Published online Dec 28, 2016. doi: 10.3748/wjg.v22.i48.10532
Peer-review started: July 21, 2016
First decision: August 22, 2016
Revised: September 15, 2016
Accepted: October 10, 2016
Article in press: October 10, 2016
Published online: December 28, 2016
To investigate the capacity of Saccharomyces cerevisiae (S. cerevisiae) and Saccharomyces boulardii (S. boulardii) yeasts to reverse or to treat acute stress-related intestinal dysmotility.
Adult Swiss Webster mice were stressed for 1 h in a wire-mesh restraint to induce symptoms of intestinal dysmotility and were subsequently killed by cervical dislocation. Jejunal and colon tissue were excised and placed within a tissue perfusion bath in which S. cerevisiae, S. boulardii, or their supernatants were administered into the lumen. Video recordings of contractility and gut diameter changes were converted to spatiotemporal maps and the velocity, frequency, and amplitude of propagating contractile clusters (PCC) were measured. Motility pre- and post-treatment was compared between stressed animals and unstressed controls.
S. boulardii and S. cerevisiae helped to mediate the effects of stress on the small and large intestine. Restraint stress reduced jejunal transit velocity (mm/s) from 2.635 ± 0.316 to 1.644 ± 0.238, P < 0.001 and jejunal transit frequency (Hz) from 0.032 ± 0.008 to 0.016 ± 0.005, P < 0.001. Restraint stress increased colonic transit velocity (mm/s) from 0.864 ± 0.183 to 1.432 ± 0.329, P < 0.001 and frequency to a lesser degree. Luminal application of S. boulardii helped to restore jejunal and colonic velocity towards the unstressed controls; 1.833 ± 0.688 to 2.627 ± 0.664, P < 0.001 and 1.516 ± 0.263 to 1.036 ± 0.21, P < 0.001, respectively. S. cerevisiae also had therapeutic effects on the stressed gut, but was most apparent in the jejunum. S. cerevisiae increased PCC velocity in the stressed jejunum from 1.763 ± 0.397 to 2.017 ± 0.48, P = 0.0031 and PCC frequency from 0.016 ± 0.009 to 0.027 ± 0.007, P < 0.001. S. cerevisiae decreased colon PCC velocity from 1.647 ± 0.187 to 1.038 ± 0.222, P < 0.001. Addition of S. boulardii or S. cerevisiae supernatants also helped to restore motility to unstressed values in similar capacity.
There is a potential therapeutic role for S. cerevisiae and S. boulardii yeasts and their supernatants in the treatment of acute stress-related gut dysmotility.
Core tip: The use of Saccharomyces cerevisiae and Saccharomyces boulardii yeasts as therapeutic agents were tested for their ability to reverse the intestinal discomfort caused by acute stress. Most studies investigate the role of microbes in the prevention of stress, however the yeasts showed promising acute therapeutic effects for the treatment of stress. Additionally, the residual supernatant (Snt) after centrifugation of the yeasts was able to recapitulate much of the effect of the microbes themselves. Saccharomyces yeasts or Snt may be potential probiotic therapies in the treatment of acute stress-related intestinal dysmotility.
- Citation: West C, Stanisz AM, Wong A, Kunze WA. Effects of Saccharomyces cerevisiae or boulardii yeasts on acute stress induced intestinal dysmotility. World J Gastroenterol 2016; 22(48): 10532-10544
- URL: https://www.wjgnet.com/1007-9327/full/v22/i48/10532.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i48.10532
Most studies on beneficial ingested microbes including probiotics have focused primarily on bacteria. However, beneficial roles have been ascribed to certain yeasts, such as the sugar-fermenting Saccharomyces[1]. Saccharomyces boulardii (S. boulardii) and Saccharomyces cerevisiae (S. cerevisiae) are two closely related strains used either as a probiotic or in the preparation of food and wine. The two strains have been closely examined; revealing that although they are nearly identical at a molecular level, S. boulardii shows more physiological resistance to heat and acid stressors[2]. It should also be noted that S. boulardii does not produce ascospores or use galactose, while S. cerevisiae does[3,4].
S. boulardii has been systematically studied for its beneficial and probiotic effects[5], but S. cerevisiae has limited research supporting a probiotic role[1]. S. boulardii has been used to help in the prevention of antibiotic or Clostridium difficile induced diarrhea, and there is evidence that it may be useful in attenuating acute gastroenteritis and traveller’s diarrhea[5,6]. Another study showed that treatment with S. boulardii helped to shorten the duration of acute diarrhea in children and to normalize the frequency and consistency of stool[7]. S. boulardii may also help in the treatment of bowel inflammation and infection by reversing mucosal injury[8].
S. cerevisiae food and wine strains have a long history in the food and wine industry[9] and are generally considered safe for consumption[1]. Suggestive for a potential beneficial role for S. cerevisiae are reports indicating that this strain may provoke immune stimulation in mice infected with Staphylococcus aureus[10]. Supplementation with a S. cerevisiae I-3856 strain may improve symptoms in constipation-predominant irritable bowel syndrome patients[11]. There is also evidence that a S. cerevisiae UFMG 905 strain can bind to bacteria and modulate inflammation pathways in a murine model of Salmonella enterica serovar Typhimurium infection[12]. In summary, there appears to be published data supporting roles for S. boulardii or S. cerevisiae as beneficial or probiotic microbes. The evidence seems to be stronger for S. boulardii than S. cerevisiae, perhaps because the latter has been less frequently investigated in this regard.
We used an acute ex vivo, before and after, motility recording paradigm similar to that used previously to test for the effects of JB-1™ on stress-induced dysmotility[13]. Saccharomyces or Saccharomyces supernatant (Snt) were added to the Krebs buffer perfusing the lumen of isolated, previously stressed or unstressed, mouse intestinal segments. Treatment effects were interpreted by comparing propagating contractile clusters (PCC) of control with treatment recording periods. The ex vivo design allowed us to localize any effects to the intestine, thus avoiding multisystem homeostatic feedback between the gut and its extrinsic nervous system. This design also allowed us to separate treatment effects from confounding preventative actions, as would have been the case if the yeasts had been fed to the animal.
The preventative effect of Saccharomyces strains in relation to diarrhea suggests a possible action on disordered gut motility. It has not been experimentally tested whether S. boulardii or S. cerevisiae are able to treat (effectively reverse) stress-related dysmotility in an experimental model. We have recently shown that restraint stress induces colonic propulsive hypermotility, while disorganizing and reducing motility in the small intestine[13]. The effects of stress on motility could be reversed ex vivo by introducing a bacterial probiotic (Lactobacillus rhamnosus JB-1™) into the lumen[13]. The example of JB-1™ in treating stress-induced dysmotility provides a way to compare putative beneficial actions of the Saccharomyces strains with a probiotic bacterium whose effects on motility and the enteric nervous system have been previously studied[14-20].
We used 6-8-wk-old adult male Swiss Webster mice (20-30 g) from Charles River Laboratories (Wilmington, MA, United States). All procedures following acute restraint were ex vivo[20] and their conduct were approved by the Animal Research Ethics Board of McMaster University (AUP 12-05-17).
The following experiments and data analysis were performed as described as in West et al[13]. Mice were placed in a wire mesh restraint device for 1 h or kept in their cage for 1 h; after which they were killed by cervical dislocation. A 4 cm long segment of jejunum or colon was excised and placed into a tissue bath perfusion chamber[20]. The oral and anal ends were cannulated with silicone tubing and the oral end was attached to a stopcock manifold, allowing inflow with oxygenated Krebs buffer or buffer to which yeast or Snt had been added. Krebs buffer was of the following composition (mmol/L): 118 NaCl, 4.8 KCl, 25 NaHCO3, 1.0 NaH2PO4, 1.2 MgSO4, 11.1 glucose, and 2.5 CaCl2 bubbled with carbogen gas (95% O2 and 5% CO2). PCCs were evoked by filling the lumen with Krebs buffer using a pressure differential of 2 hPa (cmH2O) for the inflow and 3 hPa for outflow for jejunum, and 2-3 hPa for colon inflow with the outflow raised 1 cm above the inflow.
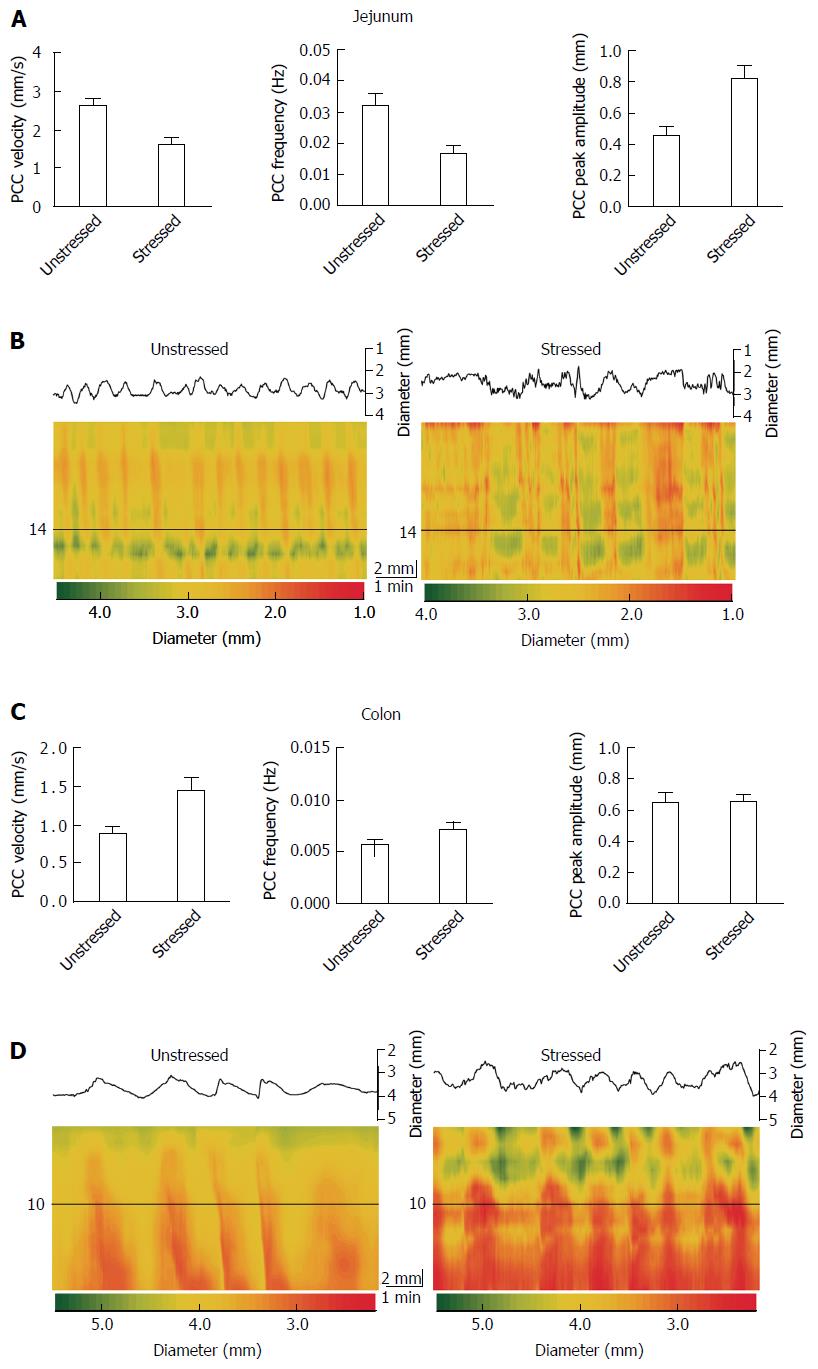
Contractions of the gut were video-recorded on a JVC camcorder placed 10 cm above the tissue bath. Conversion of videos to spatiotemporal diameter maps (Dmaps) were performed using Image J software, as described in Wu et al[20]. Dmaps are a form of heat map in which the oral to anal propagation of the intestine runs down the vertical axis and time runs across the horizontal axis. The intestine’s diameter is colour coded using red to represent contraction, and yellow to green to represent varying degrees of relaxation (Figure 1). PCCs are identified in Dmaps as described in West et al[13]. The PCCs appear as broad bands[20] that propagate in the oral to anal direction. They are believed to require ENS activity because they are abolished by the Na channel blocker tetrodotoxin[18-23]. From these Dmaps, velocity can be measured as the slope of the bands (distance/time), frequency from the intervals between the bands, and amplitude as the difference between gut diameters.
Lyophilized S. boulardii CNCM I-1079 or S. cerevisiae LYCC 6029 were obtained from Lallemand Health Solutions (Montreal, QC, CA). The microbes (starter counts) were diluted in 50 mL Krebs buffer and incubated for 45 min at 37 °C. In pilot experiments, 5 × 107, 5 × 108 and 5 × 109 starter counts of S. boulardii reduced PCC velocity in unstressed colon segments by 5%, 25% and 27%. We thus used 5 × 108-lyophilized microbes for dilution in all other experiments as this starter count produced near maximal effect in the pilots. In some experiments we used S. boulardii or S. cerevisiae Snt after completion of the incubation period. The microbes were diluted and incubated as previously described above. The Snt was separated from the post-incubation yeast microbe solution by centrifugation at 1400 g. using a Beckman Model TJ-6 centrifuge for 30 min. After sediment removal, the mixture was passed through a 0.2 μm pore-size filter and the filtered Snt was applied to the lumen of gut segment to be tested.
Effects of restraint stress on motility were measured in unpaired experiments by comparing velocity, frequency and peak amplitude for PCCs. The same parameters were compared before and after application of S. boulardii, S. cerevisiae, or their Snt. Control recordings were made in stressed or unstressed mice perfused with Krebs for a maximum of 20 min. Treatment recordings were made during and after addition of one of the stimuli (yeast or Snt) to the same segment for additional 20 min duration. Descriptive statistics were given as mean ± SD (N, where N denotes the number of mice used). In the results, treatment effect sizes are presented as % mean differences and the probability of superiority (PS) based on distribution of difference scores and standard deviations is presented in brackets[24]. Differences were also presented in the tables using unpaired or paired t tests under the null hypothesis of no difference.
Stress had different effects on the jejunum vs the colon. Stress decreased propulsive motility and decreased the regularity of PCCs in jejunum, but increased motility in colon (Figure 1). Stress decreased jejunal PCC velocity by 38% (PS = 99%, P < 0.001), but increased it by 66% (PS = 98%, P < 0.001) for colon (Table 1 and Figure 1). Frequency decreased by 50% (PS = 99%, P < 0.001) for jejunum, but increased by 27% (PS = 93%, P = 0.008) for colon. Peak amplitude increased by 81% (PS = 99%, P < 0.001) for jejunum and by 1% (PS = 51%, P = 0.902) for colon.
| Parameter | Tissue | Unstressed | Stressed | P value (t test) |
| Velocity (mm/s) | Jejunum | 2.635 ± 0.316 (17) | 1.644 ± 0.238 (17) | < 0.001 |
| Colon | 0.864 ± 0.183 (17) | 1.432 ± 0.329 (17) | < 0.001 | |
| Frequency (Hz) | Jejunum | 0.032 ± 0.008 (17) | 0.016 ± 0.005 (17) | < 0.001 |
| Colon | 0.005 ± 0.001 (17) | 0.007 ± 0.001 (17) | 0.008 | |
| Peak amplitude (mm) | Jejunum | 0.437 ± 0.020 (17) | 0.790 ± 0.015 (17) | < 0.001 |
| Colon | 0.640 ± 0.118 (17) | 0.645 ± 0.101 (17) | 0.902 |
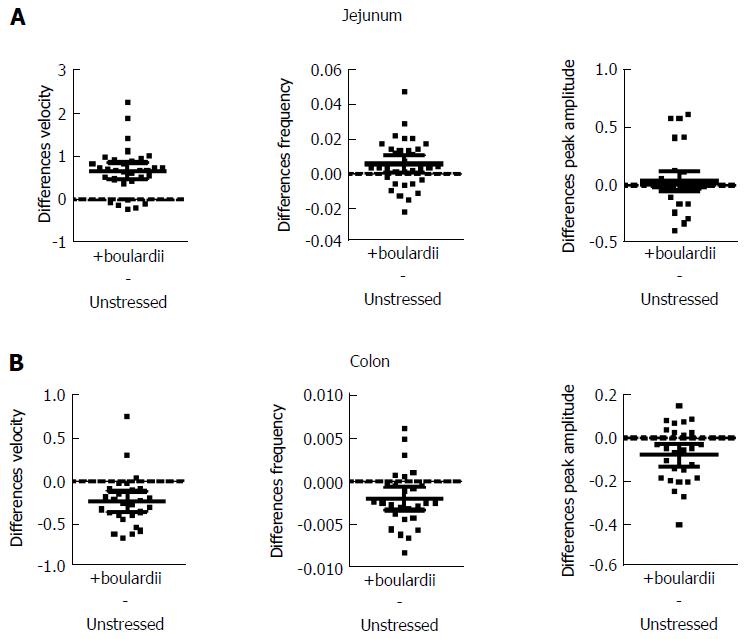
S. boulardii increased PCC velocity in unstressed jejunum by 26% (PS = 94%, P < 0.001), but decreased velocity by 27% (PS = 89%, P < 0.001) for colon. Frequency was increased by 19% (PS = 74%, P = 0.030) for jejunum, but decreased by 22% (PS = 85%, P = 0.005) for colon. Peak amplitude increased by 6% (PS = 60%, P = 0.290) for jejunum, but decreased by 13% (PS = 85%, P = 0.005) for colon (Table 2). Figure 2 also shows paired mean differences (Unstressed + S. boulardii Unstressed) with 95%CIs. When the confidence intervals did not straddle the “difference = 0” line, the value of no paired difference was excluded for these intervals with 95% confidence[25]; when it did straddle the “difference = 0” line the value of no paired difference was included within the 95%CIs.
| Parameter | Tissue | Unstressed | +boulardii | P value (t test) |
| Velocity (mm/s) | Jejunum | 2.556 ± 0.299 (32) | 3.229 ± 0.620 (32) | < 0.001 |
| Colon | 0.865 ± 0.148 (27) | 0.633 ± 0.225 (27) | < 0.001 | |
| Frequency (Hz) | Jejunum | 0.030 ± 0.018 (33) | 0.035 ± 0.025 (33) | 0.030 |
| Colon | 0.009 ± 0.004 (27) | 0.007 ± 0.003 (27) | 0.005 | |
| Peak amplitude (mm) | Jejunum | 0.587 ± 0.241 (33) | 0.623 ± 0.291 (33) | 0.290 |
| Colon | 0.621 ± 0.190 (27) | 0.543 ± 0.179 (27) | 0.005 |
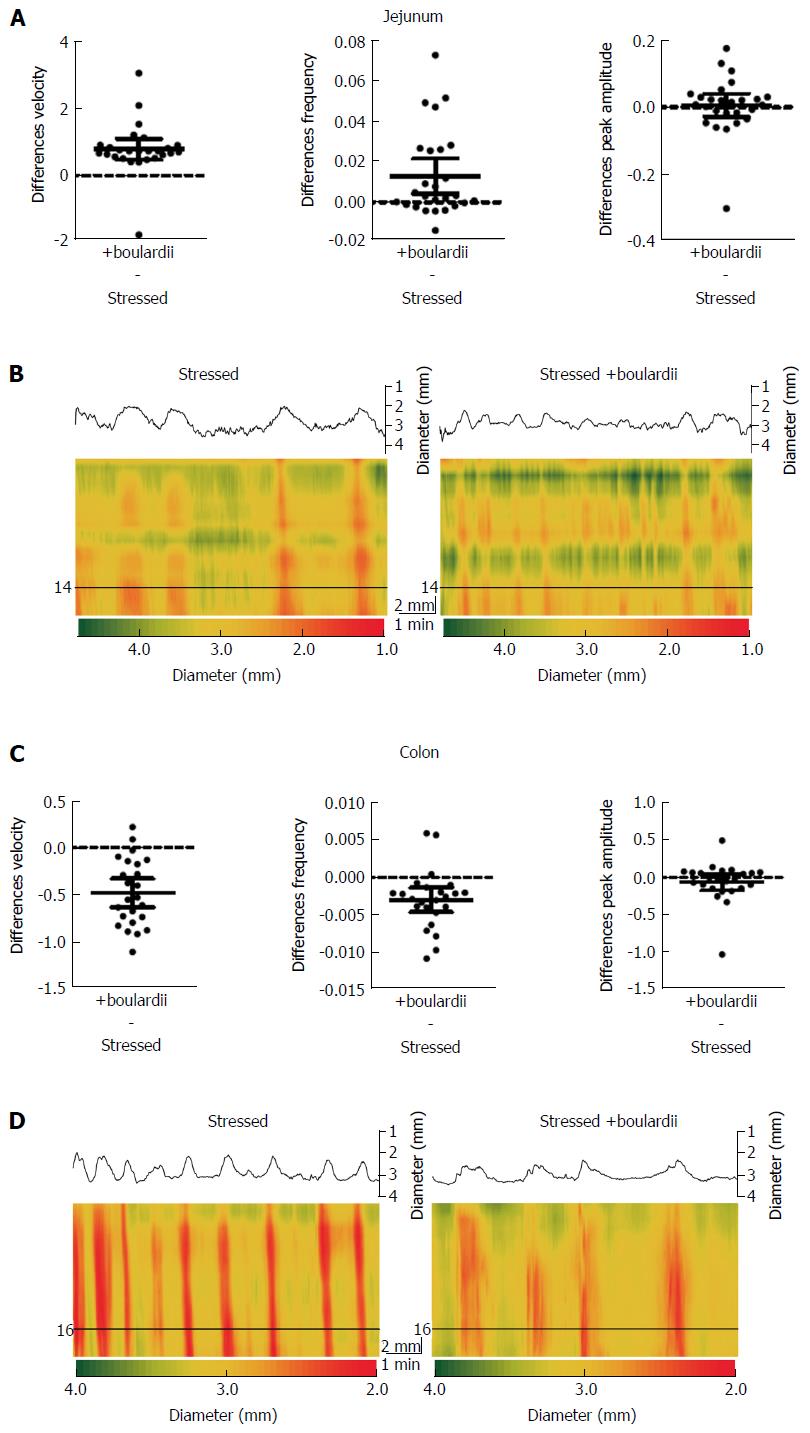
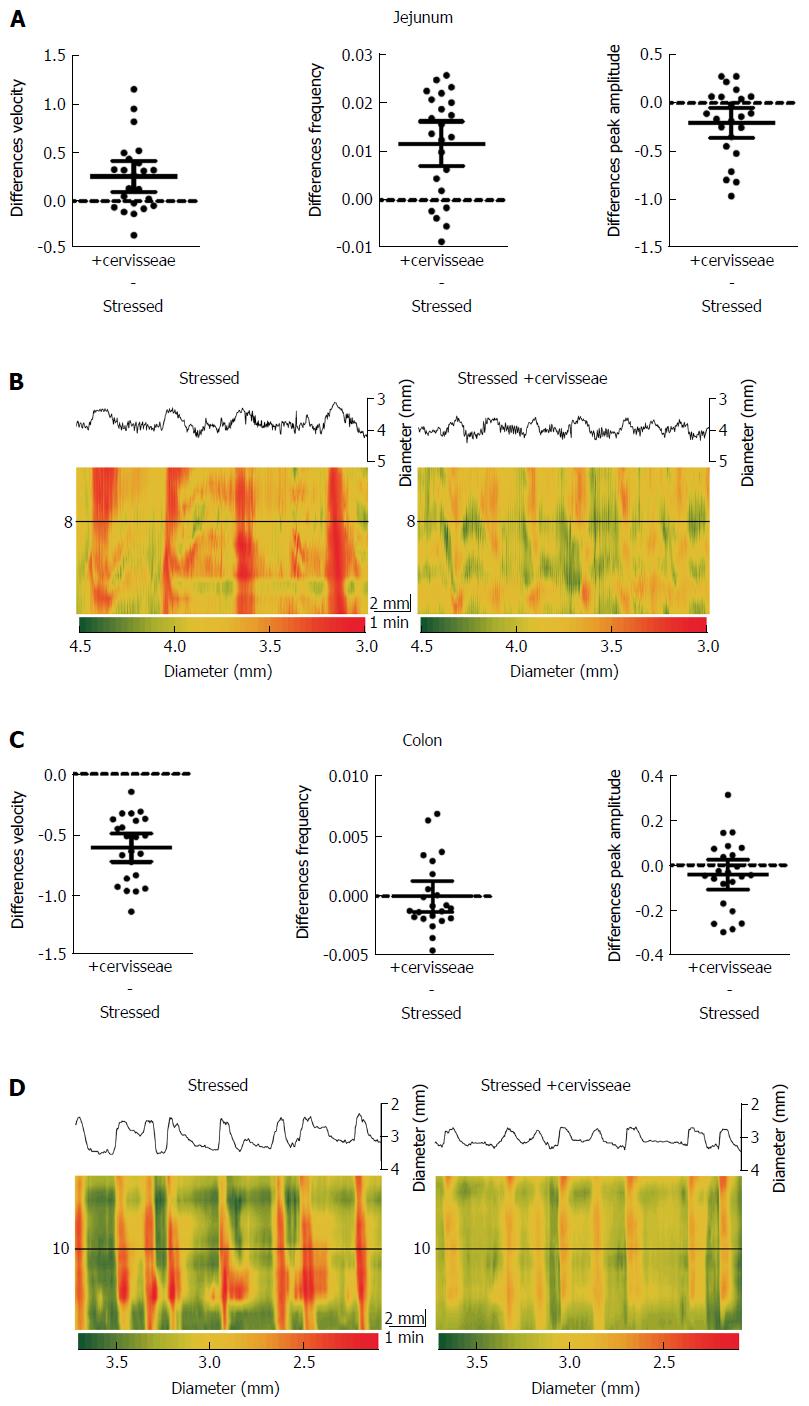
S. boulardii counter the effects of stress in both jejunal and colon segments, with the exception of peak amplitude (Figure 3 and Table 3). S. boulardii restored the regularity and frequency of contractions in both tissues, as shown in Figure 3. There is a marked increase in frequency of bands in the jejunal Dmap and a decrease in the colon Dmap. Addition of S. boulardii increased velocity by 43% (PS = 93%, P < 0.001) for stressed jejunum and decreased velocity by 32% (PS = 96%, P < 0.001) for colon. PCC frequency was increased by 69% (PS = 85%, P = 0.005) for jejunum but decreased by 29% (PS = 90%, P = 0.001) for colon. Peak amplitude changed by +1% (PS = 56%, P = 0.705) for jejunum and decreased by 9% (PS = 68%, P = 0.259) for colon. Mean paired differences (Stressed + S. boulardii Stressed) are given in Figure 3B and D with their 95%CIs. Washout with Krebs buffer for 20 min during the control period did not moderate the effects of S. boulardii on the stressed segments.
| Parameter | Tissue | Stressed | +boulardii | P value (t test) |
| Velocity (mm/s) | Jejunum | 1.833 ± 0.688 (26) | 2.627 ± 0.664 (26) | < 0.001 |
| Colon | 1.516 ± 0.263 (24) | 1.036 ± 0.210 (24) | < 0.001 | |
| Frequency (Hz) | Jejunum | 0.019 ± 0.013 (26) | 0.032 ± 0.024 (26) | 0.005 |
| Colon | 0.010 ± 0.003 (24) | 0.007 ± 0.003 (24) | 0.001 | |
| Peak amplitude (mm) | Jejunum | 0.692 ± 0.215 (26) | 0.698 ± 0.212 (26) | 0.705 |
| Colon | 0.719 ± 0.249 (24) | 0.657 ± 0.152 (24) | 0.259 |
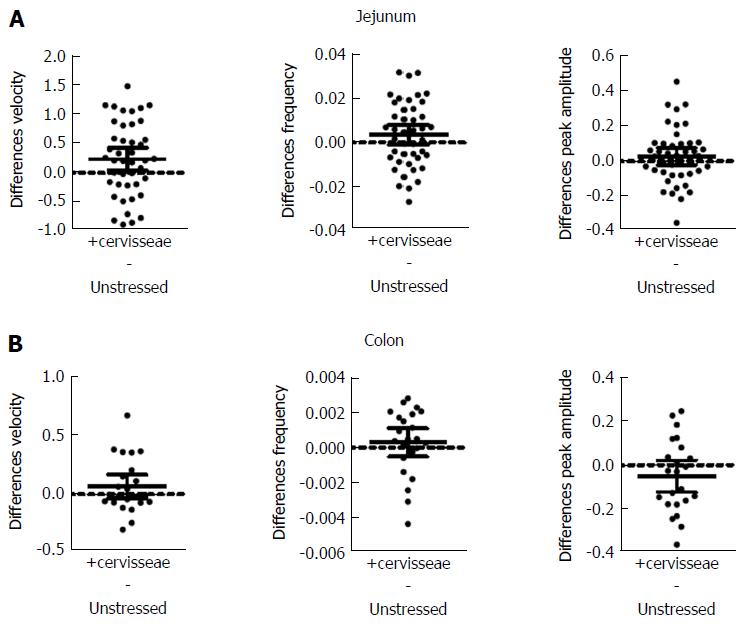
S. cerevisiae had the most noticeable effect on the jejunum (Figure 4 and Table 4). Velocity increased 9% (PS = 71%, P = 0.017) for jejunum and 9% (PS = 73%, P = 0.161) for colon. Frequency increased by 13% (PS = 64%, P = 0.003) for jejunum and by 6% (PS = 63%, P = 0.462) for colon, while peak amplitude increased by 7% (PS = 61%, P = 0.242) for jejunum, but decreased by 9% (PS = 73%, P = 0.164) for colon.
| Parameter | Tissue | Unstressed | +cerevisiae | P value (t test) |
| Velocity (mm/s) | Jejunum | 2.779 ± 0.499 (43) | 3.017 ± 0.457 (43) | 0.017 |
| Colon | 0.788 ± 0.212 (23) | 0.858 ± 0.257 (23) | 0.161 | |
| Frequency (Hz) | Jejunum | 0.027 ± 0.012 (43) | 0.031 ± 0.008 (43) | 0.003 |
| Colon | 0.0051 ± 0.001 (23) | 0.0054 ± 0.001 (23) | 0.462 | |
| Peak amplitude (mm) | Jejunum | 0.409 ± 0.111 (43) | 0.438 ± 0.098 (43) | 0.242 |
| Colon | 0.559 ± 0.176 (23) | 0.508 ± 0.15 (23) | 0.164 |
S. cerevisiae reduced most of the effects of stress, except for those of colon PCC frequency and amplitude (Figure 5 and Table 5). Velocity was increased by 14% (PS = 89%, P = 0.0031) for jejunum and decreased by 37% (PS = 98%, P < 0.001) for colon. Frequency was increased by 74% (PS = 95%, P < 0.001) for jejunum, but there was only a 0.1% (PS = 50%, P = 0.994) change for colon. Peak amplitude decreased by 27% (PS = 86%, P = 0.013) for jejunum and 7% (PS = 72%, P = 0.190) for colon. The Dmaps in Figure 5B and D show the regulation of jejunal motility by S. cerevisiae, with less potent effects on the colon. However the degree in which the contractions change in diameter appear to be lessened in the colon after addition of S. cerevisiae, and is apparent by the reduced frequency of red, strong contractions.
| Parameter | Tissue | Stressed | +cerevisiae | P value (t test) |
| Velocity (mm/s) | Jejunum | 1.763 ± 0.397 (23) | 2.017 ± 0.480 (23) | 0.0031 |
| Colon | 1.647 ± 0.187 (23) | 1.038 ± 0.222 (23) | < 0.001 | |
| Frequency (Hz) | Jejunum | 0.016 ± 0.009 (23) | 0.027 ± 0.007 (23) | < 0.001 |
| Colon | 0.008 ± 0.002 (23) | 0.008 ± 0.003 (23) | 0.994 | |
| Peak amplitude (mm) | Jejunum | 0.761 ± 0.316 (23) | 0.559 ± 0.148 (23) | 0.013 |
| Colon | 0.640 ± 0.101 (23) | 0.597 ± 0.103 (23) | 0.190 |
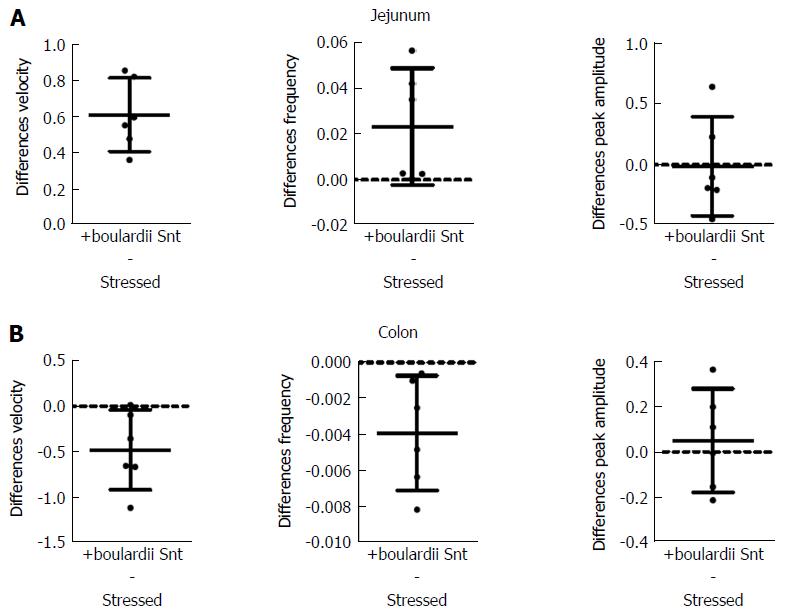
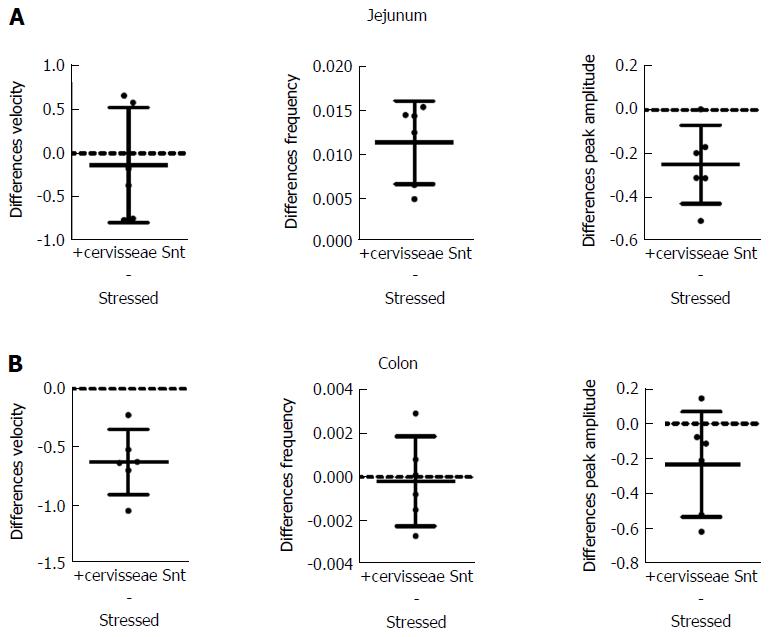
S. boulardii Snt decreased the effects of stress on PCC parameters, except for peak amplitude (Figure 6 and Table 6). Velocity increased by 31% (PS = 99.9%, P < 0.001) for jejunum, but decreased by 30% (PS = 99.9%, P = 0.038) for colon. Frequency increased by 114% (PS = 99.9%, P = 0.067) for jejunum and decreased by 37% (PS = 99%, P = 0.024) for colon. However, peak amplitude only decreased by 2% (PS = 56%, P = 0.930) for jejunum and increased 8% (PS = 74%, P = 0.590) for colon.
| Parameter | Tissue | Stressed | +boulardii Snt | P value (t test) |
| Velocity (mm/s) | Jejunum | 1.97 ± 0.39 (6) | 2.585 ± 0.468 (6) | < 0.001 |
| Colon | 1.588 ± 0.194 (6) | 1.11 ± 0.383 (6) | 0.038 | |
| Frequency (Hz) | Jejunum | 0.020 ± 0.014 (6) | 0.044 ± 0.027 (6) | 0.067 |
| Colon | 0.010 ± 0.001 (6) | 0.007 ± 0.003 (6) | 0.024 | |
| Peak amplitude (mm) | Jejunum | 0.75 ± 0.285 (6) | 0.737 ± 0.187 (6) | 0.93 |
| Colon | 0.65 ± 0.188 (6) | 0.701 ± 0.09 (6) | 0.59 |
S. cerevisiae Snt reduced the effects of stress for colon velocity, jejunal frequency and peak amplitude for jejunum and colon (Figure 7 and Table 7). Effects on other parameters were minor. Velocity decreased by 7% (PS = 56%, P = 0.970) for jejunum but decreased by 38% (PS = 99%, P = 0.002) for colon. Frequency increased by 64% (PS = 83%, P = 0.003) for jejunum and decreased by 2% (PS = 60%, P = 0.830); also, peak amplitude decreased by 35% (PS = 99%, P = 0.0016) for jejunum while it decreased by 31% (PS = 99%, P = 0.100) for colon.
| Stressed | +cerevisiae Snt | P value (t test) | ||
| Velocity (mm/s) | Jejunum | 0.713 ± 0.109 (6) | 0.466 ± 0.122 (6) | 0.97 |
| Colon | 1.639 ± 0.18 (6) | 1.018 ± 0.245 (6) | 0.002 | |
| Frequency (Hz) | Jejunum | 0.018 ± 0.004 (6) | 0.029 ± 0.004 (6) | 0.003 |
| Colon | 0.0076 ± 0.001 (6) | 0.0074 ± 0.001 (6) | 0.83 | |
| Peak amplitude (mm) | Jejunum | 0.713 ± 0.109 (6) | 0.466 ± 0.122 (6) | 0.0016 |
| Colon | 0.741 ± 0.177 (6) | 0.510 ± 0.202 (6) | 0.10 |
We have used an ex vivo intestinal segment perfusion setup to show that S. boulardii or S. cerevisiae reverse much of the jejunal or colonic dysmotility induced by restraint stress. There is sometimes a potential for normal commensal, or otherwise beneficial, yeasts to adopt a pathological role in immune-compromised individuals. It is therefore of interest that the Snt from either Saccharomyces strains recapitulate much of the treatment effects of the live yeasts. Because the yeasts were applied intraluminally in ex vivo intestinal segments the treatment effect must have occurred locally within the intestine[13]. Therefore, it could not have involved the hypothalamic-pituitary-adrenal axis or other central structures. The relative short latency approximately 10 min) for the onset of the therapeutic effect is consistent with a drug-like pharmacological mode of action, however, fast indirect modes of action involving immune cells cannot be excluded, although we consider the latter possibility to be less likely than the former.
There have been previous reports of the acute ex vivo actions of bacterial, but not fungal microbes on intestinal motility (see discussion in[20]). Such effects appear to be region specific with different actions for small vs large intestine[20]. It is important to note that others have also reported a short (approximately 10 min) latency in onset for microbial effects on intestinal propulsive motility[18,26], consistent with a drug-like pharmacological action of the microbes on the neuromuscular machinery. This supporting finding as well as the region-specific, local effect on the intestine leads us to believe the effect is most likely pharmacological in action.
Wrap restraint has been reported to increase the contractile amplitudes in the small intestine and colon, while decreasing frequency in small intestine and increasing frequency in colon[13,27]. As far as we are aware the only other publication to date referring to treatment effects of beneficial microbes on stress-induced dysmotility is West et al[13] in which Lactobacillus rhamnosus JB-1™ reversed the effects of prior stress. S. boulardii or S. cerevisiae were also effective in reversing most, but not all of the stress-induced dysmotility. S. boulardii was particularly effective in reversing the effects of stress in both the jejunum and colon. S. boulardii also had similar effects in the unstressed gut as the stressed, increasing PCC frequency and velocity in the jejunum and decreasing PCC frequency and velocity in the colon. These effects on the unstressed gut were predictive of the restoration of the stressed gut towards unstressed measures.
S. boulardii and S. cerevisiae are nearly identical genetically, but differ in resistance to temperature and acidic stressors and growth characteristics[2]. Similarly, the treatment effects of S. boulardii and S. cerevisiae were not identical, indicating that despite their genetic similarity the functional interaction of these microbes or their Snt with the host intestine is functionally different and region specific. There is clearly a need to identify the bioactive molecules released by the yeasts and those that mediate the motility modifying effects on the host tissue. This is likely a difficult task since there is no reason to suppose that only a single molecule is the mediator for anyone strain; yeasts like other microbes produce a multitude of molecules, of which any combination could potentially be effective. A similar logic applies to bacterial microbes with motility modifying effects. Previous research on Lactobacillus rhamnosus JB-1™ isolated the molecule-containing microvesicles of the bacteria and tested them for their individual effect. Application of the microvesicles to the gut epithelium replicated the effects of the JB-1™ bacteria on enteric neurons[28]. In similar fashion, further isolating components of the Snt and evaluating their effects may help to narrow down the underlying bioactive molecules.
The results of the present paper suggest that there may be other yeasts with potential therapeutic actions in animal models of stress. Our ex vivo perfusion setup may provide a relatively simple, though not high throughput, method to screen yeasts or fungi for beneficial effects on the host intestine. Previously discussed results of S. cerevisiae and S. boulardii clinical trials further support the therapeutic potential of Saccharomyces yeasts in humans[7,11]. We predict that the beneficial or probiotic potential of fungi and yeasts is set to expand with increased research, initially in animal models followed by human trials.
Stress has adverse effects on intestinal motility causing irregular propagating contractile clusters (PCCs) that decrease motility in the jejunum and increase motility in the colon. Saccharomyces yeasts have been shown to have potential beneficial and probiotic effects in the intestine and in the treatment of some gastrointestinal disorders.
Most stress and probiotic research studies focus on the prevention of stress-related symptoms on the intestinal tract. The use of probiotics, including yeasts, is an emerging treatment option for gastrointestinal disorders, particularly in exchange for antibiotics.
This is first evidence of Saccharomyces yeasts acting in the pre-clinical treatment of stress-related gut dysmotility. It is also the first evidence of Saccharomyces supernatant (yeast microbes removed) having a beneficial effect on gut motility and the treatment of stress.
The data suggests that Saccharomyces yeasts may be potential therapeutic treatments for stress-related dysmotility in the intestine. Additionally, Saccharomyces supernatants cause similar effects as their respective yeasts, and might also play a therapeutic role. However, more animal experiments are needed to better support these results.
PCCs or propagating contractile clusters are sweeping bands of intestinal contraction that move in the oral to anal direction and are likely stimulated by the ENS.
The authors investigated the possible effects of Saccharomyces boulardii or Saccharomyces cerevisiae on reducing the stress-related intestinal dysmotility. The study is well designed and well implemented. The manuscript should be accepted for publication with the following revision.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bi X, Garcia-Olmo D, Islek A, Soyturk M, Welch MG, Zhang ZJ S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Moslehi-Jenabian S, Pedersen LL, Jespersen L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients. 2010;2:449-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Fietto JL, Araújo RS, Valadão FN, Fietto LG, Brandão RL, Neves MJ, Gomes FC, Nicoli JR, Castro IM. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can J Microbiol. 2004;50:615-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Edwards-Ingram L, Gitsham P, Burton N, Warhurst G, Clarke I, Hoyle D, Oliver SG, Stateva L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl Environ Microbiol. 2007;73:2458-2467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | McFarland LV. Saccharomyces boulardii is not Saccharomyces cerevisiae. Clin Infect Dis. 1996;22:200-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Czerucka D, Piche T, Rampal P. Review article: yeast as probiotics -- Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26:767-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 312] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 6. | Barnes D, Yeh AM. Bugs and Guts: Practical Applications of Probiotics for Gastrointestinal Disorders in Children. Nutr Clin Pract. 2015;30:747-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Htwe K, Yee KS, Tin M, Vandenplas Y. Effect of Saccharomyces boulardii in the treatment of acute watery diarrhea in Myanmar children: a randomized controlled study. Am J Trop Med Hyg. 2008;78:214-216. [PubMed] [Cited in This Article: ] |
| 8. | Canonici A, Siret C, Pellegrino E, Pontier-Bres R, Pouyet L, Montero MP, Colin C, Czerucka D, Rigot V, André F. Saccharomyces boulardii improves intestinal cell restitution through activation of the α2β1 integrin collagen receptor. PLoS One. 2011;6:e18427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Legras JL, Merdinoglu D, Cornuet JM, Karst F. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol Ecol. 2007;16:2091-2102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 399] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 10. | Ochigava I, Kalandarishvili L, Zhvania M. Immunological and central nervous system changes in mice suffering from Staphyloccocus aureus and treated with Saccharomyces cerevisiae var. vini living cells. Folia Microbiol (Praha). 2006;51:659-663. [PubMed] [Cited in This Article: ] |
| 11. | Spiller R, Pélerin F, Cayzeele Decherf A, Maudet C, Housez B, Cazaubiel M, Jüsten P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: improvement in abdominal pain and bloating in those with predominant constipation. United European Gastroenterol J. 2016;4:353-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Martins FS, Elian SD, Vieira AT, Tiago FC, Martins AK, Silva FC, Souza EL, Sousa LP, Araújo HR, Pimenta PF. Oral treatment with Saccharomyces cerevisiae strain UFMG 905 modulates immune responses and interferes with signal pathways involved in the activation of inflammation in a murine model of typhoid fever. Int J Med Microbiol. 2011;301:359-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | West C, Wu RY, Wong A, Stanisz AM, Yan R, Min KK, Pasyk M, McVey Neufeld KA, Karamat MI, Foster JA. Lactobacillus rhamnosus strain JB-1 reverses restraint stress-induced gut dysmotility. Neurogastroenterol Motil. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Kunze WA, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, Bienenstock J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13:2261-2270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 15. | Ma X, Mao YK, Wang B, Huizinga JD, Bienenstock J, Kunze W. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am J Physiol Gastrointest Liver Physiol. 2009;296:G868-G875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Mao YK, Kasper DL, Wang B, Forsythe P, Bienenstock J, Kunze WA. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat Commun. 2013;4:1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Perez-Burgos A, Wang B, Mao YK, Mistry B, McVey Neufeld KA, Bienenstock J, Kunze W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2013;304:G211-G220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Wang B, Mao YK, Diorio C, Pasyk M, Wu RY, Bienenstock J, Kunze WA. Luminal administration ex vivo of a live Lactobacillus species moderates mouse jejunal motility within minutes. FASEB J. 2010;24:4078-4088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Wang B, Mao YK, Diorio C, Wang L, Huizinga JD, Bienenstock J, Kunze W. Lactobacillus reuteri ingestion and IK(Ca) channel blockade have similar effects on rat colon motility and myenteric neurones. Neurogastroenterol Motil. 2010;22:98-107, e33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Wu RY, Pasyk M, Wang B, Forsythe P, Bienenstock J, Mao YK, Sharma P, Stanisz AM, Kunze WA. Spatiotemporal maps reveal regional differences in the effects on gut motility for Lactobacillus reuteri and rhamnosus strains. Neurogastroenterol Motil. 2013;25:e205-e214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Kakunaga T. The role of cell division in the malignant transformation of mouse cells treated with 3-methylcholanthrene. Cancer Res. 1975;35:1637-1642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil. 1997;9:99-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Smith TK, Oliver GR, Hennig GW, O’Shea DM, Vanden Berghe P, Kang SH, Spencer NJ. A smooth muscle tone-dependent stretch-activated migrating motor pattern in isolated guinea-pig distal colon. J Physiol. 2003;551:955-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Simpson JL, Nadler HL. Maternal serum alpha-fetoprotein screening in 1987. Obstet Gynecol. 1987;69:134-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3596] [Cited by in F6Publishing: 4141] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 25. | Cumming G, Finch S. Inference by eye: confidence intervals and how to read pictures of data. Am Psychol. 2005;60:170-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 605] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 26. | Massi M, Ioan P, Budriesi R, Chiarini A, Vitali B, Lammers KM, Gionchetti P, Campieri M, Lembo A, Brigidi P. Effects of probiotic bacteria on gastrointestinal motility in guinea-pig isolated tissue. World J Gastroenterol. 2006;12:5987-5994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Burks TF, Peterson JM, Williams , CL , Kramer TH. Restraint stress and intestinal transit in rats. In: Bueno L, Collins, S, Junien JL. Stress and digestive motility. London: John Libbey, 1989: 115-122. . [Cited in This Article: ] |
| 28. | Al-Nedawi K, Mian MF, Hossain N, Karimi K, Mao YK, Forsythe P, Min KK, Stanisz AM, Kunze WA, Bienenstock J. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2015;29:684-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |