammatory response[28].
HS is characterized by epidermal alterations such as psoriasiform epidermal hyperplasia and keratin pluggings. In HS lesions, the epidermis is an active source of proinflammatory cytokines. It shows inflammasome activation and can be stimulated by IL-17+ cells. The inflammatory process in HS involves the recruitment of innate immune cells, particularly IL-17-expressing neutrophils[29].
Impaired Notch signalling has been proposed to be a crucial pathomechanism of HS, capable of compromising apocrine gland homoeostasis and leading to subsequent stimulation of TLR-mediated innate immunity[30]. This mechanism has been hypothesized not only as an inducer of inflammation in HS but also as responsible for an insufficient feedback regulation of overstimulated innate immunity, linking HS to other Th17-driven comorbidities.
On the other hand, an alteration of immune imbalance with a prevalence of inflammatory cytokines has been clearly stated for inflammatory bowel disease and, at the moment, strongly affects therapeutic approach[1-4].
Some items, generally involved in IBD pathogenesis, are invoked also for HS.
Smoking is one of the most relevant risk factors for both HS and CD, representing a predictor of their severity[4,6].
In a recent meta-analysis enclosing 33 cohort studies[31], CD smoker patients showed increased risks of disease activity flares [odds ratio (OR) = 1.97; 95%CI: 1.21-2.01], post-surgical flares (OR = 1.97; 95%CI: 1.36-2.85), need for both first surgery (OR = 1.68; 95%CI: 1.33-2.12) and second surgery (OR = 2.17; 95%CI: 1.63-2.89). Conversely, the risk of such events was significantly reduced by smoking discontinuation[31-33]. Moreover, smoking has been reported as a well-established risk factor in HS by the European S1 guidelines for the treatment of HS/acne inversa[34]. An association between prevalence of HS and current smoking was found in a French cohort comprising about 10000 subjects (OR = 12.55; 95%CI: 8.58-18.38). This association was not demonstrated in former smokers[35]. Despite this evidence, some aspects of the correlation between HS severity and smoking remain controversial. Indeed, Sartorius et al[36] demonstrated a more severe course in active smokers as compared to non-smokers (P = 0.03), even though no statistical difference with former smokers was observed. Conversely, no effect of smoking on disease severity was found in a cohort study enclosing 268 HS patients[37].
Although the relationship between smoking and both diseases is supported by evidence, a hypothetical shared pathogenetic mechanism remains unclear and may be different for HS and CD. Indeed, nicotine may act in HS by multiple pathways, i.e., over-stimulation of the sweat gland with a possible duct obstruction and consequent inflammation, chemotaxis for neutrophils, over-expression of tumor necrosis factor (TNF) alpha in keratinocytes and thickening of epidermidis by means of non-neuronal acetylcholine[38]. Simultaneously, in CD nicotine determines a more aggressive disease pattern, probably causing ischemia of microvessels, due to the implementation of carbon monoxide concentration, and by decreasing the expression of anti-inflammatory cytokines[37]. Finally, smoking cessation improves CD course, however this topic has not been largely investigated in HS[36].
On the other hand, it is well known that smoking does not affect UC course. In detail, nicotine may modulate the immune system by means of its binding to nicotine acetylcholine receptor α7 subunit expressed on macrophage, leading to a reduction of TNF-alpha and inflammation[39].
In conclusion, even if smoking represents a crucial pathogenic factor for both CD and HS, there is currently a lack of studies analyzing a possible shared pathway.
A role for inheritance in HS and CD pathogenesis has been supposed. Up to 40% of patients with HS show a familial history and an autosomal dominant pattern of inheritance has been observed in some familial cases[6]. Two loci on chromosome 6 and 19, and another one on chromosome 1 (1p21.1-1q25.3) have been linked to HS[6,40,41]. However, a recent report by Al-Ali et al[42] did not report any association between the locus 1p21.1-1q25.3 and this disease. Additionally, mutations involving presenilin-1 (PSEN1), presenilin enhancer-2 (PSENEN) and nicastrin (NCSTN) genes, which determine the inactivation of the gamma-secretase enzyme complex, have also been related to HS. The mutation of this enzyme complex is involved in HS pathogenesis via aberrant trichilemmal keratinization[6,41-44].
As for CD, the nucleotide-binding oligomerization domain containing 2 (NOD2) gene has been described as a possible inherited factor. Three different mutations have been identified in Caucasian CD patients: one frameshift and two missense mutations[45,46]. This gene is involved in intestinal homeostasis by detecting peptidoglycan released from the gut microbiota and driving a nuclear factor-κB (NF-κB)-mediated inflammatory response. The alteration of this process is supposed to play a role in the development of chronic intestinal inflammation[46].
A recent study by Janse et al[26] tried to identify a genetic link between HS and CD. The authors evaluated three different genes, i.e., ELOVL fatty acid elongase 7 (ELOVL7) gene on chromosome 5, sulfotransferase family cytosolic 1B member 1 (SULT1B1) and sulfotransferase family 1E member 1(SULT1E1) genes on chromosome 4. These genes on chromosome 4 originate from the sulfotransferase family, encoding for enzymes that catalyze the sulphate conjugation of hormones, drugs, neurotransmitters and xenobiotic compounds. SULT1E1 encodes for an enzyme regulating estrogen homeostasis[47]. These hormones seem to be involved in HS clinical course. Indeed, the reactivation of the disease usually occurs during hypoestrogenic states, thus estrogens seem to play a protective role[48]. Additionally, since adiposity is another supposed risk factor for HS, the expression of SULT1E1 in the abdominal subcutaneous tissue of obese people may be considered further evidence of the role of obesity[6]. Moreover, Ahima et al[47] demonstrated the co-expression of estrogen sulfotransferase and TNF-alpha in abdominal adipose tissue of obese subjects. This last pro-inflammatory cytokine has a role in HS and CD pathogenesis as well as representing a therapeutic target for both diseases[49].
However, further studies are needed to investigate the genetic association between HS and CD.
Although the pathogenesis of IBD and HS is generally linked to alterations of the immune response[4,42], recent findings suggest a role for intestinal and skin microbiota, respectively[50,51].
The frequent finding of Staphylococcus aureus (S. aureus) and coagulase-negative staphylococci (CoNS) on HS cutaneous lesions suggests a bacterial involvement in disease pathogenesis[49].
Kurzen et al[52] supposed that nicotine may stimulate the growth of S. aureus. Jemec et al[53] suggested that S. aureus could induce the initial development process of HS, since it influences a series of anatomical alterations in the hair follicles facilitating inflammation and necrosis.
CoNS, in particular Staphylococcus epidermidis (S. epidermidis), usually are non-pathogenic microorganisms and commensals of the normal skin flora[54]. Lapins et al[55] found CoNS in 21 patients with HS. Sixteen out of the 21 patients showed CoNS in the deep levels of the skin, and in 9 of them CoNS were the only bacteria isolated, thus presuming a promoting activity for these germs in HS inflammation. A histological retrospective study analyzing 27 patients with HS showed the presence of S. epidermidis-related biofilm (i. e., an extracellular matrix used by bacteria as a protective cover against host defense mechanisms and antimicrobial agents) in one-fifth of the samples located in hair follicles and sinus tracts[56].
Since microflora varies in the different cutaneous regions of the body, in relation to different distributions of hair follicles and glands, two different profiles of HS patients have been identified in a recent report by Guet-Revillet et al[57]. Staphylococcus lugdunensis was cultured from 58% of HS lesions, that were almost exclusively Hurley stage 1 lesions and more frequently located on the buttocks and the breasts, whereas a polymicrobial flora (strict anaerobes and/or anaerobic actinomycetes and/or streptococci of the milleri group) was predominantly associated with Hurley stage 2 and stage 3 lesions, especially in the axilla, and inguinal and gluteal folds.
Finally, antibiotics represent a treatment option for HS. In this regard, both topic and oral administrations act by killing involved bacteria and determining an indirect immunomodulation with reduction of pro-inflammatory cytokines and induction of neutrophil apoptosis[6].
With regard to IBD pathogenesis, modification of intestinal microflora, including about 1000 bacterial species, has been proposed as a promoting factor. Moreover, different bacterial compositions affect different sites of digestive system inflammation in animal models[51]. Indeed, in germ IL-10-/-germ free mice, bacterial colonization of Escheria coli or Bilophila wadworthia led to cecum or distal colon involvement, respectively[58]. Couturier-Maillard et al[59] described a potential link between genetic factors and microbiome modulation. They transplanted fecal microbiota from healthy wild-type mice to NOD2 deficient ones, obtaining a reduction of IBD risk. Conversely, disease risk rose in wild-type mice that received fecal microbiota from NOD2-deficient ones.
Smoking, as previously described for HS, could determine microbiota alterations, also in the gut with a reduction of Firmicutes and Actinobacteria and an increase of Proteobacteria and Bacteroides[60,61].
The modulation of gut microbiota is a potential therapeutic target in IBD and antibiotics, such as metronidazole and ciprofloxacin, which are currently used in Crohn’s colitis, ileocolitis and pouchitis[3,51]. Nevertheless, tetracyclines, antibiotics largely used for HS, showed a Hazard Ratio for developing IBD, for any exposure to these drugs, of 1.39 (95%CI: 1.02-1.90) even if no clear explanation of the mechanism was found[62]. Additionally, a meta-analysis[63] of 11 observational studies, including 7208 IBD patients, demonstrated an OD of 1.57 (95%CI: 1.27-1.94) for IBD development after the exposure to any antibiotic. This risk was higher for CD (OR = 1.74; 95%CI: 1.35-2.23), metronidazole (OR = 5.01, 95%CI: 1.65-15.25), fluoroquinolones (OR = 1.79, 95%CI: 1.03-3.12) and in children (OR = 2.75; 95%CI: 1.72-4.38). Only the penicillin class was not associated with IBD onset.
IBD and HS may show some similarities in the therapeutic management, since they may be controlled by some immunomodulatory drugs.
Indeed, HS may benefit from anti-TNF-alpha biologic therapy, similarly to IBD. Numerous case reports have demonstrated that infliximab improves skin lesions in patients with both CD and HS[18,20,21,64,65]. On these bases, patients suffering from HS have been treated off-label with infliximab and etanercept, with a remission rate of about 35% and a decrease in HS activity of 50%[49,66]. In a systematic review by Haslund et al[67], almost all HS treated patients experienced a positive effect. Infliximab therapy is indicated in moderate-severe HS and is well tolerated, reduces skin pain, decreases disease severity and improves quality of life[49]. However, the long-term results are rather poor. Adalimumab has been recently approved by Food and Drug Administration for HS treatment. This FDA approval is based on the results of two pivotal Phase 3 studies, PIONEER I and PIONEER II[68-70].
Additionally, Ustekinumab is a monoclonal antibody that selectively targets IL-12 and IL-23, which has been proposed for both IBD and HS treatment. In a setting of 17 HS patients, Ustekinumab allowed, after 40 wk, a moderate improvement in the 82% and a complete clinical response in the 47%[71]. A similar success rate, ranging from 46% to 65%, has been found in patients affected by CD who did not benefit from other anti-TNF alpha biologic agents[72,73].
Finally, other immunomodulators, such as corticosteroids and cyclosporine, have been proven to be effective for HS[73-76], similarly to IBD, thus supporting a possible link. However, the general level of evidence for these drugs is very low, given the small number of HS patients described in the literature so far and the lack of randomized controlled studies.
IBD and HS share a chronic inflammatory trait. Despite an association between these two conditions having been reported only anecdotally, in recent years novel clinical investigations performed on large scale have shed new light on their association. The link between HS and IBD - CD in particular - could be stronger than expected. However, epidemiologic data is not supported by strong basic studies. Despite some evidence having shown that immune dysregulation, alteration of microbiota, genetic factors and tobacco smoking may underlie both diseases[52,59,77], a convincing in vivo proof has not yet been found. Additionally, the common therapeutic scenario described for IBD and HS might be another clue for their association.
An unregulated inflammation leading to microscopic granulomatous wounds may cause the lesions typical of both diseases, particularly when they coexist. However, this is still a largely unexplored field, and further studies are required to elucidate their pathogenesis and possible therapeutic approaches, as well as the interconnection between the disorders and the consequent practical implications. Indeed, despite the association between HS and IBD having been under-evaluated up to now, our pooled results show that the mean prevalence of HS in IBD is 12.8%, with a peak for CD (17.3%). Therefore, an existent link between these two conditions may be argued. On these bases, a careful skin examination should usually be performed in IBD patients, since the association CD-HS may be very disabling. Therefore, an early detection of HS in IBD could prevent the worsening of the skin disorder, thus avoiding the need of for some toxic medications.
P- Reviewer: Actis GC, Ahluwalia NK, Capasso R, Zezos P S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1247] [Cited by in F6Publishing: 1242] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 2. | Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:235-241. [PubMed] [Cited in This Article: ] |
| 3. | Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, Beaugerie L, Gomollón F, Häuser W, Herrlinger K. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 329] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 4. | Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis. 2010;4:63-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 526] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 5. | Dessinioti C, Katsambas A, Antoniou C. Hidradenitis suppurrativa (acne inversa) as a systemic disease. Clin Dermatol. 2014;32:397-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Nazary M, van der Zee HH, Prens EP, Folkerts G, Boer J. Pathogenesis and pharmacotherapy of Hidradenitis suppurativa. Eur J Pharmacol. 2011;672:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 395] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 9. | Church JM, Fazio VW, Lavery IC, Oakley JR, Milsom JW. The differential diagnosis and comorbidity of hidradenitis suppurativa and perianal Crohn’s disease. Int J Colorectal Dis. 1993;8:117-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kamal N, Cohen BL, Buche S, Delaporte E, Colombel JF. Features of Patients With Crohn’s Disease and Hidradenitis Suppurativa. Clin Gastroenterol Hepatol. 2016;14:71-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Ostlere LS, Langtry JA, Mortimer PS, Staughton RC. Hidradenitis suppurativa in Crohn’s disease. Br J Dermatol. 1991;125:384-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Burrows NP, Jones RR. Crohn’s disease in association with hidradenitis suppurativa. Br J Dermatol. 1992;126:523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Gower-Rousseau C, Maunoury V, Colombel JF, Coulom P, Piette F, Cortot A, Paris JC. Hidradenitis suppurativa and Crohn’s disease in two families: a significant association? Am J Gastroenterol. 1992;87:928. [PubMed] [Cited in This Article: ] |
| 14. | Attanoos RL, Appleton MA, Hughes LE, Ansell ID, Douglas-Jones AG, Williams GT. Granulomatous hidradenitis suppurativa and cutaneous Crohn’s disease. Histopathology. 1993;23:111-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Tsianos EV, Dalekos GN, Tzermias C, Merkouropoulos M, Hatzis J. Hidradenitis suppurativa in Crohn’s disease. A further support to this association. J Clin Gastroenterol. 1995;20:151-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Roy MK, Appleton MA, Delicata RJ, Sharma AK, Williams GT, Carey PD. Probable association between hidradenitis suppurativa and Crohn’s disease: significance of epithelioid granuloma. Br J Surg. 1997;84:375-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Martínez F, Nos P, Benlloch S, Ponce J. Hidradenitis suppurativa and Crohn’s disease: response to treatment with infliximab. Inflamm Bowel Dis. 2001;7:323-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Roussomoustakaki M, Dimoulios P, Chatzicostas C, Kritikos HD, Romanos J, Panayiotides JG, Kouroumalis EA. Hidradenitis suppurativa associated with Crohn’s disease and spondyloarthropathy: response to anti-TNF therapy. J Gastroenterol. 2003;38:1000-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Yazdanyar S, Miller IM, Jemec GB. Hidradenitis suppurativa and Crohn’s disease: two cases that support an association. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:23-25. [PubMed] [Cited in This Article: ] |
| 20. | Goertz RS, Konturek PC, Naegel A, Janka R, Amann K, Maennlein G, Wein A, Hahn EG, Boxberger F. Experiences with a long-term treatment of a massive gluteal acne inversa with infliximab in Crohn’s disease. Med Sci Monit. 2009;15:CS14-CS18. [PubMed] [Cited in This Article: ] |
| 21. | dos Santos CH, Netto PO, Kawaguchi KY, Parreira Alves JA, de Alencar Souza VP, Reverdito S. Association and management of Crohn’s disease plus hidradenitis suppurativa. Inflamm Bowel Dis. 2012;18:E801-E802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Hiraiwa T, Hanami Y, Yamamoto T. Hidradenitis suppurativa and multiple dermatofibromas in a patient with ulcerative colitis. J Dermatol. 2013;40:1071-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | van der Zee HH, van der Woude CJ, Florencia EF, Prens EP. Hidradenitis suppurativa and inflammatory bowel disease: are they associated? Results of a pilot study. Br J Dermatol. 2010;162:195-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | van der Zee HH, de Winter K, van der Woude CJ, Prens EP. The prevalence of hidradenitis suppurativa in 1093 patients with inflammatory bowel disease. Br J Dermatol. 2014;171:673-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Yadav S, Singh S, Edakkanambeth Varayil J, Harmsen WS, Zinsmeister AR, Tremaine WJ, Davis MD, Wetter DA, Colombel JF, Loftus EV. Hidradenitis Suppurativa in Patients With Inflammatory Bowel Disease: A Population-Based Cohort Study in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2016;14:65-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Janse IC, Koldijk MJ, Spekhorst LM, Vila AV, Weersma RK, Dijkstra G, Horváth B. Identification of Clinical and Genetic Parameters Associated with Hidradenitis Suppurativa in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:106-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Esmann S, Dufour DN, Jemec GB. Questionnaire-based diagnosis of hidradenitis suppurativa: specificity, sensitivity and positive predictive value of specific diagnostic questions. Br J Dermatol. 2010;163:102-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Schlapbach C, Hänni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 29. | Melnik BC, Plewig G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: an approach to pathogenesis by evidence from translational biology. Exp Dermatol. 2013;22:172-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Lima AL, Karl I, Giner T, Poppe H, Schmidt M, Presser D, Goebeler M, Bauer B. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment Pharmacol Ther. 2016;43:549-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Johnson GJ, Cosnes J, Mansfield JC. Review article: smoking cessation as primary therapy to modify the course of Crohn’s disease. Aliment Pharmacol Ther. 2005;21:921-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Nunes T, Etchevers MJ, Domènech E, García-Sánchez V, Ber Y, Peñalva M, Merino O, Nos P, Garcia-Planella E, Casbas AG. Smoking does influence disease behaviour and impacts the need for therapy in Crohn’s disease in the biologic era. Aliment Pharmacol Ther. 2013;38:752-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, Lapins J, Matusiak L, Prens EP, Revuz J. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 622] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 35. | Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, Poli F, Faye O, Roujeau JC, Bonnelye G. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 395] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 36. | Sartorius K, Emtestam L, Jemec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 334] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 37. | Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 38. | Kelly G, Sweeney CM, Tobin AM, Kirby B. Hidradenitis suppurativa: the role of immune dysregulation. Int J Dermatol. 2014;53:1186-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2256] [Cited by in F6Publishing: 2310] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 40. | Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Gao M, Wang PG, Cui Y, Yang S, Zhang YH, Lin D, Zhang KY, Liang YH, Sun LD, Yan KL. Inversa acne (hidradenitis suppurativa): a case report and identification of the locus at chromosome 1p21.1-1q25.3. J Invest Dermatol. 2006;126:1302-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Al-Ali FM, Ratnamala U, Mehta TY, Naveed M, Al-Ali MT, Al-Khaja N, Sheth JJ, Master DC, Maiti AK, Chetan GK. Hidradenitis suppurativa (or Acne inversa) with autosomal dominant inheritance is not linked to chromosome 1p21.1-1q25.3 region. Exp Dermatol. 2010;19:851-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Pink AE, Simpson MA, Brice GW, Smith CH, Desai N, Mortimer PS, Barker JN, Trembath RC. PSENEN and NCSTN mutations in familial hidradenitis suppurativa (Acne Inversa). J Invest Dermatol. 2011;131:1568-1570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Pan Y, Lin MH, Tian X, Cheng HT, Gridley T, Shen J, Kopan R. gamma-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell. 2004;7:731-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 45. | Hugot JP, Laurent-Puig P, Gower-Rousseau C, Olson JM, Lee JC, Beaugerie L, Naom I, Dupas JL, Van Gossum A, Orholm M. Mapping of a susceptibility locus for Crohn’s disease on chromosome 16. Nature. 1996;379:821-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 649] [Cited by in F6Publishing: 665] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 46. | Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1158] [Cited by in F6Publishing: 1195] [Article Influence: 66.4] [Reference Citation Analysis (1)] |
| 47. | Ahima RS, Stanley TL, Khor VK, Zanni MV, Grinspoon SK. Estrogen sulfotransferase is expressed in subcutaneous adipose tissue of obese humans in association with TNF-alpha and SOCS3. J Clin Endocrinol Metab. 2011;96:E1153-E1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Harrison BJ, Read GF, Hughes LE. Endocrine basis for the clinical presentation of hidradenitis suppurativa. Br J Surg. 1988;75:972-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 50. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2085] [Cited by in F6Publishing: 2046] [Article Influence: 102.3] [Reference Citation Analysis (1)] |
| 51. | Bringiotti R, Ierardi E, Lovero R, Losurdo G, Di Leo A, Principi M. Intestinal microbiota: The explosive mixture at the origin of inflammatory bowel disease? World J Gastrointest Pathophysiol. 2014;5:550-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 49] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Kurzen H, Kurokawa I, Jemec GB, Emtestam L, Sellheyer K, Giamarellos-Bourboulis EJ, Nagy I, Bechara FG, Sartorius K, Lapins J. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455-456; discussion 457-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Jemec GB, Faber M, Gutschik E, Wendelboe P. The bacteriology of hidradenitis suppurativa. Dermatology. 1996;193:203-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 115] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Ring HC, Riis Mikkelsen P, Miller IM, Jenssen H, Fuursted K, Saunte DM, Jemec GB. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 55. | Lapins J, Jarstrand C, Emtestam L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br J Dermatol. 1999;140:90-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Jahns AC, Killasli H, Nosek D, Lundskog B, Lenngren A, Muratova Z, Emtestam L, Alexeyev OA. Microbiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patients. APMIS. 2014;122:804-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Guet-Revillet H, Coignard-Biehler H, Jais JP, Quesne G, Frapy E, Poirée S, Le Guern AS, Le Flèche-Matéos A, Hovnanian A, Consigny PH. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg Infect Dis. 2014;20:1990-1998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 58. | Kim SC, Tonkonogy SL, Albright CA, Tsang J, Balish EJ, Braun J, Huycke MM, Sartor RB. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 59. | Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 285] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 60. | Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, Steurer-Stey C, Frei A, Frei P, Scharl M. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8:e59260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 269] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 61. | Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 488] [Article Influence: 48.8] [Reference Citation Analysis (1)] |
| 62. | Margolis DJ, Fanelli M, Hoffstad O, Lewis JD. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, Shaw S, Van Kruiningen H, Colombel JF, Atreja A. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. 2014;109:1728-1738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 223] [Article Influence: 22.3] [Reference Citation Analysis (1)] |
| 64. | Lebwohl B, Sapadin AN. Infliximab for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2003;49:S275-S276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Katsanos KH, Christodoulou DK, Tsianos EV. Axillary hidradenitis suppurativa successfully treated with infliximab in a Crohn’s disease patient. Am J Gastroenterol. 2002;97:2155-2156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Pelekanou A, Kanni T, Savva A, Mouktaroudi M, Raftogiannis M, Kotsaki A, Giamarellos-Bourboulis EJ. Long-term efficacy of etanercept in hidradenitis suppurativa: results from an open-label phase II prospective trial. Exp Dermatol. 2010;19:538-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Haslund P, Lee RA, Jemec GB. Treatment of hidradenitis suppurativa with tumour necrosis factor-alpha inhibitors. Acta Derm Venereol. 2009;89:595-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 68. | Abbvie . Drug molecule: Adalimumab (Humira). 2015. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=608D4F0D-B19F-46D3-749A-7159AA5F933D. [Cited in This Article: ] |
| 69. | Kimball AB, Kerdel F, Adams D, Mrowietz U, Gelfand JM, Gniadecki R, Prens EP, Schlessinger J, Zouboulis CC, van der Zee HH. Safety and Efficacy of Adalimumab in Patients with Moderate to Severe Hidradenitis Suppurativa: Results from First 12 Weeks of PIONEER I, a Phase 3, Randomized, Placebo-Controlled Trial. Abstract #210 44th. Copenhagen, Denmark: Annual Meeting of the European Society for Dermatological Research (ESDR) 2014; . [Cited in This Article: ] |
| 70. | Kimball AB, Kerdel F, Adams D, Mrowietz U, Gelfand JM, Gniadecki R, Prens EP, Schlessinger J, Zouboulis CC, van der Zee HH. Efficacy and Safety of Adalimumab in Patients with Moderate to Severe Hidradenitis Suppurativa: Results from PIONEER II, a Phase 3, Randomized, Placebo-Controlled Trial. Abstract FC08.2. 22nd. Amsterdam, Netherlands: Congress of the European Dermatology and Venereology (EADV) Meeting 2014; . [Cited in This Article: ] |
| 71. | Blok JL, Li K, Brodmerkel C, Horvátovich P, Jonkman MF, Horváth B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. 2016;174:839-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 72. | Harris KA, Horst S, Gadani A, Nohl A, Annis K, Duley C, Beaulieu D, Ghazi L, Schwartz DA. Patients with Refractory Crohn’s Disease Successfully Treated with Ustekinumab. Inflamm Bowel Dis. 2016;22:397-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Wils P, Bouhnik Y, Michetti P, Flourie B, Brixi H, Bourrier A, Allez M, Duclos B, Grimaud JC, Buisson A. Subcutaneous Ustekinumab Provides Clinical Benefit for Two-Thirds of Patients With Crohn’s Disease Refractory to Anti-Tumor Necrosis Factor Agents. Clin Gastroenterol Hepatol. 2016;14:242-250.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 74. | Buckley DA, Rogers S. Cyclosporin-responsive hidradenitis suppurativa. J R Soc Med. 1995;88:289P-290P. [PubMed] [Cited in This Article: ] |
| 75. | Gupta AK, Ellis CN, Nickoloff BJ, Goldfarb MT, Ho VC, Rocher LL, Griffiths CE, Cooper KD, Voorhees JJ. Oral cyclosporine in the treatment of inflammatory and noninflammatory dermatoses. A clinical and immunopathologic analysis. Arch Dermatol. 1990;126:339-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 130] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Danto JL. Preliminary studies of the effect of hydrocortisone on hidradenitis suppurativa. J Invest Dermatol. 1958;31:299-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Giudici F, Maggi L, Santi R, Cosmi L, Annunziato F, Nesi G, Barra G, Bassotti G, De Palma R, Tonelli F. Perianal Crohn’s disease and hidradenitis suppurativa: a possible common immunological scenario. Clin Mol Allergy. 2015;13:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |