Published online Aug 7, 2016. doi: 10.3748/wjg.v22.i29.6706
Peer-review started: May 19, 2016
First decision: June 13, 2016
Revised: June 22, 2016
Accepted: July 6, 2016
Article in press: July 6, 2016
Published online: August 7, 2016
AIM: To observe the alterations in gut microbiota in high-fat diet (HFD)-induced diabetes recurrence after duodenal-jejunal bypass (DJB) in rats.
METHODS: We assigned HDF- and low-dose streptozotocin-induced diabetic rats into two major groups to receive DJB and sham operation respectively. When the DJB was completed, we used HFD to induce diabetes recurrence. Then, we grouped the DJB-operated rats by blood glucose level into the DJB-remission (DJB-RM) group and the DJB-recurrence (DJB-RC) group. At a sequence of time points after operations, we compared calorie content in the food intake (calorie intake), oral glucose tolerance test, homeostasis model assessment of insulin resistance (HOMA-IR), concentrations of glucagon-like peptide 1 (GLP-1), serum insulin, total bile acids (TBAs) and lipopolysaccharide (LPS) and alterations in colonic microbiota.
RESULTS: The relative abundance of Firmicutes in the control (58.06% ± 11.12%; P < 0.05 vs sham; P < 0.05 vs DJB-RC) and DJB-RM (55.58% ± 6.16%; P < 0.05 vs sham; P < 0.05 vs DJB-RC) groups was higher than that in the sham (29.04% ± 1.36%) and DJB-RC (27.44% ± 2.17%) groups; but the relative abundance of Bacteroidetes was lower (control group: 33.46% ± 10.52%, P < 0.05 vs sham 46.88% ± 2.34%, P < 0.05 vs DJB-RC 47.41% ± 5.67%. DJB-RM group: 34.63% ± 3.37%, P < 0.05 vs sham; P < 0.05 vs DJB-RC). Escherichia coli was higher in the sham (15.72% ± 1.67%, P < 0.05 vs control, P < 0.05 vs DJB-RM) and DJB-RC (16.42% ± 3.00%; P < 0.05 vs control; P < 0.05 vs DJB-RM) groups than in the control (3.58% ± 3.67%) and DJB-RM (4.15% ± 2.76%) groups. Improved HOMA-IR (2.82 ± 0.73, P < 0.05 vs DJB-RC 4.23 ± 0.72), increased TBAs (27803.17 ± 4673.42 ng/mL; P < 0.05 vs DJB-RC 18744.00 ± 3047.26 ng/mL) and decreased LPS (0.12 ± 0.04 ng/mL, P < 0.05 vs DJB-RC 0.19 ± 0.03 ng/mL) were observed the in DJB-RM group; however, these improvements were reversed in the DJB-RC group, with the exception of GLP-1 (DJB-RM vs DJB-RC P > 0.05).
CONCLUSION: Alterations in gut microbiota may be responsible for the diabetes remission and recurrence after DJB, possibly by influencing serum LPS and TBAs.
Core tip: To determine the alteration in gut microbiota during diabetes recurrence after the performance of duodenal-jejunal bypass (DJB), high-fat diet (HFD)-fed and low-dose streptozotocin-injected diabetic rats received DJB. We used postoperative HFD to induce diabetes recurrence. Relative abundance of Firmicutes in diabetes-recurrence rats is lower than that in diabetes-remission rats, whereas higher relative abundance of Bacteroidetes and Escherichia coli is observed in diabetes-recurrence rats. Alterations in gut microbiota may cause diabetes to reappear postoperatively by influencing levels of serum lipopolysaccharide and total bile acids, which have links with low-grade inflammation and glycolipid metabolism in diabetes.
- Citation: Zhong MW, Liu SZ, Zhang GY, Zhang X, Liu T, Hu SY. Alterations in gut microbiota during remission and recurrence of diabetes after duodenal-jejunal bypass in rats. World J Gastroenterol 2016; 22(29): 6706-6715
- URL: https://www.wjgnet.com/1007-9327/full/v22/i29/6706.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i29.6706
Bariatric surgery can provide the most significant effectiveness in treating type 2 diabetes mellitus (T2DM)[1,2], and the past decade saw a steady growth in the amount of bariatric procedures performance all over the world[3]. Although bariatric surgery offers a rapid resolution of T2DM, the long-term remission rate usually decreases over time, and several previous studies have observed that, a part of patients who achieved initial postoperative resolution, experienced T2DM again[4-7]. Several factors have been found to be closely related to diabetes recurrence, including preoperative body mass index (BMI), age, course and gravity of T2DM, percentage of excess body weight loss (%EBWL), weight regain, postoperative diet and lifestyle[8-12].
Approximately 100 trillion bacterial cells, 10 times the number of human cells, colonize the human gut[13]. According to recent data, gut microbiota can regulate host physiology and metabolism by harvesting more energy from diet, modulating lipid metabolism, regulating bile acid biosynthesis, and increasing inflammatory tone[14]. Gut microbial dysbiosis, a considered environmental factor, can regulate obesity and T2DM[15,16]. Gastric bypass, the most effective way for treating obesity and T2DM, can affect the host’s gut microbiota. A study including individuals undergoing gastric bypass surgery has reported the associated changes that occur in the abundance of specific gut microbes[17]. By the transformation of gut microbiota from mice with gastric bypass to germ-free mice, one research study demonstrated the causal link between gut microbiota and the effect of gastric bypass[18].
A previous study by our lab demonstrated an effect in deteriorating glucose tolerance of a high-fat diet (HFD) after initial improvement in diabetic rats[19]. HFD seems to be one of the primary factors in obesity and T2DM[20]. And, the diet, serving as the major force, contributes much to the formation of the composition of the gut microbiota[21-23]. Alterations in gut microbiota, furthermore, exert considerable influence on diabetes’ pathogenesis and postoperative remission. The association between gut microbiota and postoperative diabetes recurrence has not been explored so far. We also speculated that the postoperative diabetes recurrence may be involved with alterations in gut microbiota.
In our study, we performed DJB on HFD- and STZ-induced diabetic rats. Postoperatively, the HFD was used to induce diabetes recurrence. The gut microbiota, glucose profiles and serum parameters including levels of glucagon-like peptide 1 (GLP-1), insulin, total bile acids (TBAs) and lipopolysaccharide (LPS) were determined and compared between the groups.
With the condition that constant temperature is 24 to 26 °C, humidity is 50% to 60%, and light/dark alternates each 12 h, 8-wk-old Wistar rats, provided by the Laboratory Animal Center of Shandong University, were separately housed in independently ventilated cages. Then, we picked 7 from 30 male rats as the control group, which were given free access to standard chow (14% calories from fat; Shandong University Laboratory Animal Center) till the study ended. In order to induce insulin resistance, we fed the remaining rats for 4 wk with a HFD (Huafukang Biotech Company, China), which contained 40% fat as calories. A 12-h fasting period was succeeded by intraperitoneal injection with 2% streptozotocin (STZ) (Sigma, United States), at the dose of 35 mg/kg weight, so the rats reached a diabetic state. After 2 wk, 21 rats met the diabetic criterion - a blood glucose level of at least 16.7 mmol/L during oral glucose tolerance test (OGTT) - and were randomly divided into two groups receiving sham (n = 7) or DJB operation (n = 14), in which rats were fed HFD postoperatively. Twelve wk after surgery, the DJB group was subdivided into the DJB-recurrence group (DJB-RC, 5 rats, defined as having blood glucose ≥ 16.7 mmol/L during OGTT) and the DJB-remission group (DJB-RM group, 6 rats).
Body weight and calorie content in the food intake (calorie intake) were measured at baseline, 4, 8 and 12 wk postoperatively. Finally, all rats were euthanized by chloral hydrate overdose (intraperitoneal injection, 15 mL/kg, 10% chloral hydrate) for tissue collection. All animal experimental procedures involved in our study were approved by the Animal Care and Utilization Committee of Qilu Hospital of Shandong University, Jinan, China.
Fifteen days after induction of diabetes, a low-residue diet was administered to the rats in the sham group and DJB group from 48 h before surgery to 72 h after surgery. We performed DJB or sham surgery on rats under anesthesia (10% chloral hydrate at the dose of 3 mL/kg). All of the surgeries were completed within 3 d. Seventy-two postoperative hours, all rats in both groups were allowed access to the HFD and water ad libitum.
DJB: We transected the duodenum just at the site of connection with the pylorus, and 7-0 silk sutures (Ningbo Medical Needle, China) were applied in the closure of the duodenum distal end, followed by transecting jejunum 15 cm at the site of distal to the Treitz’s ligament. Then, we proceeded anastomose of the proximal end of the duodenum and distal jejunum (duodenojejunal anastomosis). Moreover, we anastomosed jejunum proximal end to the antimesenteric border of the alimentary limb 15 cm distal to the duodenojejunal anastomosis, to form an end-to-side anastomosis.
Sham: We transected the intestines at the same sites with those in enterotomies of DJB, and then carried out re-anastomosis in situ. Because of similar stress from surgery and anesthesia, the duration of the sham operation was similar to DJB.
We conducted the oral glucose tolerance test (OGTT) at several time points, including baseline and postoperative wk 4 and 12. After completion of 8-hr fasting, all rats received 1 g/kg glucose by oral gavage. Then, we estimated the levels of blood glucose at six time points (baseline, 15, 30, 60, 90 and 120 min administration) respectively.
During the OGTT at 4 and 12 wk postoperatively, we respectively gathered blood samples by the retrobulbar venous plexus approach at time points of baseline, 15, 30, 60 and 120 min after gavage with glucose, to collect serum by centrifugation (1006 ×g, 4 °C, 15 min) and stored at -80 °C for further measurement. Concentrations of insulin and GLP-1 in serum and LPS in fasting serum were tested by enzyme-linked immune sorbent assay (ELISA) kits from Millipore (United States), kits from Uscn Life Science Inc. (China), and a rat LPS ELISA kit from Bio-Swamp (China) respectively. Levels of TBAs in fasting serum were detected by a Hitachi automatic biochemical analyzer (Japan).
The calculations of homeostasis model assessment of insulin resistance (HOMA-IR) at 4 and 12 wk postoperatively were performed in order to evaluate insulin resistance, using this formula: HOMA-IR=fasting insulin (mIU/L) × fasting glucose (mmol/L)/22.5[24].
Because host metabolism can be influenced by the biological activity of microbiota in the colon, we focused our study on the colonic microbiota. At 12 wk after surgery, all rats were narcotized with 10% chloral hydrate (3 mL/kg). A 3-cm segment of colon was ligatured and removed from the enterocoelia. The colon was incised longitudinally, and then we gathered the colonic contents and stored them at -80 °C. All operations were performed under aseptic conditions. Genomic DNA of colonic microbiota was isolated based on the protocol of an E.Z.N.A. Soil DNA kit (Omega, United States). The method to amplify the V4 region of microbial 16S rDNA g by PCR has been reported previously[25]. We used an Illumina MiSeq platform (BGI Technology, China) to sequence the amplified V4 region. The raw data were filtered to eliminate the adapter pollution and low quality reads so as to obtain clean reads. Then, paired-end reads with overlap were merged to tags, which were clustered, at 97% sequence similarity, to operational taxonomic units (OTUs). By using the Ribosomal Database Project (RDP) Na, Bayesian Classifier v.2.2, we assigned taxonomic ranks to OTU representative sequence. At last, the different species screening tests were analyzed based on OTU and taxonomic ranks.
All quantitative data were presented as mean ± SD. By the use of trapezoidal integration, we calculated the area under the curves for OGTT (AUCOGTT). Body weight, calorie intake, AUCOGTT, HOMA-IR, TBAs and LPS were evaluated with the use of one-way analysis of variance (ANOVA) followed by Bonferroni post hoc comparison. All the data of the insulin and total GLP-1 concentrations were analyzed by mixed model ANOVA followed by Bonferroni post hoc comparison after glucose administered by gavage to rats. All statistical calculations were processed with the use of SPSS version 19.0 (IBM, United States), at an alpha level of 0.05.
The information of the 16S rDNA-based study involving gut microbiota was analyzed by BGI Technology. Package “ade4” of software R (v3.0.3) was used in principal component analysis (PCA). Heatmap was generated using the package “gplots” of software R (v3.0.3). The relative abundance of specific microbes between groups was analyzed by the Wilcoxon test.
When the study ended 12 wk after surgery, 7, 7, 5 and 6 rats were alive in the control, sham, DJB-RC and DJB-RM groups, respectively. Three rats died of anastomotic leak after DJB.
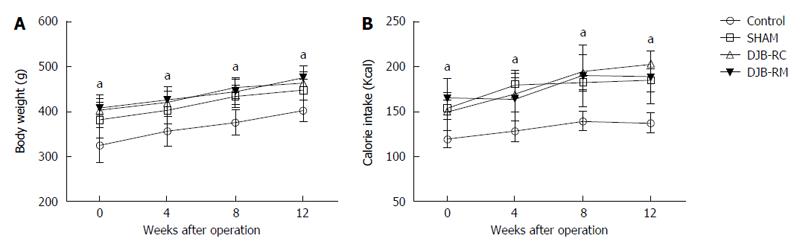
Compared with rats in the other three groups at any time points, the rats in the control group had markedly lower body weight and calorie intake (P < 0.05) (Figure 1A and B). The differences of other groups were not significant.
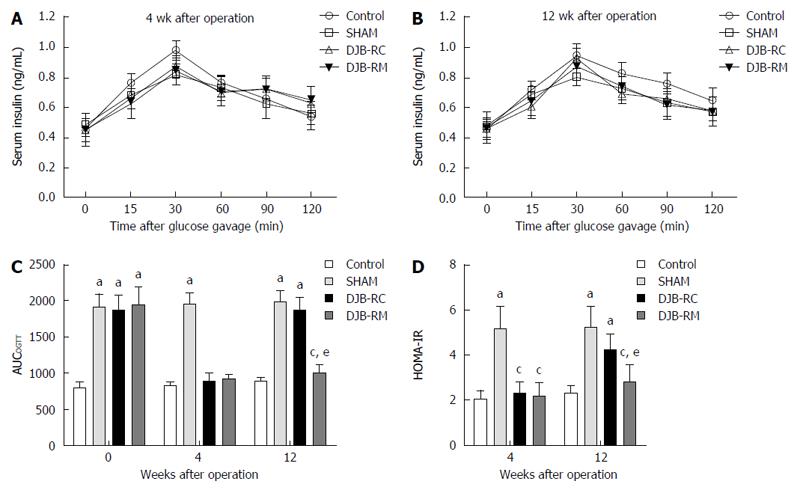
As Figure 2C shows, compared with the other three groups, preoperative AUCOGTT of the control group (797.04 ± 81.01) was statistically lower (P < 0.05 vs sham 1913.46 ± 184.55; P < 0.05 vs DJB-RC 1869.90 ± 216.37; P < 0.05 vs DJB-RM 1935.88 ± 250.73), with no differences among the other three groups. The postoperative AUCOGTT of the control group remained unchanged. Compared with the sham group (4 wk after surgery: 1935.88 ± 250.73; 12 wk after surgery: 1985.57 ± 152.56), both the DJB-RM (4 wk after surgery: 920.88 ± 62.90, P < 0.05 vs sham; 12 wk after surgery: 1009.86 ± 119.90, P < 0.05 vs sham) and control (4 wk after surgery: 827.86 ± 63.83,P < 0.05 vs sham; 12 wk after surgery: 895.39 ± 44.80, P < 0.05 vs sham) groups showed lower AUCOGTT at all postoperative time points, indicating a deterioration in diabetes in sham rats. Notably, the AUCOGTT in the DJB-RC group (890.85 ± 114.41) was comparable to that in the DJB-RM group (920.88 ± 62.90, P > 0.05 vs DJB-RC) 4 wk postoperatively, which was higher when compared with the DJB-RM group 12 wk postoperatively (P < 0.05 DJB-RC vs DJB-RM, 1876.20 ± 178.48 vs 1009.86 ± 119.90), demonstrating that the improved glucose tolerance in the DJB-RC group was reversed.
Figure 2A and B shows the postoperative curves of serum insulin levels during the OGTT. It can be seen that no statistically difference was observed in serum insulin between the groups 4 wk and 12 wk postoperatively.
Postoperative HOMA-IR is shown in Figure 2D. Four weeks after surgery, compared with the other three groups, HOMA-IR in the sham group (5.18 ± 0.98) was higher (P < 0.05 vs control 2.08 ± 0.38; P < 0.05 vs DJB-RC 2.30 ± 0.46; P < 0.05 vs DJB-RM, 2.17 ± 0.60), and no difference was observed among the control group, DJB-RC group and DJB-RM group. Consistent with the results of OGTT, 12 wk after surgery, the DJB-RC group (4.23 ± 0.72) exhibited higher HOMA-IR values than the DJB-RM group (2.82 ± 0.73, P < 0.05 vs DJB-RC), indicating re-impaired insulin sensitivity in the DJB-RC group 12 wk after surgery.
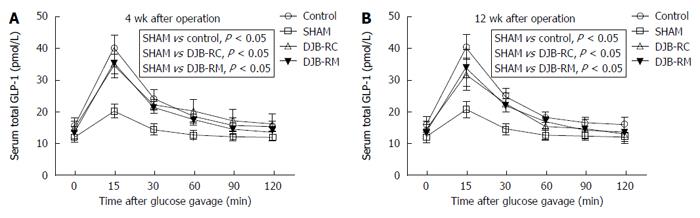
At both 4 wk (Figure 3A) and 12 wk (Figure 3B) after surgery, total serum GLP-1 secretion was comparable among the control group, DJB-RM group and DJB-RC group (P > 0.05), and rats in the sham group showed a lower total serum GLP-1 secretion (P < 0.05 vs control; P < 0.05 vs DJB-RC; P < 0.05 vs DJB-RM).
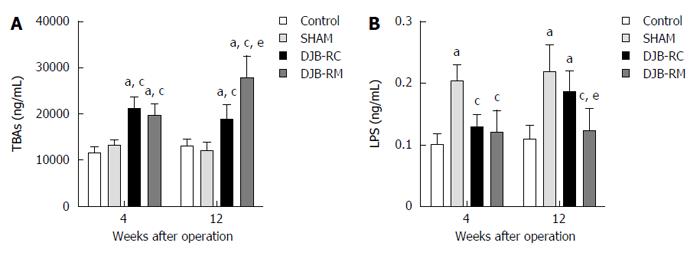
As shown in Figure 4A, at both 4 wk and 12 wk after surgery, TBAs levels were higher in the DJB-RC (4 wk after surgery: 21161.60 ± 2550.10 ng/mL, P < 0.05 vs control, P < 0.05 vs sham; 12 wk after surgery: 18744.00 ± 3047.26 ng/mL, P < 0.05 vs control, P < 0.05 vs sham) and DJB-RM (4 wk after surgery: 19543.00 ± 2639.35 ng/mL, P < 0.05 vs control, P < 0.05 vs sham; 12 wk after surgery: 27803.17 ± 4673.42 ng/mL, P < 0.05 vs control, P < 0.05 vs sham) groups than in the control (4 wk after surgery: 11608.00 ± 1248.10 ng/mL; 12 wk after surgery: 13001.86 ± 1613.55 ng/mL) and sham (4 wk after surgery: 13190.14 ± 1237.38 ng/mL; 12 wk after surgery: 12064.86 ± 1809.36 ng/mL) groups. Notably, at 12 wk postoperatively, rats in the DJB-RM group showed higher TBAs than rats in the DJB-RC group (P < 0.05).
As shown in Figure 4B, 4 wk after surgery, levels of fasting serum LPS in the sham group (0.20 ± 0.03 ng/mL) were higher compared with the other three groups (P < 0.05 vs control 0.10 ± 0.02 ng/mL; P < 0.05 vs DJB-RC 0.13 ± 0.02 ng/mL; P < 0.05 vs DJB-RM, 0.12 ± 0.04 ng/mL). Twelve weeks after surgery, the sham (0.22 ± 0.04 ng/mL) and DJB-RC (0.19 ± 0.03 ng/mL) groups exhibited similar levels of LPS, but higher levels than the control group (0.11 ± 0.02 ng/mL; P < 0.05 vs sham; P < 0.05 vs DJB-RC) and the DJB-RM (0.12 ± 0.04 ng/mL; P < 0.05 vs sham; P < 0.05 vs DJB-RC) group.
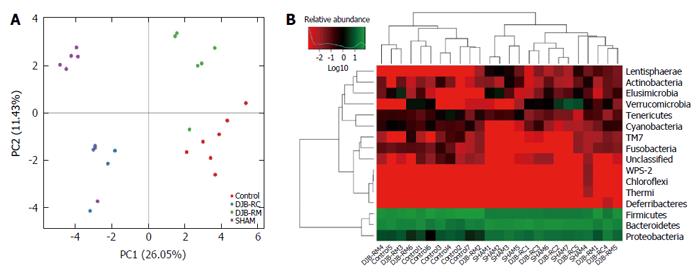
As demonstrated by PCA (Figure 5A), the gut microbiota showed marked changes in the four groups, which indicated a relative-centralized tendency intra-group and a relative-dispersed distribution inter-group. These tendencies were also observed in the Heatmap analysis at the phylum level (Figure 5B).
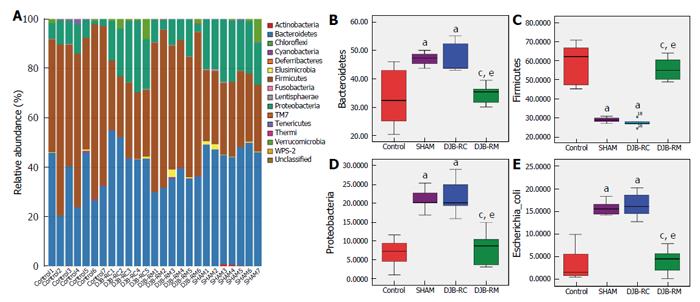
The taxonomic composition distribution histograms, at the phylum level, in each group of rats are shown in Figure 6A. Bacteroidetes were the predominant gut microbes in the sham (46.88% ± 2.34%) and DJB-RC (47.41% ± 5.67%) groups (Figure 6B), while Firmicutes were predominant in the control (58.06% ± 11.12%) and DJB-RM groups (55.58% ± 6.16%; Figure 6C). The relative abundance of Proteobacteria was higher in the sham (24.14% ± 2.89%) and DJB-RC (21.83% ± 5.09%) groups than in the control group (6.86% ± 3.79%; P < 0.05 vs sham; P < 0.05 vs DJB-RC) and the DJB-RM group (8.31% ± 4.39%; P < 0.05 vs sham; P < 0.05 vs DJB-RC; Figure 6D).
It is noteworthy that although resolution at the species level was low, the relative abundance of Escherichia coli (E. coli) in the sham group (15.72% ± 1.67%) and DJB-RC group (16.42% ± 3.00%) was remarkably higher than that in the control group (3.58% ± 3.67%; P < 0.05 vs sham; P < 0.05 vs DJB-RC) and the DJB-RM group (4.15% ± 2.76%; P < 0.05 vs sham; P < 0.05 vs DJB-RC; Figure 6E).
With the understanding of bariatric surgery developing, more and more clinicians and patients consider it as their first choice of treating obesity and T2DM, during which the procedure of Roux-en-Y gastric bypass (RYGB) is performed most frequently[3]. Bariatric surgery achieves a higher diabetes remission rate than non-surgical methods in treating diabetes[2,26]. However, the recurrence of diabetes after initial remission should not be ignored. Due to limitations in clinical research, we established an animal model to investigate the mechanisms of diabetes recurrence after surgery. Our research group previously reported that the re-impairment of insulin sensitivity was a major factor of diabetes postoperative recurrence in an animal study[19]. The HFD, a widely used insulin resistance inducer in rats, was adopted in the present and in our previous studies to reverse the improvement in diabetes[19,27]. In the model of diabetes remission and recurrence, we hoped to delineate the major mechanisms of diabetes postoperative recurrence.
In this study, statistically significant differences in body weight were not seen among the sham, DJB-RC and DJB-RM groups. Furthermore, the body weight of all DJB rats increased after surgery. Our study including the DJB model was devised to estimate whether the bypass surgery produced weight-independent anti-diabetic effects, and then the effects were proven[28]. This indicated that the remission and recurrence of diabetes after surgery were independent of body weight, which was consistent with findings from our previous studies and with the common view that the rapid anti-diabetic effect postoperatively has nothing to do with weight loss[29,30]. Some clinical trials have reported that the diabetes recurrence was linked to inadequate weight loss and weight regain[1,31], which contradicted the results obtained in our study.
T2DM is a metabolic disease featuring insulin resistance and functional loss of pancreatic beta-cells[32]. The present study has observed that impaired insulin secretion during the OGTT did not increase after surgery, demonstrating that the remission of diabetes was the result of improved insulin sensitivity represented by a lower HOMA-IR, which was in accordance with our previous study[33]. Taking into account that insulin resistance and function loss of pancreatic beta-cells could lead to T2DM, and compared with DJB-RM rats, DJB-RC rats exhibited comparable secretion of insulin after gavage and re-impaired insulin sensitivity represented by a higher HOMA-IR at the end of this study, we concluded that the recurrence of diabetes after initial remission in DJB rats was due to the re-deterioration of improved insulin sensitivity. Interestingly, both DJB-RC and DJB-RM rats experienced the same pre- and post-operative management and procedures; these rats, however, showed opposite glucose profiles and different insulin sensitivity. To analyze the intrinsic mechanisms involved in the re-deterioration of insulin sensitivity, factors related to insulin resistance, including gut microbiota, serum TBAs, LPS and GLP-1, were measured.
The gut microbiota, known as an environmental factor, can affect obesity and diabetes, and it can take effect between the host genotype and diet, responsible for the metabolic process of host glucose and lipid being modulated[14]. Larsen et al[34] recently reported that gut microbial composition was related to T2DM, and observed that, compared with non-diabetic people, Firmicutes in T2DM patients was significantly decreased. Moreover, a positive correlation existed between the Bacteroidetes-to-Firmicutes ratio and the plasma glucose level, rather than existing between the ratio and BMI. The present study has observed that, compared with control group, diabetic rats in sham groups offered fewer Firmicutes and more Bacteroidetes in terms of relative abundance.
After surgery, Firmicutes, compared with sham rats, increased remarkably in DJB-RM rats, whereas Bacteroidetes decreased, and these results were consistent with that reported by Ryan et al[35]. Compared with DJB-RM rats, DJB-RC rats showed the reverse microbial composition in terms of relative abundance of Firmicutes and Bacteroidetes; the trend was similar to that in sham rats. Based on the above findings, a conclusion could be made that there was a close connection between the altered Firmicutes-to-Bacteroidetes and diabetes’ postoperative remission and recurrence.
Except for Firmicutes and Bacteroidetes, compared with the control group and DJB-RM group, the sham group and DJB-RC group had greater relative abundance of Proteobacteria. Further species annotation indicated that the difference in Proteobacteria was due to alterations in E. coli. In addition, serum LPS levels showed the same tendency as the relative abundance of E. coli, where sham and DJB-RC rats had higher serum LPS than control and DJB-RM rats. LPS, a structural material in the cell wall of E. coli is a low-grade inflammation and insulin resistance inducer. As a result of chronic infusion of LPS through 4 wk, the chow-fed mice demonstrated increased adiposity and infiltration of macrophages in the adipose tissue, and inflammation and insulin resistance developed in liver[36]. According to the evidence shown above, we surmised that the alteration in E. coli may be a major factor in triggering postoperative diabetes by influencing serum LPS levels. However, our study only observed a similar trend of LPS to the trend of the relative abundance of E. coli. The alterations in LPS may be the result of combined effects of gut microbiota, gut barrier function and host immunity. We could not demonstrate the exact causal relationship between LPS and E. coli, which needs to be verified in further studies.
Gut microbiota exerts a role of regulation in synthetizing bile acid synthesis and production[37-40]. Bile acids can convey signaling information and regulate metabolic process of lipid, glucose and energy by farnesoid X receptor (FXR) and G-protein-coupled receptor 5 (TGR5)[41]. Our study showed that fasting serum TBAs levels increased 4 wk after surgery in all DJB rats, which showed no difference between the control and sham groups. Similar changes were observed by other researchers and in our previous studies[42,43]. When the study ended 12 wk after surgery, the fasting serum TBAs levels in DJB-RC rats decreased and were lower than those in DJB-RM rats, which was consistent with changes in insulin sensitivity as assessed by HOMA-IR. These results suggested that the changes in TBAs may be due to other factors influencing the remission and recurrence of diabetes after DJB.
GLP-1 is one of the most significant gut hormones, the source of which is L cells mostly existing in the epithelium of distal ileum and colon[44]. GLP-1 functions as a regulator of glucose homeostasis through the improvement of insulin secretion, inhibition of glucagon secretion and apoptosis of beta-cells, and promotion of proliferation of beta-cells[45]. Mounting evidence has confirmed that postprandial GLP-1 secretion would be enhanced after DJB[46], which is reconfirmed by our study. No statistically significant difference, however, between the DJB-RC group and DJB-RM group, indicated that GLP-1 was not related to the recurrence of diabetes, and enhancement of GLP-1 secretion after surgery partially contributed to diabetes remission.
There are some limitations in this research. First, the gut microbiota is a dynamically changing process, which is distributed throughout the entire digestive tract. Based on measurements of the gut microbiota composition in different segments and at different time points, the function of gut microbiota after surgery could be well explained. Second, this study mainly focused on the alterations in gut microbiota during the remission and recurrence of diabetes after surgery, and LPS and TBAs were examined to help understand the role of microbes in the changes of glucose tolerance. Further studies on the mechanisms of diabetes remission and recurrence with alterations in gut microbiota are anticipated. Third, assessment of calorie intake in our study did not show any difference among the sham, DJB-RC and DJB-RM groups. However, it is a pity that the calorie content in feces was not measured, and as a consequence the calories absorbed from food intake could not be calculated.
In conclusion, a postoperative HFD can re-exacerbate insulin resistance and induce recurrence of diabetes after initial remission in DJB-operated rats. Alterations in gut microbiota may be responsible for the recurrence of diabetes after DJB, possibly by influencing serum LPS and TBAs.
Bariatric surgery can contribute to remission of type 2 diabetes mellitus. Some patients, however, experience diabetes again postoperatively. Several factors have been found to be closely related to diabetes recurrence, including percentage of excess body weight loss, body mass index before operation, age, duration and severity of diabetes, weight regain, postoperative diet and lifestyle. The intrinsic mechanism of diabetes postoperative recurrence, however, remains unclear.
Not only can gut microbiota modulate host metabolism, but it also exerts an influence on diabetes postoperative remission. Alterations in gut microbiota after bariatric surgery may be responsible for diabetes remission possibly by influencing serum levels of lipopolysaccharide (LPS) and total bile acids (TBAs).
This study created the high-fat diet-induced rat models of diabetes recurrence after duodenal-jejunal bypass (DJB) and determined the alterations in gut microbiota during diabetes recurrence after DJB. The rats with diabetes recurrence presented the phenomena with a reduced relative abundance of Firmicutes and an increased Bacteroidetes and Escherichia coli. Alterations in gut microbiota may be responsible for the diabetes postoperative recurrence, possibly by influencing serum LPS and TBAs.
The findings in this study could enable investigators to focus more on the link between gut microbiota and diabetes postoperative recurrence. A new design of therapeutic interventions aiming to target gut microbiota may prevent diabetes postoperative recurrence.
DJB, serving as an experimental procedure, was devised to estimate the anti-diabetic effects independent from weight related to Roux-en-Y gastric surgery, which is the most effective treatment for diabetes.
This is a very well designed, performed and written experimental study for investigation of the role of alterations in gut microbiota in the pathogenesis and remission of type 2 diabetes after bariatric surgery. For investigation of this aim the authors created and used a rat model of diabetes recurrence after DJB and compared the results obtained in diabetes rats induced by high-fat diet after bypass and in the group with sham operation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chaudhury A, Mayer RJ, Vorobjova T S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH
| 1. | Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, Kennedy L, Kashyap SR, Kirwan JP, Rogula T, Kroh M. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258:628-36; discussion 636-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 364] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 2. | Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, Bouchard C, Carlsson B, Karason K, Lönroth H, Näslund I, Sjöström E, Taube M, Wedel H, Svensson PA, Sjöholm K, Carlsson LM. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297-2304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 680] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 3. | Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25:1822-1832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1090] [Cited by in F6Publishing: 1053] [Article Influence: 117.0] [Reference Citation Analysis (1)] |
| 4. | Kashyap SR, Schauer P. Clinical considerations for the management of residual diabetes following bariatric surgery. Diabetes Obes Metab. 2012;14:773-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Jiménez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A, Vidal J. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256:1023-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Campos JM, Lins DC, Silva LB, Araujo-Junior JG, Zeve JL, Ferraz ÁA. Metabolic surgery, weight regain and diabetes re-emergence. Arq Bras Cir Dig. 2013;26 Suppl 1:57-62. [PubMed] [Cited in This Article: ] |
| 7. | Yamaguchi CM, Faintuch J, Hayashi SY, Faintuch JJ, Cecconello I. Refractory and new-onset diabetes more than 5 years after gastric bypass for morbid obesity. Surg Endosc. 2012;26:2843-2847. [PubMed] [Cited in This Article: ] |
| 8. | Chikunguwo SM, Wolfe LG, Dodson P, Meador JG, Baugh N, Clore JN, Kellum JM, Maher JW. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | DiGiorgi M, Rosen DJ, Choi JJ, Milone L, Schrope B, Olivero-Rivera L, Restuccia N, Yuen S, Fisk M, Inabnet WB. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6:249-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Lee WJ, Ser KH, Chong K, Lee YC, Chen SC, Tsou JJ, Chen JC, Chen CM. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147:664-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C; Endocrine Society. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95:4823-4843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 12. | Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 533] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 13. | Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804-810. [PubMed] [Cited in This Article: ] |
| 14. | Greiner T, Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22:117-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 15. | Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11:639-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 573] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 16. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [PubMed] [Cited in This Article: ] |
| 17. | Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1323] [Cited by in F6Publishing: 1320] [Article Influence: 88.0] [Reference Citation Analysis (1)] |
| 18. | Liou AP, Paziuk M, Luevano JM, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 671] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 19. | Liu SZ, Sun D, Zhang GY, Wang L, Liu T, Sun Y, Li MX, Hu SY. A high-fat diet reverses improvement in glucose tolerance induced by duodenal-jejunal bypass in type 2 diabetic rats. Chin Med J (Engl). 2012;125:912-919. [PubMed] [Cited in This Article: ] |
| 20. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [PubMed] [Cited in This Article: ] |
| 21. | Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 768] [Article Influence: 51.2] [Reference Citation Analysis (1)] |
| 22. | Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, Pan C, Civelek M, Rau CD, Bennett BJ. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17:141-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 23. | Zhang C, Zhang M, Pang X, Zhao Y, Wang L, Zhao L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012;6:1848-1857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 333] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 24. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [PubMed] [Cited in This Article: ] |
| 25. | Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621-1624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5437] [Cited by in F6Publishing: 5111] [Article Influence: 425.9] [Reference Citation Analysis (0)] |
| 26. | Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 811] [Cited by in F6Publishing: 849] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 27. | Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390-1394. [PubMed] [Cited in This Article: ] |
| 28. | Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1-11. [PubMed] [Cited in This Article: ] |
| 29. | Liu S, Zhang G, Wang L, Sun D, Chen W, Yan Z, Sun Y, Hu S. The entire small intestine mediates the changes in glucose homeostasis after intestinal surgery in Goto-Kakizaki rats. Ann Surg. 2012;256:1049-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Breen DM, Yue JT, Rasmussen BA, Kokorovic A, Cheung GW, Lam TK. Duodenal PKC-δ and cholecystokinin signaling axis regulates glucose production. Diabetes. 2011;60:3148-3153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248-256.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1816] [Cited by in F6Publishing: 1664] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 32. | Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998;19:491-503. [PubMed] [Cited in This Article: ] |
| 33. | Han H, Hu C, Wang L, Zhang G, Liu S, Li F, Sun D, Hu S. Duodenal-jejunal bypass surgery suppresses hepatic de novo lipogenesis and alleviates liver fat accumulation in a diabetic rat model. Obes Surg. 2014;24:2152-2160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1783] [Cited by in F6Publishing: 1889] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 35. | Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 704] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 36. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [PubMed] [Cited in This Article: ] |
| 37. | Claus SP, Tsang TM, Wang Y, Cloarec O, Skordi E, Martin FP, Rezzi S, Ross A, Kochhar S, Holmes E. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol. 2008;4:219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 251] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 38. | Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4523-4530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 502] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 39. | Madsen D, Beaver M, Chang L, Bruckner-Kardoss E, Wostmann B. Analysis of bile acids in conventional and germfree rats. J Lipid Res. 1976;17:107-111. [PubMed] [Cited in This Article: ] |
| 40. | Wostmann BS. Intestinal bile acids and cholesterol absorption in the germfree rat. J Nutr. 1973;103:982-990. [PubMed] [Cited in This Article: ] |
| 41. | Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 510] [Cited by in F6Publishing: 598] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 42. | Sachdev S, Wang Q, Billington C, Connett J, Ahmed L, Inabnet W, Chua S, Ikramuddin S, Korner J. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes Surg. 2016;26:957-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 43. | Han H, Wang L, Du H, Jiang J, Hu C, Zhang G, Liu S, Zhang X, Liu T, Hu S. Expedited Biliopancreatic Juice Flow to the Distal Gut Benefits the Diabetes Control After Duodenal-Jejunal Bypass. Obes Surg. 2015;25:1802-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [PubMed] [Cited in This Article: ] |
| 45. | Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24-32. [PubMed] [Cited in This Article: ] |
| 46. | Rhee NA, Vilsbøll T, Knop FK. Current evidence for a role of GLP-1 in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes Obes Metab. 2012;14:291-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |