Published online Oct 21, 2016. doi: 10.3748/wjg.v22.i39.8812
Peer-review started: May 5, 2016
First decision: May 27, 2016
Revised: June 30, 2016
Accepted: July 31, 2016
Article in press: July 31, 2016
Published online: October 21, 2016
To establish a threshold value for liver fat content between healthy children and those with non-alcoholic fatty liver disease (NAFLD) by using magnetic resonance imaging (MRI), with liver biopsy serving as a reference standard.
The study was approved by the local ethics committee, and written informed consent was obtained from all participants and their legal guardians before the study began. Twenty-seven children with NAFLD underwent liver biopsy to assess the presence of nonalcoholic steatohepatitis. The assessment of liver fat fraction was performed using MRI, with a high field magnet and 2D gradient-echo and multiple-echo T1-weighted sequence with low flip angle and single-voxel point-resolved ¹H MR-Spectroscopy (¹H-MRS), corrected for T1 and T2* decays. Receiver operating characteristic curve analysis was used to determine the best cut-off value. Lin coefficient test was used to evaluate the correlation between histology, MRS and MRI-PDFF. A Mann-Whitney U-test and multivariate analysis were performed to analyze the continuous variables.
According to MRS, the threshold value between healthy children and those with NAFLD is 6%; using MRI-PDFF, a cut-off value of 3.5% is suggested. The Lin analysis revealed a good fit between the histology and MRS as well as MRI-PDFF.
MRS is an accurate and precise method for detecting NAFLD in children.
Core tip: Differentiating normal from pathologic liver fat storage in children could depend on technical measurements. Using MR-spectroscopy, a cut-off value of 6% demonstrates the best diagnostic performance, otherwise magnetic resonance imaging (MRI)-PDFF cut-off value of 3.5% better discriminates normal weight from obese children. It is confirmed that MRS is an accurate and precise method for detecting non-alcoholic fatty liver disease in children. However, MRI-PDFF- is a feasible alternative to MRS for quantifying liver steatosis.
- Citation: Di Martino M, Pacifico L, Bezzi M, Di Miscio R, Sacconi B, Chiesa C, Catalano C. Comparison of magnetic resonance spectroscopy, proton density fat fraction and histological analysis in the quantification of liver steatosis in children and adolescents. World J Gastroenterol 2016; 22(39): 8812-8819
- URL: https://www.wjgnet.com/1007-9327/full/v22/i39/8812.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i39.8812
Non-alcoholic fatty liver disease (NAFLD) is emerging as a leading cause of chronic liver disease in children and adolescents, with a prevalence in the general population ranging from 3%-10% in normal weight children and 80% in obese children[1-2]. Children with NAFLD are usually asymptomatic and garner clinical attention because of elevated liver enzymes or fatty liver being observed during an ultrasound examination. The measurement of liver enzymes alone is not sufficient for accurate fatty liver screening in overweight children because enzymatic abnormalities correlates poorly or not at all with early steatosis[3,4]. At present, liver biopsy represents the reference standard for diagnosing liver steatosis, although the drawbacks of this invasive technique are well known. It is associated with morbidity and mortality, it has sampling errors, and it is not appropriate for screening, longitudinal monitoring, or evaluating treatment response[4]. Among the currently available imaging modalities, magnetic resonance imaging (MRI) and ¹H MR spectroscopy (MRS) are the most reproducible, safe, and accurate imaging techniques that can be used in clinical trials and epidemiologic studies[5]. However, MRS has limited clinical applicability or availability because it requires sophisticated post-processing methods, and not every MRI is routinely equipped with MRS capabilities. Recent improvements in MRI can provide the magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF), which is a novel biomarker that has demonstrated robust correlation and equivalency with MRS[6-10]. In addition, MRI-PDFF allows fat mapping of the entire liver, and it can be used with any clinical MRI platform, whereas MRS measures fat biochemically in small regions of interest (ROIs). To the best of our knowledge, few studies have used MRS and histology to investigate liver fat content in adolescents[11-14]. A recently published paper suggested similar fat-storage between overweight children and adults, and the study proposed the same cut-off value for normal and pathologic storage[15]. The aim of our study was to validate a cut-off value for a pediatric population and to correlate the data with laboratory/chemistry results and subcutaneous and visceral adipose tissue (SAT and VAT) findings.
The study has been approved by the ethics committee, and written informed consent was obtained from all participants and their legal guardians before the study began. From October 2013 to December 2014, 93 Caucasian obese children and adolescents [body mass index (BMI) above the 95th percentile for age and gender] were referred to the Hepatology Outpatient Unit of the Department of Pediatrics to confirm or rule out the presence of NALFD. All enrolled subjects underwent the following measurements: fast blood samples [glucose, cholesterol, triglycerides, alanine aminotransferase (ALT), aspartate aminotransferase (AST), insulin and and γ-glutamyl transferase (γ-GT)] and BMI. They also underwent MRI to quantify liver steatosis and evaluate SAT and VAT. Five children were excluded because their data imaging was not suitable for post-processing due to several motion artifacts. The study population included 27 patients (16 males and 11 females; mean age, 13 years; range, 9-18 years) who were scheduled for liver biopsy to assess the presence of nonalcoholic steatohepatitis (NASH) or other liver diseases. Liver biopsy was performed within two weeks of the MR examination to avoid any bias, such as diet modification. An age- and sex-matched control group of 27 healthy Caucasian children, who also had blood chemistry results available, were recruited (11 males and 16 females; mean age, 12 years; range 8-18 years).
MRI exams were using a 3T magnet system (GE Discovery 750; General Electric Healthcare, Milwaukee, WI, United States), with a peak gradient amplitude of 50 mT/m and a 200 μsec time to peak. An eight-element body torso-array coil system was used. Before the spectroscopy acquisition, a T2-weighted image in the coronal plane (TR 1300, TE 125, FA 90°, slice thickness 6 mm, matrix 288 × 192) and a T1-weighted axial image (TR 4 ms/TE 1 ms, FA 60°, slice thickness 6 mm, matrix 288 × 192) were acquired. To quantify the hepatic fat fraction (HFF), an axial breath-hold low-flip angle, T1-weighted, 2D multiple-echo, spoiled gradient-echo (GRE) sequence (TR5.1/TE from 0.8 to 3.8, flip angle 5°, field of view 33 cm; section thickness, 10 mm; intersection gap, 0, matrix 128 × 128, acquisition time 2 × 17 s) was used was used. MRS was performed using one 20 mm × 20 mm × 20 mm voxels placed in segment VI-VII (as close as possible to the site of liver biopsy) and avoiding the artifact, major blood vessels and biliary ducts. All spectra were obtained in the stimulated echo acquisition mode (STEAM, TR 4000 ms), using a breath hold sequence with an acquisition time of approximately 24 s. Field homogeneity was automatically adjusted for each voxel. The T2 relaxation times of both metabolites were determined from their peak amplitudes at each echo time using an exponential least-squares fitting algorithm, and saturation bands were used[8]. For the quantification of subcutaneous and visceral adipose tissue (VAT and SAT), a 3D GRE T1-weighted sequence on an axial plane (TR4.1, TE 1.1, flip angle 15°, matrix 320 × 192, section thickness 6 mm reconstructed 3 mm, intersection gap 0) was acquired using the IDEAL imaging and Dixon method, which enabled the separation between water and fat components using the chemical shift MR technique.
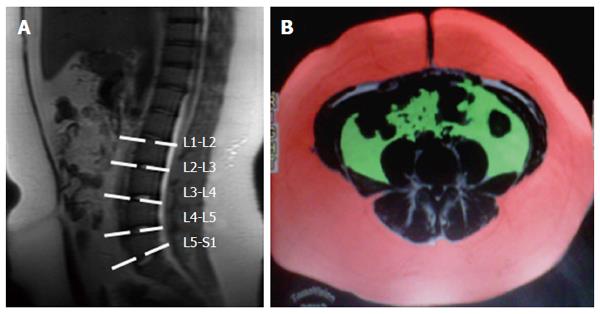
Images from multiple-echo GRE sequencing were subsequently analyzed with software provided by the manufacturer (Functool, GE Healthcare, Milwaukee, WI, United States); this procedure has been previously published[16]. A ROI measuring 2-3 cm in diameter was drawn at the same site as the voxel used for 1H-MRS to avoid extrahepatic fat and large vessels. Magnetic resonance spectra were reconstructed on a dedicated workstation using the SAGE Dev2 0017.1 software (General Electric Healthcare, Milwaukee, WI, United States). Raw data were zero-filled once, and no filter was used. Spectra were referenced to residual water and the dominant methylene lipid (-CH3 and -CH2) peak at δ 4.8-5.2 and δ 0.9-1.1 and 1.3-1.6 and 2.1-2.3, respectively. The fat fraction percentage (FF) was defined as follows: FF = FA/(FA + WA) x 100, where FA is the area under the fat peak and WA is the area under the water peak. The fat-only dataset from the T1-weighted sequence was transferred to a personal computer and analyzed using commercially available software (Slice-O-Matic; Tomovision Inc, Montreal Canada), the procedure for which has been described elsewhere[17,18]. Briefly, SAT and VAT were calculated from 5 images extending from 5 cm below L4-L5 to 15 cm above L4-L5. A free-form ROI and manual thresholding were used to select tissue of fat within the subcutaneous and visceral adipose tissue (Figure 1).
With ultrasound guidance and within two weeks of the MR examination, a percutaneous needle liver biopsy was performed using an 18-gauge needle, with the patients under local anesthesia. To obtain an adequate sample, biopsy specimens were obtained twice from all patients at two different sites in the right hepatic lobe (VI and VII segments). Liver specimens that were at least 1.5 cm in length and contained at least 10-11 complete portal tracts were considered to be adequate for histological assessment. Liver steatosis was determined by estimating the percentage of fat-containing hepatocytes on hematoxylin-eosin-stained specimens. The grading system for liver steatosis was based on the NASH Clinical Research Network criteria[19]: grade 0, less than 5% steatosis; grade 1, 6%-33% steatosis; grade 2, 34%-66% steatosis; and grade 3, greater than 66% steatosis. The grading system incorporates the accepted normal value for histopathologic liver fat, which is less than 5%, and it is the standard applied in the clinical assessment of severity of liver steatosis by hepatologists and gastroenterologists.
To estimate the proper sample size in the correlation between the MR imaging and liver biopsy, a power analysis was conducted, considering a coefficient correlation of 0.6, α error= 0.05 and power 1-β = 0.90; the number of subjects was 21. Receiver operating characteristic (ROC) curve analysis was used to determine the best cut-off values for MRS and MRI-PDHFF between the control group and children with NAFLD. The Pearson correlation coefficientwas calculated among histology, MRS and MRI-PDHFF We also estimated agreement by using the 95% limit-of-agreement method developed by Bland and Altman[20]. An analysis of the study population and control groups was performed to determine the median ± SD, and comparisons were made using the Mann-Whitney U test. Multivariate analysis of the continuous variables was performed to evaluate which variables were useful for predicting liver steatosis. A P value less than 0.05 was considered to indicate significant difference. Statistical analysis was performed using the MedCalc Software (V.13.1.2, Acacialaan 22 m 8400, Onsted, Belgium), except for the multivariate analysis, which was calculated using the JMP software (JMP.11, SAS Institute Inc., Cary, NC, United States).
The clinical characteristics of the study population and control group are presented in Table 1.
| Patients with NAFLD | Control group | |||||
| Median | SD | 95%CI | Median | SD | 95%CI | |
| BMI | 28.71 | 4.07 | 26.2-29.58 | 23.75 | 3.5 | 21.86-25.84 |
| ALP | 185 | 104.54 | 122.21-244.81 | 219.0 | 103.00 | 136.13-303.8 |
| ALT | 31 | 52.21 | 30-59.5 | 16.5 | 50.7 | 15-20.45 |
| AST | 25 | 25.78 | 21.14-30.85 | 22 | 25.21 | 20-26 |
| Bilirubin | 0.53 | 0.4074 | 0.38-0.68 | 0.59 | 0.21 | 0.37-0.67 |
| γ-GT | 21 | 12.24 | 17.14-28.97 | 11 | 9.98 | 10-12 |
| Insuline | 17.61 | 11.46 | 12.12-24.14 | 11.6 | 3.4 | 8.17-12.34 |
| Cholesterol | 154 | 38.56 | 130.44-168.55 | 142.5 | 28.8 | 136-167 |
| Blood glucose | 85 | 7.08 | 83.14-89.70 | 83 | 3.6 | 81-84 |
| Triglycerides | 77 | 68.86 | 68.68-114.65 | 60.5 | 24.64 | 50-84.45 |
| VAT | 368.351 | 258.23 | 334.9-501.7 | 275.9 | 76.02 | 252.4-299.9 |
| SAT | 1949.221 | 1184.9 | 1743.6-2886.0 | 1352.9 | 746.95 | 840.3-1539.4 |
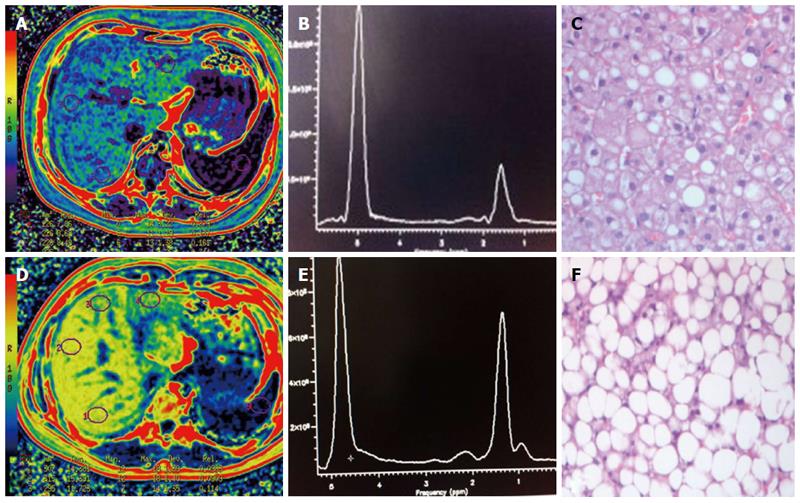
Of the 27 patients who underwent liver biopsy, 11 (40.7%) children demonstrated grade 1 steatosis, 9 (33.3%) children demonstrated grade 2 and 7 (26%) demonstrated grade 3 (mean, 43.3% ± 26; range, 10%-90%). The mean lipid content for MRS was 30% ± 18 (median, 30%; range, 5%-66%), whereas the MRI-PDFF mean fat fraction was 11% ± 7 (median, 10%; range, 1%-33%) (Figure 2). The following HFF values were recorded in the control group: MRS (mean, 4.4% ± 2.5; median, 4%; range, 0.8%-13%) and MRI-PDFF (mean, 2.5 ± 2; median, 1.9%; range, 0.7%-11%).
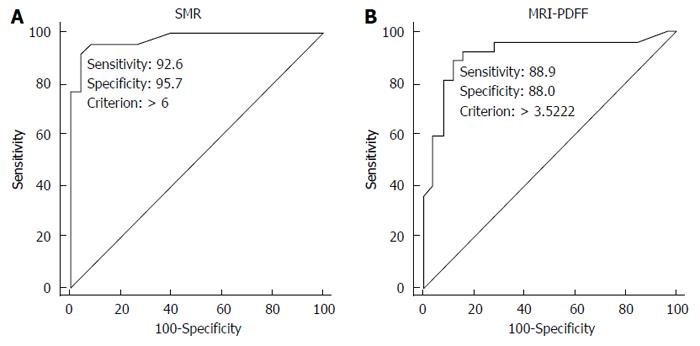
The ROC curve analysis suggested a cut-off value of 6% for MRS to discriminate between patients with and without steatosis (sensitivity, 92.6%; specificity, 95.7%; 6 false positive calls); conversely, MRI-PDFF suggested a cut-off value of 3.5% (sensitivity, 89%; specificity, 88%; 4 false positive calls) (Figure 3).
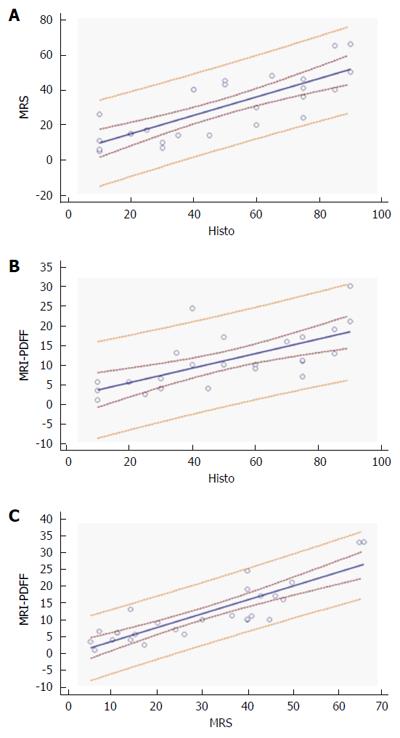
For MR spectroscopy, compared with the histology results, the Pearson test revealed a correlation of 0.68, (P = 0.0001) MRI-PDFF showed slightly lower values of 0.63, 0 (P = 0.0005).
Excellent correlation was reported between the MR techniques (ρ 0.81, P < 0.0001). Bland Altman plot reveals all points within were within the 95% limit of agreement, a possible bias encountered in the evaluation of MR-PDFF at medium and high level of hepatic steatosis (Figure 4).
Compared to the control group, significantly higher BMI (0.0002), VAT (< 0.0001), SAT (0.0001), ALT (< 0.0001) and insulin (0.0008) values were reported in the study population. Multivariate analysis of the quantitative variables demonstrated good correlation among VAT, SAT, BMI and insulin in terms of predicting liver steatosis (0.80, 0.51, 0.50 and 0.52, respectively).
Our results suggest that MRS is an accurate, non-invasive diagnostic technique for quantifying liver steatosis in a pediatric population and that MRI-PDFF is a feasible alternative technique. For MRS, the same cut-off value of 5% can be used to diagnosis liver steatosis in adolescents and adults; in contrast, when MRI-PDFF is used to quantify liver steatosis, a cut-off value of 3.5% is more appropriate. For clinical and morphologic analyses, the BMI, VAT, SAT and insulin levels should be jointly considered promising tools for predicting liver steatosis.
In a pediatric population, precise measurement of the degree of liver steatosis is of clinical interest because it has demonstrated an association with subclinical signs of atherosclerosis and changes in myocardial functions[21-24]. At present, no laboratory test can be used for the non-invasive quantification of liver steatosis, and ALT values may be elevated only in severe cases[25]. Although our analysis reported significantly higher levels of ALT and insulin in predicting liver steatosis, only in severe cases were the results beyond normal ranges. Anthropometry data revealed significantly higher BMI, SAT and VAT values in children with NAFLD, as previously described in the literature[26,27]; unfortunately, multivariate analysis demonstrated only moderate to good values in predicting liver steatosis (range, 0.80 - 0.50).
Among the imaging modalities, MRS can provide a reliable estimation of the weight fraction of liver fat content, and it is now considered to be the most accurate non-invasive method; for these reasons, it is used extensively in published reports[28-32]. In addition, in our experience, MRS demonstrated excellent correlation with liver biopsy, with good concordance. However, MRS is an expensive and primarily research-based tool that is not always available[33,34]. MRI-PDFF has been proposed as a feasible and simple alternative method for quantifying liver steatosis[10,11,35-37]. Our data demonstrated good correlation between histology. However a probable error should be develop in the differentiation between moderate and high grade of steatosis: this limit of chemical shift technique is attributed to the presence of both water and fat (fat-water signal dominance ambiguity) in the single voxel and it is not of clinical relevance for patients treatment and managment[38,39]. MR-PDFF suggests a lower threshold (3.5%) to define hepatic steatosis in children, in agreement with a recent publication by Rehm et al[40]. Our multiple-echo sequence has several advantages over MRS. First, the acquisition time is short, easily fitting within a breath hold. Second, the fat content can be measured throughout the liver instead of in only one voxel or a few voxels; this point is of major importance because fat distribution is often heterogeneous. Third, no spatial miss-registration errors occur because the OP and IP images are acquired simultaneously. Fourth, post-processing is considerably easier and faster than with MRS.
Some study limitations should be noted. First, the site-to-site reproducibility of our technique was evaluated only in segment VII. Second, our control group did not have histologically confirmed healthy livers because it would have been unethical to subject normal individuals to liver biopsy assessments. Thirdly, the three parameters (MRS, MR-PDFF and histology) tested in our study assess different aspects of steatosis. Histology reveals the percentage of hepatocytes that contain vesicles of fat, MR-PDFF determines the proportion of mobile protons contained within fat molecules and MRS shows the peaks of methyl and methylene (-CH2 and CH3) protons in the triglyceride molecule.
Finally, Bland-Altman plot analysis demonstrates a difficulty of MR-PDFF in the differentiation between moderate to severe steatosis; however this drawback has not clinical relevance becasuse treatment and managmetn of patients is quite similar.
In conclusion, MRS was confirmed as the most accurate non-invasive method for quantifying liver steatosis in obese children.
Multiple-echo gradient-echo MR sequence may also be an easily and rapidly performed technique used to quantify liver steatosis in a pediatric population; this technique demonstrated excellent agreement with MRS and should be considered as reference standard in longitudinal studies or clinical trials. Together, BMI, VAT, SAT and insulin measurements provide the most powerful test for predicting liver steatosis. Further studies should be conducted to better match data from histological and MRI analyses and to establish MRI thresholds for different grades of steatosis.
Non-alcoholic fatty liver disease (NAFLD) is emerging as a leading cause of chronic liver disease in children and adolescents, with a prevalence in the general population ranging from 3%-10% in normal weight children and 80% in obese children. Children with NAFLD are usually asymptomatic and garner clinical attention because of elevated liver enzymes or fatty liver being observed during an ultrasound examination.
A recently published paper suggested similar fat-storage between overweight children and adults, and the study proposed the same cut-off value for normal and pathologic storage.
Body mass index, subcutaneous and visceral adipose tissue and insulin measurements provide the most powerful test for predicting liver steatosis.
This study should be conducted to better match data from histological and MRI analyses and to establish MRI thresholds for different grades of steatosis.
Interesting study, reasonable N. Needs more description of methods, results; I think with a careful revision, these authors can justify their conclusions with the data presented.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shah RV, Wang CY S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Giorgio V, Prono F, Graziano F, Nobili V. Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. 2013;13:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Widhalm K, Ghods E. Nonalcoholic fatty liver disease: a challenge for pediatricians. Int J Obes (Lond). 2010;34:1451-1467. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538-1544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 511] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 4. | Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 586] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 6. | Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, Changchien C, Brenner DA, Sirlin C. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930-1940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 387] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 7. | Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:spcone. [Cited in This Article: ] |
| 8. | Yokoo T, Shiehmorteza M, Hamilton G, Wolfson T, Schroeder ME, Middleton MS, Bydder M, Gamst AC, Kono Y, Kuo A. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258:749-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 9. | Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, Reeder SB. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 10. | Hines CD, Frydrychowicz A, Hamilton G, Tudorascu DL, Vigen KK, Yu H, McKenzie CA, Sirlin CB, Brittain JH, Reeder SB. T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging. 2011;33:873-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, Middleton MS. In vivo characterization of the liver fat ¹H MR spectrum. NMR Biomed. 2011;24:784-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 398] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 12. | Cali AM, De Oliveira AM, Kim H, Chen S, Reyes-Mugica M, Escalera S, Dziura J, Taksali SE, Kursawe R, Shaw M. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49:1896-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Chabanova E, Bille DS, Thisted E, Holm JC, Thomsen HS. (1)H MRS assessment of hepatic steatosis in overweight children and adolescents: comparison between 3T and open 1T MR-systems. Abdom Imaging. 2013;38:315-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 366] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 15. | Larson-Meyer DE, Newcomer BR, VanVrancken-Tompkins CL, Sothern M. Feasibility of assessing liver lipid by proton magnetic resonance spectroscopy in healthy normal and overweight prepubertal children. Diabetes Technol Ther. 2010;12:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Idilman IS, Aniktar H, Idilman R, Kabacam G, Savas B, Elhan A, Celik A, Bahar K, Karcaaltincaba M. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 17. | Demerath EW, Ritter KJ, Couch WA, Rogers NL, Moreno GM, Choh A, Lee M, Remsberg K, Czerwinski SA, Chumlea WC. Validity of a new automated software program for visceral adipose tissue estimation. Int J Obes (Lond). 2007;31:285-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab. 2002;87:5044-5051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 752] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 20. | Guiu B, Petit JM, Loffroy R, Ben Salem D, Aho S, Masson D, Hillon P, Krause D, Cercueil JP. Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology. 2009;250:95-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 452] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 22. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1326] [Cited by in F6Publishing: 1361] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 23. | Pacifico L, Di Martino M, De Merulis A, Bezzi M, Osborn JF, Catalano C, Chiesa C. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology. 2014;59:461-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Santoro N, Caprio S. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis in obese adolescents: a looming marker of cardiac dysfunction. Hepatology. 2014;59:372-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Sagi R, Reif S, Neuman G, Webb M, Phillip M, Shalitin S. Nonalcoholic fatty liver disease in overweight children and adolescents. Acta Paediatr. 2007;96:1209-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Taksali SE, Caprio S, Dziura J, Dufour S, Calí AM, Goodman TR, Papademetris X, Burgert TS, Pierpont BM, Savoye M. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 27. | Maffeis C, Manfredi R, Trombetta M, Sordelli S, Storti M, Benuzzi T, Bonadonna RC. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93:2122-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Fonvig CE, Bille DS, Chabanova E, Nielsen TR, Thomsen HS, Holm JC. Muscle fat content and abdominal adipose tissue distribuition investigated by magnetic resonance spectroscopy and imaging in obese children and youths. Pediatr Rep. 2012;4:e11. [Cited in This Article: ] |
| 29. | Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, Lundbom N, Rissanen A, Ridderstråle M, Groop L. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 543] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 30. | Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, Crocè LS, Grigolato P, Paoletti S, de Bernard B. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 295] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 31. | Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977-E989. [PubMed] [Cited in This Article: ] |
| 32. | van Werven JR, Marsman HA, Nederveen AJ, Smits NJ, ten Kate FJ, van Gulik TM, Stoker J. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256:159-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 33. | Chalasani N. Nonalcoholic fatty liver disease liver fat score and fat equation to predict and quantitate hepatic steatosis: promising but not prime time! Gastroenterology. 2009;137:772-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16:1560-1566. [DOI] [Cited in This Article: ] [Cited by in CrossRef: 88] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Reeder SB, Robson PM, Yu H, Shimakawa A, Hines CD, McKenzie CA, Brittain JH. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009;29:1332-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 36. | Machann J, Thamer C, Schnoedt B, Stefan N, Haring HU, Claussen CD, Fritsche A, Schick F. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med. 2006;55:913-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Cowin GJ, Jonsson JR, Bauer JD, Ash S, Ali A, Osland EJ, Purdie DM, Clouston AD, Powell EE, Galloway GJ. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging. 2008;28:937-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 38. | Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, Hassanein T, Patton HM, Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251:67-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 263] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 39. | Africa JA, Newton KP, Schwimmer JB. Lifestyle Interventions Including Nutrition, Exercise, and Supplements for Nonalcoholic Fatty Liver Disease in Children. Dig Dis Sci. 2016;61:1375-1386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 40. | Rehm JL, Wolfgram PM, Hernando D, Eickhoff JC, Allen DB, Reeder SB. Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol. 2015;25:2921-2930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |