Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.5927
Peer-review started: April 13, 2016
First decision: May 12, 2016
Revised: May 30, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 14, 2016
Endoscopic submucosal dissection (ESD) has become widely accepted as a standard method of treatment for superficial gastrointestinal neoplasms because it enables en block resection even for large lesions or fibrotic lesions with minimal invasiveness, and decreases the local recurrence rate. Moreover, specimens resected in an en block fashion enable accurate histological assessment. Taking these factors into consideration, ESD seems to be more advantageous than conventional endoscopic mucosal resection (EMR), but the associated risks of perioperative adverse events are higher than in EMR. Bleeding after ESD is the most frequent among these adverse events. Although post-ESD bleeding can be controlled by endoscopic hemostasis in most cases, it may lead to serious conditions including hemorrhagic shock. Even with preventive methods including administration of acid secretion inhibitors and preventive hemostasis, post-ESD bleeding cannot be completely prevented. In addition high-risk cases for post-ESD bleeding, which include cases with the use of antithrombotic agents or which require large resection, are increasing. Although there have been many reports about associated risk factors and methods of preventing post-ESD bleeding, many issues remain unsolved. Therefore, in this review, we have overviewed risk factors and methods of preventing post-ESD bleeding from previous studies. Endoscopists should have sufficient knowledge of these risk factors and preventive methods when performing ESD.
Core tip: Antithrombotic agents and large resection are known to be significant risk factors for post-endoscopic submucosal dissection (post-ESD) bleeding, and as the indications for antithrombotic agents increase, and the indications for endoscopic resection are expanded, endoscopists have a chance to face an increasing number of patients with a high risk of post-ESD bleeding. Acid secretion inhibitors and preventive hemostasis are effective for the prevention of post-ESD bleeding, but do not seem to be completely effective in its prevention. Developing additional preventive methods which can reduce post-ESD bleeding more effectively will become an increasingly important issue in the future.
- Citation: Kataoka Y, Tsuji Y, Sakaguchi Y, Minatsuki C, Asada-Hirayama I, Niimi K, Ono S, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Bleeding after endoscopic submucosal dissection: Risk factors and preventive methods. World J Gastroenterol 2016; 22(26): 5927-5935
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/5927.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.5927
Endoscopic submucosal dissection (ESD) has become a well-established method of treatment for superficial neoplasms in the gastrointestinal tract. ESD was first developed as an advanced technique which was intended to overcome the limitations of conventional endoscopic mucosal resection (EMR) in the 1990s[1-3]. ESD is curatively advantageous over EMR in that it enables en block fashion, regardless of tumor size, shape, ulceration or location, which contributes to the decrease in local recurrence rate. Moreover, specimens obtained by en block resection enable accurate histological diagnosis of target lesions[4-11].
However, ESD is technically more difficult and requires a longer procedure time than EMR. In addition, ESD is accompanied by a relatively high risk of procedure-related adverse events[4,5,7,11]. Especially, bleeding after ESD is one of the most severe adverse events because post-ESD bleeding may lead to serious conditions including hemorrhagic shock. Moreover, post-ESD bleeding can occur later than other adverse events, and may require additional treatment even after discharge[12-14]. Therefore, in this review article, we will focus on risk factors and preventive methods of post-ESD bleeding.
Post-ESD bleeding, or bleeding after ESD is the most frequent adverse event associated with ESD. The incidence of bleeding after gastric ESD has been reported to range from 1.8% to 15.6%[4,9,15-18]. On the other hand, there have been many reports that bleeding rates after esophageal or colorectal ESD are a much smaller percentages[19-25] (Tables 1 and 2). Therefore, the reports listed in the following section are focused on the risk factors and methods of preventing bleeding after gastric ESD.
| Ref. | Organ | Year | Case No. | Post-ESD bleeding | Perforation | En block resection |
| Ichiro et al[13] | Stomach | 2005 | 1033 | 6.2% | 3.7% | 98.0% |
| Isomoto et al[17] | Stomach | 2009 | 589 | 1.8% | 4.5% | 94.9% |
| Chung et al[9] | Stomach | 2009 | 1000 | 15.6% | 1.2% | 95.3% |
| Mannen et al[26] | Stomach | 2009 | 478 | 8.2% | 3.6% | |
| Tsuji et al[14] | Stomach | 2010 | 398 | 5.8% | - | - |
| Higashiyama et al[31] | Stomach | 2011 | 924 | 3.0% | 4.0% | - |
| Okada et al[27] | Stomach | 2011 | 647 | 4.3% | - | - |
| Toyokawa et al[32] | Stomach | 2012 | 1123 | 5.0% | 2.4% | 93.5% |
| Goto et al[18] | Stomach | 2012 | 1814 | 5.5% | - | - |
| Lim et al[16] | Stomach | 2012 | 1591 | 5.9% | - | - |
| Koh et al[15] | Stomach | 2013 | 1166 | 5.3% | - | 98.5% |
| Ref. | Organ | Year | Case No. | Postoperative bleeding | Perforation | En block resection |
| Ono et al[25] | Esophagus | 2009 | 107 | 0.0% | 4.0% | 100.0% |
| Isomoto et al[19] | Esophagus | 2013 | 291 | 0.7% | 0.0% | 99.7% |
| Tsujii et al[20] | Esophagus | 2015 | 373 | 0.0% | 5.2% | 96.7% |
| Saito et al[21] | Colon | 2010 | 1111 | 1.5% | 4.9% | 88.0% |
| Niimi et al[79] | Colon | 2010 | 310 | 1.6% | 4.8% | 90.3% |
| Oka et al[80] | Colon | 2010 | 688 | 1.7% | 3.3% | - |
| Toyonaga et al[22] | Colon | 2012 | 1143 | 1.2% | 1.4% | 99.3% |
| Takeuchi et al[81] | Colon | 2012 | 348 | 4.6% | 2.3% | 91.1% |
| Lee et al[24] | Colon | 2013 | 1000 | 0.4% | 5.3% | 97.5% |
| Nakajima et al[23] | Colon | 2013 | 816 | 2.2% | 2.0% | 94.5% |
Post-ESD bleeding is generally defined as the condition that presents any clinical signs of bleeding such as hematemesis, melena, hemodynamic deterioration or downtick of > 2 g/dL in hemoglobin level and requires endoscopic hemostasis[12,13,26].
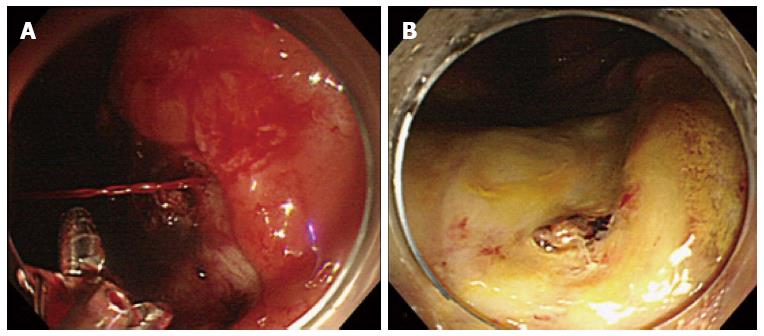
Oda et al reported that 76% of post-ESD bleeding occurred within 24 h of ESD, but it can occur as late as two weeks after the procedure[12,13,27,28]. Post-ESD bleeding can be controlled by endoscopic hemostasis in most cases (Figure 1), but it sometimes leads to life-threatening conditions that require blood transfusion or emergency surgery[12,29]. Therefore, endoscopists should have sufficient knowledge of risk factors for this adverse event and be fully prepared for it.
When performing gastric ESD, endoscopists should know whether their cases have a high risk of post-ESD bleeding. There have been many reports concerning the risk factors for post-ESD bleeding[9,12-15,26,27,29-32]. Although other factors are still controversial, several studies have revealed that antithrombotic agents and resection size are significant risk factors for post-ESD bleeding[9,14,15,26,27,29,30,33].
Because the number of patients taking antithrombotic agents has been increasing worldwide[34,35], there will be an increasing necessity to perform ESD for these patients in the future. Endoscopists should pay attention to both the risks of bleeding and thromboembolism when performing ESD in this situation.
Tentative guidelines concerning the continuation and cessation of antithrombotic agents during endoscopy have been published from several societies including the Japan Gastroenterological Endoscopy Society, American Society for Gastrointestinal Endoscopy, and European Society of Gastrointestinal Endoscopy[36-38]. ESD for patients taking antithrombotic agents is performed according to these guidelines, but currently data supporting ESD under these guidelines is still insufficient. It is a clinically important, but unsolved question whether antithrombotic agents increase the risk of post-ESD bleeding. Several retrospective studies have shown that antithrombotic agents as a whole are risk factors for post-ESD bleeding[14,15,33]. Adversely, there is also data which suggests that antithrombotic agents do not significantly increase post-ESD bleeding[26,27,29,32]. However, the types of antithrombotic agents and cessation periods differed among these studies. Each antithrombotic agent has its own mechanism and carries a different risk of bleeding. So the post-ESD bleeding risk for each agent must be analyzed individually.
Aspirin is known to be one of the most commonly administered antiplatelet agents. Initial reports demonstrated the safety of colonoscopic polypectomy in patients taking aspirin[39-41]. Similarly the rate of post-ESD bleeding does not significantly increase with the cessation of aspirin from one week before ESD[16,42]. Although available data concerning continued aspirin use is still lacking, guidelines permit ESD without aspirin cessation in patients with a high-risk of thromboembolism. Recently, Lim et al[16], Matsumura et al[30] and Sanomura et al[43] reported that the continued use of aspirin did not increase the risk of bleeding after gastric ESD. However, Cho et al[44] reported that continued aspirin use increased the risk of bleeding after gastric ESD; post-ESD bleeding occurred at a rate of 21.1% (4 out of 19 continued aspirin users). It is controversial whether the continued use of aspirin is a risk factor of post-ESD bleeding. More accumulation of data on ESD without aspirin cessation is required.
As for thienopyridine, recent guidelines recommend cessation of thienopyridine from at least 5 d before ESD, according to the data that continued use of thienopyridine increased the rate of bleeding after colon polypectomy[45]. Although data on thienopyridine monotherapy during the ESD procedure is insufficient, there are two available reports concerning gastric ESD in patients receiving dual antiplatelet therapy. Thienopyridine is principally used in dual antiplatelet therapy (DAPT) in patients undergoing implantation of drug-eluting coronary stents. Tounou et al[46] reported that DAPT markedly increased the rate of post-ESD bleeding (35.5%). Ono et al[47] reported a prospective study regarding bleeding after endoscopic procedure including polypectomy, EMR and ESD. Twenty-eight patients continuing aspirin therapy during endoscopic treatment were enrolled in this study, and 7 patients experienced major bleeding events after ESD (stomach: 6, colon: 1). Subanalysis of gastric ESD showed that all the 6 cases of post-ESD bleeding occurred after the resumption of thienopyridine derivatives[47]. These reports showed that DAPT is a significant risk factor of bleeding after ESD.
Heparin bridge therapy is commonly performed during cessation of warfarin in patients with a high risk of thromboembolism, but evidence concerning whether heparin bridge therapy can prevent thromboembolism is lacking, and in addition heparin bridge therapy is known to increase the incidence of bleeding events[48]. Two retrospective studies showed that post-ESD bleeding in patients undergoing heparin bridge therapy occurred at the rate of 23% to 38%[30,49]. Additionally, Douketis et al[50] reported a randomized trial to evaluate the risks of thromboembolism and bleeding events after operations or other invasive procedures in patients taking warfarin for chronic atrial fibrillation or flutter. This study suggested that forgoing bridging anticoagulation is non-inferior to perioperative heparin bridging for the prevention of arterial thromboembolism and decreases the risk of major bleeding[50]. Therefore it may be necessary to reconsider whether bridging is necessary for the management of patients taking warfarin.
Recently direct oral anticoagulant drugs (DOACs) have become increasingly used in clinical practice, and the association between DOACs and the risk of post-ESD bleeding needs to be assessed. However, data about DOACs is still being accumulated and is currently still insufficient. The risk of DOACs for post-ESD bleeding remains to be investigated hereafter.
There have been several reports that specimen size > 40 mm is a significant risk factor for post-ESD bleeding[9,15,27,30]. Owing to the acceptance of expanded indications for larger lesions, there have been increasingly more cases of large ESD in our practices[51-53]. The reason why larger resection causes more bleeding is simply considered to derive from the fact that more vessels would be exposed on the ulcer bases after large ESD.
Patients receiving hemodialysis are known to be prone to bleed from gastroduodenal ulcers[54]. A few studies showed hemodialysis is a risk factor for post-ESD bleeding[30,31,55,56]. Numata et al[55] reported that two ESD-related deaths occurred among hemodialysis patients in an evaluation of ESD outcomes in 63 patients with chronic kidney disease; post-ESD bleeding triggered femoral infarction in one case, and alveolar hemorrhage occurred in the other case. More careful management after ESD may be required for patients on hemodialysis because post-ESD bleeding may lead to secondary adverse events.
Two studies have also shown that long procedure time is an independent risk factor for post-ESD bleeding[31,32]. A longer procedure time was required in these studies when intraoperative bleeding was frequent and difficult to control, which might mean more vessels exist in the submucosal layer in these cases.
As for the location, it has been generally reported that the lower part of the stomach is a risk factor for post-ESD bleeding. Tsuji et al[14] and Miyahara et al[29] reported that post-ESD bleeding occurred more frequently in the lower part of the stomach than in the upper or middle part. That may be partly because more careful endoscopic hemostasis is required during the ESD procedure in the upper and middle part of the stomach where intraoperative bleeding frequently occurs, which may ultimately prevent post-ESD bleeding[13,14,29]. Although intraoperative bleeding may be associated to submucosal artery diameters, arteries of the upper and middle part of the stomach are known to be thicker in diameter than in the lower part as evaluated in human resected gastric specimens and dog models[57,58]. In addition, antral active peristalsis and bile reflux may contribute to a high incidence of post-ESD bleeding in the lower part of stomach[12,14]. Adversely, Chung et al[9] reported that the upper part of the stomach was a risk factor. They performed hemostasis on all vessels likely to bleed regardless of the location[9]. Tsuji et al[14] showed that post-ESD bleeding occurred more often when beginners performed coagulation of the ulcer floor after ESD. These discrepancies might occur due to the amount of remnant exposed vessels on the mucosal defect of ESD.
In summary, according to available evidence, DAPT and heparin bridge therapy significantly increase post-ESD bleeding, but it is unclear whether other antithrombotic agents are risk factors. In terms of other risk factors for post-ESD bleeding, large resection size would be a reliable risk factor, but there have been an insufficient number of prospective studies and there is not enough well-established data. Large-scale prospective analyses concerning this issue are essential.
Massive post-ESD bleeding occasionally leads to a severe condition that requires blood transfusion, such as hemorrhagic shock[12]. Therefore, prevention of post-ESD bleeding is imperative. According to previous studies, there are only two well-established effective methods of prevention with supportive evidence: the use of acid secretion inhibitors and preventive coagulation of the ESD-induced ulcer bed.
Acid secretion inhibitors including proton pump inhibitors (PPI) or histamine-2 receptor antagonists (H2RA) are normally used to facilitate healing of ulcers after gastric ESD. It is still unclear whether PPIs can reduce post-ESD bleeding more effectively than H2RAs although several studies have reported that PPIs may be superior to H2RAs[59-63].
Niimi et al[64] reported that 2-wk administration of PPI resulted in 80% of the transitional rate to scarring-stage ulcers at 8 wk after ESD. The study suggested 2-wk administration of a maintenance dosage of PPI may be sufficient in cases without deteriorating factors such as concomitant use of antithrombotic agents or ulcerative findings in the tumor. Further studies are required to determine optimum doses and duration of PPI administration.
Endoscopic preventive coagulation or clipping after ESD may prevent post-ESD bleeding. Takizawa et al[12] reported that post-ESD coagulation of visible vessels (PEC) prevented post-ESD bleeding (with PEC, 3.1% vs without, 7.1%, P < 0.01). Mukai et al[65] reported that PEC plus artery-selective clipping may reduce delayed bleeding after gastric ESD (PEC, 4.5% vs PEC plus artery-selective clipping, 1.3%, P = 0.17). Uedo et al[66] reported that Doppler US may be helpful to search vessels in the post-ESD ulcers.
However, repeated coagulation by hemostatic forceps can lead to coagulation syndrome or delayed perforation[67]. A patient with coagulation syndrome presents fever, abdominal pain or leukocytosis as a result of electrocoagulation injury to the gastrointestinal wall. Therefore, endoscopists should take care not to perform excessive coagulation.
It was originally reported that a second-look endoscopy (SLE) after the initial endoscopic hemostasis for peptic ulcer bleeding significantly reduces the risk of recurrent bleeding[68]. According to such findings, SLE after ESD is performed in many facilities in Japan. However, recent studies have implied that SLE has little influence on the prevention of post-ESD bleeding[28,69]. Mochizuki et al[70] reported that SLE was not routinely recommended for patients with an average bleeding risk (the incidence of postoperative bleeding of SLE group vs non-SLE groups; 5.4% vs 3.8%). On the other hand, Jung et al[71] reported the efficacy of SLE with prophylactic hemostasis.
Nishizawa et al[72] systematically evaluated the efficacy of second-look endoscopy for gastric ESD, and they concluded in their systematic review and meta-analysis that second-look endoscopy has no advantage for the prevention of post-ESD bleeding in patients without a high risk of bleeding.
As for patients at low-risk for post-ESD bleeding, it seems that SLE is not routinely recommended. However, there is insufficient data to evaluate the efficacy of SLE in patients with a high risk of post-ESD bleeding.
Even with the above mentioned preventive methods, the rate of postoperative bleeding is still approximately 4.5%[4]. Therefore, the development of a novel technique that decreases post-ESD bleeding more effectively is essential.
In order to prevent post-ESD bleeding, methods of closing or shielding the ESD-induced ulcer seem to be promising. As for the closing method, conventional clipping closure is technically difficult in cases where the mucosal defect is large. Lee et al[73] reported that mucosal closure with a detachable snare and clips supports earlier healing of ulcers after ESD. Kantsevoy et al[74] reported that endoscopic suturing closure is a feasible technique which can eliminate the need for hospitalization after the ESD procedure.
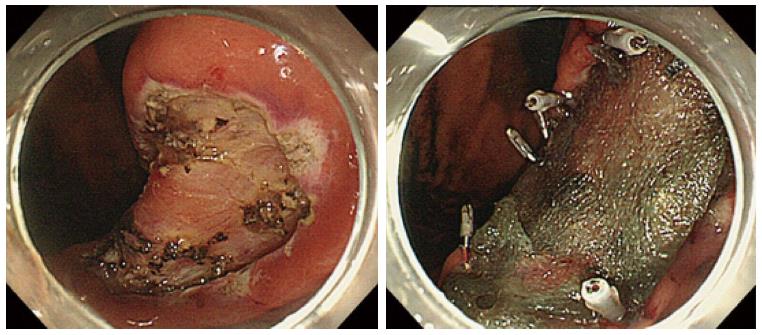
Recently, the utility of a shielding method using polyglycolic acid (PGA) sheets and fibrin glue to manage ulcers after ESD procedure has been reported. PGA sheets are widely used in the surgical field as an absorbable material to reinforce suturing. Takimoto et al[75] originally reported the efficacy of shielding a mucosal defect after duodenal ESD using PGA sheets and fibrin glue to prevent delayed perforation. Furthermore, Tsuji et al[76,77] also reported the possibility of reducing postoperative adverse events, such as post-ESD bleeding or delayed perforation (Figure 2). In addition to PGA shielding, other shielding methods have been reported. There has been a report concerning bio-sheet graft therapy for post-ESD ulcer in an animal experiment[78]. According to the study, this bio-sheet graft therapy might be effective in attenuating the degree of inflammation in the ESD-induced ulcers.
However, there has been no randomized controlled trial to investigate the efficacy of these novel methods to prevent postoperative bleeding. Therefore, further research on its efficacy is required.
Although ESD has been established as an excellent method of treatment for superficial gastrointestinal neoplasms, the prevention and management of post-ESD adverse events is an issue still to be solved. Especially, controlling bleeding after ESD should be considered one of the top priorities because its occurrence rate is relatively high and sometimes leads to a severe condition. It is imperative for all endoscopists who perform ESD to get acquainted with the risk factors of post-ESD bleeding. To date, some risk factors, such as antithrombotic drug use and large resection size, have been recognized, but optimum management of these risk factors is still to be clarified. Concerning prevention of post-ESD bleeding, PEC and PPI use are widely established as effective preventive methods, but have not been able to prevent bleeding completely. Currently there are several ongoing studies concerning novel techniques for preventing bleeding with the ultimate goal of achieving zero risk for post-ESD bleeding. Further research is required.
Manuscript source: Invited manuscript
P- Reviewer: Harada H, Imai K, Yan SL S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
| 1. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1134] [Cited by in F6Publishing: 1104] [Article Influence: 48.0] [Reference Citation Analysis (4)] |
| 2. | Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. 1999;50:560-563. [PubMed] [Cited in This Article: ] |
| 3. | Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490-4498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 443] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 4. | Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666-2677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 5. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 7. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 8. | Urabe Y, Hiyama T, Tanaka S, Yoshihara M, Arihiro K, Chayama K. Advantages of endoscopic submucosal dissection versus endoscopic oblique aspiration mucosectomy for superficial esophageal tumors. J Gastroenterol Hepatol. 2011;26:275-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 447] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 10. | Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 529] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 12. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 13. | Ichiro O, Takuji G, Hisanao H, Takeo E, Yutaka S, Takahisa M, Pradeep B, Fabian E, Daizo S, Hiroyuki O. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Digest Endosc. 2005;17:54-58. [Cited in This Article: ] |
| 14. | Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, Matsuhashi N. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913-2917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 79] [Cited by in F6Publishing: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Lim JH, Kim SG, Kim JW, Choi YJ, Kwon J, Kim JY, Lee YB, Choi J, Im JP, Kim JS. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc. 2012;75:719-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 496] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 18. | Goto O, Fujishiro M, Oda I, Kakushima N, Yamamoto Y, Tsuji Y, Ohata K, Fujiwara T, Fujiwara J, Ishii N. A multicenter survey of the management after gastric endoscopic submucosal dissection related to postoperative bleeding. Dig Dis Sci. 2012;57:435-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Isomoto H, Yamaguchi N, Minami H, Nakao K. Management of complications associated with endoscopic submucosal dissection/ endoscopic mucosal resection for esophageal cancer. Dig Endosc. 2013;25 Suppl 1:29-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Tsujii Y, Nishida T, Nishiyama O, Yamamoto K, Kawai N, Yamaguchi S, Yamada T, Yoshio T, Kitamura S, Nakamura T. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy. 2015;47:775-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 21. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 535] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 22. | Toyonaga T, Nishino E, Man-I M, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc. 2012;45:362-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Lee EJ, Lee JB, Lee SH, Kim do S, Lee DH, Lee DS, Youk EG. Endoscopic submucosal dissection for colorectal tumors--1,000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc. 2013;27:31-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 319] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 26. | Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, Noda T, Shimoda R, Sakata H, Ogata S. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45:30-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Okada K, Yamamoto Y, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2011;25:98-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Goto O, Fujishiro M, Kodashima S, Ono S, Niimi K, Hirano K, Yamamichi N, Koike K. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: a retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest Endosc. 2010;71:241-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Miyahara K, Iwakiri R, Shimoda R, Sakata Y, Fujise T, Shiraishi R, Yamaguchi K, Watanabe A, Yamaguchi D, Higuchi T. Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors: analysis of 1190 lesions in low- and high-volume centers in Saga, Japan. Digestion. 2012;86:273-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Matsumura T, Arai M, Maruoka D, Okimoto K, Minemura S, Ishigami H, Saito K, Nakagawa T, Katsuno T, Yokosuka O. Risk factors for early and delayed post-operative bleeding after endoscopic submucosal dissection of gastric neoplasms, including patients with continued use of antithrombotic agents. BMC Gastroenterol. 2014;14:172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Higashiyama M, Oka S, Tanaka S, Sanomura Y, Imagawa H, Shishido T, Yoshida S, Chayama K. Risk factors for bleeding after endoscopic submucosal dissection of gastric epithelial neoplasm. Dig Endosc. 2011;23:290-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | Takeuchi T, Ota K, Harada S, Edogawa S, Kojima Y, Tokioka S, Umegaki E, Higuchi K. The postoperative bleeding rate and its risk factors in patients on antithrombotic therapy who undergo gastric endoscopic submucosal dissection. BMC Gastroenterol. 2013;13:136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Vandvik PO, Lincoff AM, Gore JM, Gutterman DD, Sonnenberg FA, Alonso-Coello P, Akl EA, Lansberg MG, Guyatt GH, Spencer FA. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e637S-e668S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 35. | Lansberg MG, O’Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, Sonnenberg FA, Schulman S, Vandvik PO, Spencer FA. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e601S-e636S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 36. | Fujimoto K, Fujishiro M, Kato M, Higuchi K, Iwakiri R, Sakamoto C, Uchiyama S, Kashiwagi A, Ogawa H, Murakami K. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 304] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 37. | Veitch AM, Vanbiervliet G, Gershlick AH, Boustiere C, Baglin TP, Smith LA, Radaelli F, Knight E, Gralnek IM, Hassan C. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48:385-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 38. | Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 388] [Article Influence: 48.5] [Reference Citation Analysis (1)] |
| 39. | Hui AJ, Wong RM, Ching JY, Hung LC, Chung SC, Sung JJ. Risk of colonoscopic polypectomy bleeding with anticoagulants and antiplatelet agents: analysis of 1657 cases. Gastrointest Endosc. 2004;59:44-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 40. | Shiffman ML, Farrel MT, Yee YS. Risk of bleeding after endoscopic biopsy or polypectomy in patients taking aspirin or other NSAIDS. Gastrointest Endosc. 1994;40:458-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 133] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Yousfi M, Gostout CJ, Baron TH, Hernandez JL, Keate R, Fleischer DE, Sorbi D. Postpolypectomy lower gastrointestinal bleeding: potential role of aspirin. Am J Gastroenterol. 2004;99:1785-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Technical feasibility of endoscopic submucosal dissection for early gastric cancer in patients taking anti-coagulants or anti-platelet agents. Dig Liver Dis. 2009;41:725-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Sanomura Y, Oka S, Tanaka S, Numata N, Higashiyama M, Kanao H, Yoshida S, Ueno Y, Chayama K. Continued use of low-dose aspirin does not increase the risk of bleeding during or after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2014;17:489-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Cho SJ, Choi IJ, Kim CG, Lee JY, Nam BH, Kwak MH, Kim HJ, Ryu KW, Lee JH, Kim YW. Aspirin use and bleeding risk after endoscopic submucosal dissection in patients with gastric neoplasms. Endoscopy. 2012;44:114-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Singh M, Mehta N, Murthy UK, Kaul V, Arif A, Newman N. Postpolypectomy bleeding in patients undergoing colonoscopy on uninterrupted clopidogrel therapy. Gastrointest Endosc. 2010;71:998-1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Tounou S, Morita Y, Hosono T. Continuous aspirin use does not increase post-endoscopic dissection bleeding risk for gastric neoplasms in patients on antiplatelet therapy. Endosc Int Open. 2015;3:E31-E38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Ono S, Fujishiro M, Yoshida N, Doyama H, Kamoshida T, Hirai S, Kishihara T, Yamamoto Y, Sakae H, Imagawa A. Thienopyridine derivatives as risk factors for bleeding following high risk endoscopic treatments: Safe Treatment on Antiplatelets (STRAP) study. Endoscopy. 2015;47:632-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Rechenmacher SJ, Fang JC. Bridging Anticoagulation: Primum Non Nocere. J Am Coll Cardiol. 2015;66:1392-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 49. | Yoshio T, Nishida T, Kawai N, Yuguchi K, Yamada T, Yabuta T, Komori M, Yamaguchi S, Kitamura S, Iijima H. Gastric ESD under Heparin Replacement at High-Risk Patients of Thromboembolism Is Technically Feasible but Has a High Risk of Delayed Bleeding: Osaka University ESD Study Group. Gastroenterol Res Pract. 2013;2013:365830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373:823-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 695] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 51. | Peng LJ, Tian SN, Lu L, Chen H, Ouyang YY, Wu YJ. Outcome of endoscopic submucosal dissection for early gastric cancer of conventional and expanded indications: systematic review and meta-analysis. J Dig Dis. 2015;16:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Nakamura K, Honda K, Akahoshi K, Ihara E, Matsuzaka H, Sumida Y, Yoshimura D, Akiho H, Motomura Y, Iwasa T. Suitability of the expanded indication criteria for the treatment of early gastric cancer by endoscopic submucosal dissection: Japanese multicenter large-scale retrospective analysis of short- and long-term outcomes. Scand J Gastroenterol. 2015;50:413-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Song HJ, Lee GH. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 54. | Luo JC, Leu HB, Huang KW, Huang CC, Hou MC, Lin HC, Lee FY, Lee SD. Incidence of bleeding from gastroduodenal ulcers in patients with end-stage renal disease receiving hemodialysis. CMAJ. 2011;183:E1345-E1351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Numata N, Oka S, Tanaka S, Higashiyama M, Sanomura Y, Yoshida S, Arihiro K, Chayama K. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer in patients with chronic kidney disease. J Gastroenterol Hepatol. 2013;28:1632-1637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Goto O, Fujishiro M, Kodashima S, Ono S, Niimi K, Yamamichi N, Omata M. Feasibility of endoscopic submucosal dissection for patients with chronic renal failure on hemodialysis. Dig Endosc. 2010;22:45-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Hirao M, Asanuma T, Masuda K. Endoscopic resection of early gastric cancer following locally injecting hypertonic saline-epinephrine. Stomach Intest. 1988;23:399-409. [Cited in This Article: ] |
| 58. | Narimiya N, Sato H, Joki M, Odagiri M, Iwasaki M. An experimental study of submucosal vascular structure of the stomach after endoscopic mucosal resection. Gastroenterol Endosc. 1994;36:958-962. [Cited in This Article: ] |
| 59. | Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 60. | Tomita T, Kim Y, Yamasaki T, Okugawa T, Kondo T, Toyoshima F, Sakurai J, Tanaka J, Morita T, Oshima T. Prospective randomized controlled trial to compare the effects of omeprazole and famotidine in preventing delayed bleeding and promoting ulcer healing after endoscopic submucosal dissection. J Gastroenterol Hepatol. 2012;27:1441-1446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Fujishiro M, Chiu PW, Wang HP. Role of antisecretory agents for gastric endoscopic submucosal dissection. Dig Endosc. 2013;25 Suppl 1:86-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Yang Z, Wu Q, Liu Z, Wu K, Fan D. Proton pump inhibitors versus histamine-2-receptor antagonists for the management of iatrogenic gastric ulcer after endoscopic mucosal resection or endoscopic submucosal dissection: a meta-analysis of randomized trials. Digestion. 2011;84:315-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Imaeda H, Hosoe N, Suzuki H, Saito Y, Ida Y, Nakamura R, Iwao Y, Ogata H, Hibi T. Effect of lansoprazole versus roxatidine on prevention of bleeding and promotion of ulcer healing after endoscopic submucosal dissection for superficial gastric neoplasia. J Gastroenterol. 2011;46:1267-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Niimi K, Fujishiro M, Goto O, Kodashima S, Minatsuki C, Hirayama I, Mochizuki S, Ono S, Yamamichi N, Kakushima N. Prospective single-arm trial of two-week rabeprazole treatment for ulcer healing after gastric endoscopic submucosal dissection. Dig Endosc. 2012;24:110-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Mukai S, Cho S, Nakamura S, Hatano Y, Kotachi T, Shimizu A, Matsuura G, Azakami T, Takaba A, Hamada T. Postprocedural combined treatment using the coagulation plus artery-selective clipping (2C) method for the prevention of delayed bleeding after ESD. Surg Endosc. 2013;27:1292-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Uedo N, Takeuchi Y, Ishihara R, Hanaoka N, Inoue T, Kizu T, Higashino K, Iishi H, Tatsuta M, Chak A. Endoscopic Doppler US for the prevention of ulcer bleeding after endoscopic submucosal dissection for early gastric cancer: a preliminary study (with video). Gastrointest Endosc. 2010;72:444-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Hirasawa K, Sato C, Makazu M, Kaneko H, Kobayashi R, Kokawa A, Maeda S. Coagulation syndrome: Delayed perforation after colorectal endoscopic treatments. World J Gastrointest Endosc. 2015;7:1055-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 60] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding: Is a second look necessary? A meta-analysis. Gastrointest Endosc. 2003;57:62-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Kim JS, Chung MW, Chung CY, Park HC, Ryang DY, Myung DS, Cho SB, Lee WS, Joo YE. The need for second-look endoscopy to prevent delayed bleeding after endoscopic submucosal dissection for gastric neoplasms: a prospective randomized trial. Gut Liver. 2014;8:480-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Mochizuki S, Uedo N, Oda I, Kaneko K, Yamamoto Y, Yamashina T, Suzuki H, Kodashima S, Yano T, Yamamichi N. Scheduled second-look endoscopy is not recommended after endoscopic submucosal dissection for gastric neoplasms (the SAFE trial): a multicentre prospective randomised controlled non-inferiority trial. Gut. 2015;64:397-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 71. | Jung JH, Kim BJ, Choi CH, Kim JG. Second-look endoscopy with prophylactic hemostasis is still effective after endoscopic submucosal dissection for gastric neoplasm. World J Gastroenterol. 2015;21:13518-13523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 72. | Nishizawa T, Suzuki H, Kinoshita S, Goto O, Kanai T, Yahagi N. Second-look endoscopy after endoscopic submucosal dissection for gastric neoplasms. Dig Endosc. 2015;27:279-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Lee BI, Kim BW, Kim HK, Choi H, Ji JS, Hwang SM, Cho YS, Chae HS, Choi KY. Routine mucosal closure with a detachable snare and clips after endoscopic submucosal dissection for gastric epithelial neoplasms: a randomized controlled trial. Gut Liver. 2011;5:454-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Kantsevoy SV, Bitner M, Mitrakov AA, Thuluvath PJ. Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos). Gastrointest Endosc. 2014;79:503-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 75. | Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26 Suppl 2:46-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 76. | Tsuji Y, Ohata K, Gunji T, Shozushima M, Hamanaka J, Ohno A, Ito T, Yamamichi N, Fujishiro M, Matsuhashi N. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to cover wounds after colorectal endoscopic submucosal dissection (with video). Gastrointest Endosc. 2014;79:151-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Tsuji Y, Fujishiro M, Kodashima S, Ono S, Niimi K, Mochizuki S, Asada-Hirayama I, Matsuda R, Minatsuki C, Nakayama C. Polyglycolic acid sheets and fibrin glue decrease the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms (with video). Gastrointest Endosc. 2015;81:906-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Kwon CI, Kim G, Ko KH, Jung Y, Chung IK, Jeong S, Lee DH, Hong SP, Hahm KB. Bio-sheet graft therapy for artificial gastric ulcer after endoscopic submucosal dissection: an animal feasibility study. Gastrointest Endosc. 2015;81:989-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Niimi K, Fujishiro M, Kodashima S, Goto O, Ono S, Hirano K, Minatsuki C, Yamamichi N, Koike K. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010;42:723-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 80. | Oka S, Tanaka S, Kanao H, Ishikawa H, Watanabe T, Igarashi M, Saito Y, Ikematsu H, Kobayashi K, Inoue Y. Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc. 2010;22:376-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 81. | Takeuchi Y, Ohta T, Matsui F, Nagai K, Uedo N. Indication, strategy and outcomes of endoscopic submucosal dissection for colorectal neoplasm. Dig Endosc. 2012;24 Suppl 1:100-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Ono S, Tsuji Y, Fujishiro M, Kodashima S, Yamamichi N, Koike K. An effective technique for delivery of polyglycolic acid sheet after endoscopic submucosal dissection of the esophagus: the clip and pull method. Endoscopy. 2014;46 Suppl 1 UCTN:E44-E45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |