Published online Mar 7, 2016. doi: 10.3748/wjg.v22.i9.2678
Peer-review started: September 29, 2015
First decision: November 13, 2015
Revised: December 19, 2015
Accepted: January 11, 2016
Article in press: January 11, 2016
Published online: March 7, 2016
Pancreatic cancer (PC) is the most aggressive type of common cancers, and in 2014, nearly 40000 patients died from the disease in the United States. Pancreatic ductal adenocarcinoma, which accounts for the majority of PC cases, is characterized by an intense stromal desmoplastic reaction surrounding the cancer cells. Cancer-associated fibroblasts (CAFs) are the main effector cells in the desmoplastic reaction, and pancreatic stellate cells are the most important source of CAFs. However, other important components of the PC stroma are inflammatory cells and endothelial cells. The aim of this review is to describe the complex interplay between PC cells and the cellular and non-cellular components of the tumour stroma. Published data have indicated that the desmoplastic stroma protects PC cells against chemotherapy and radiation therapy and that it might promote the proliferation and migration of PC cells. However, in animal studies, experimental depletion of the desmoplastic stroma and CAFs has led to more aggressive cancers. Hence, the precise role of the tumour stroma in PC remains to be elucidated. However, it is likely that a context-dependent therapeutic modification, rather than pure depletion, of the PC stroma holds potential for the development of new treatment strategies for PC patients.
Core tip: Pancreatic cancer (PC), the most aggressive type of common cancers, is characterized by a limited response to chemotherapeutics, which are often directed against the PC cells. One of the histological hallmarks of PC is the extensive desmoplastic stromal reaction that surrounds the PC cells. The PC stroma is not simply a bystander of the neoplastic process but plays an active part in disease progression and metastasis. The PC cells, cancer-associated fibroblasts, inflammatory cells, endothelial cells, and the extracellular matrix engage in a complex interplay, the modulation of which could hold potential for the future development of new PC therapies.
- Citation: Nielsen MFB, Mortensen MB, Detlefsen S. Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol 2016; 22(9): 2678-2700
- URL: https://www.wjgnet.com/1007-9327/full/v22/i9/2678.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i9.2678
In 2014, pancreatic cancer (PC) was the fourth leading cause of cancer-associated deaths in the United States, with 46420 estimated new cases and 39590 estimated deaths[1]. The 5-year PC survival rate is approximately 5%, and surgical resection offers the only option for long-term survival[1]. However, even for patients who undergo surgery for localized disease, the 5-year survival rate is only approximately 20%[2].
Pancreatic ductal adenocarcinoma (PDAC) accounts for approximately 85% of all PCs, and most of the published data have indicated that PDAC arises from ductal epithelial cells[2,3]. However, an alternative hypothesis, called acinar-ductal metaplasia (ADM), suggests that PDAC arises from the centro-acinar acinar compartment (CAAC)[4]. According to a well-established progression model for the development of PDAC, the normal duct epithelium progresses to invasive adenocarcinoma through histologically well-defined stages of duct lesions, the so-called pancreatic intraepithelial neoplasias (PanINs)[5,6]. However, not all PanINs will progress to invasive cancer. The PanIN subsets are histologically distinctive and genetic alterations characteristic of invasive adenocarcinomas can be identified in early ductal lesions[5]. Molecular abnormalities, which include the overexpression of oncogenes (K-ras and HER2/neu) and the deletion of tumour-suppressor genes (p16, p53, DPC4, and BRCA2), accumulate during progression through the different PanIN stages[5,7]. In support of the credibility of this progression model, approximately two-thirds of somatic mutations have been found to be shared between PanINs and associated invasive carcinomas[8]. Animal models using rats and transgenic mice, however, found that so-called tubular complexes, which are thought to originate via ADM, might be important precursor lesions in PDAC[9-11]. In subsequent studies using genetically engineered mouse models (GEMMs) and human tissue, it was hypothesized that ADM might progress and contribute to the development of PanINs[12,13]. In support of the ADM hypothesis, ADM-associated atypical flat lesions were found to be the most likely PDAC precursor in KrasG12D/+, Ptf1a-Creex1/+ GEMMs and in patients with family histories of PDAC, but not in sporadic PDAC[4]. In sporadic PDAC, however, tubular complexes and mucinous tubular complexes were frequently found.
Studies using PC animal models, especially GEMMs, have contributed to the understanding of PDAC progression[14,15]. GEMMs are considered superior to xenograft models, in which PC tumours are formed when human PC cells are introduced into immunocompromised mice. In GEMMs of PC, endogenous expression of K-ras is switched on in the progenitor cells of the mouse pancreas[16]. Such molecular modifications result in progression to invasive PC through distinct stages of ductal lesions that mimic human PanINs. Human 3-dimensional (3D) organotypic models have contributed to the characterization of tumour-stroma crosstalk in PC[17,18]. The organotypic model is a simplified representation of the complex in vivo 3D microenvironment. Organotypic models are relatively easy manipulated, and they constitute a useful tool for the systematic examination of anticancer therapies.
The most common symptoms in patients with PC are asthenia, anorexia, weight loss, icterus, and abdominal pain[19]. The majority of PC patients are diagnosed in the late stage of disease, when surgical resection is no longer an option. Currently, effective screening tools to detect premalignant or early stages of the disease are not available. Both inherent and environmental factors are key contributors to the development of PC[20]. Cigarette smoking is the most important predisposing factor. Up to 20%-25% of all PC cases can be attributed to smoking, and current smokers have a 2.2-fold higher risk of developing PC than people who have never smoked[21,22]. Other important risk factors include type 2 diabetes, chronic pancreatitis, and heavy alcohol consumption[23-27]. According to a meta-analysis of seven case-control and two cohort studies including 6568 patients, individuals with family histories of PC had a nearly 2-fold increased risk of developing PC[28]. Approximately 10% of all PC cases have a familial background, and BRCA2, PALP2, and p16 germline mutations have been associated with familial pancreatic cancer (FPC)[20,29,30]. FPC is defined as PC occurring in a patient with two or more first-degree relatives with PC[31,32]. FPC is inherited as an autosomal dominant trait with high penetrance in family members[33]. The risk of developing PC is very high in cases with hereditary pancreatitis, which is most often caused by mutations in the cationic trypsinogen gene (PRSS1)[34].
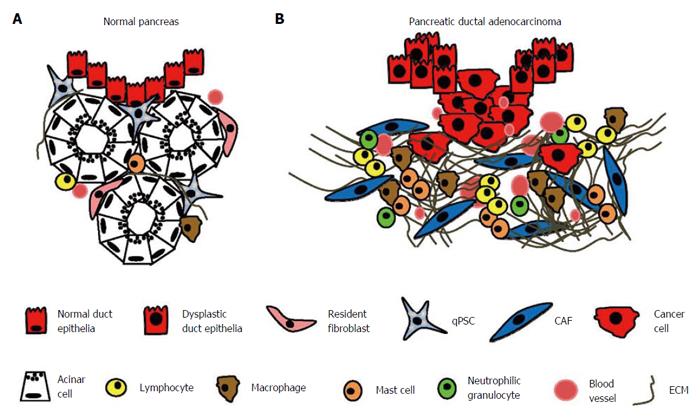
PDAC is distinguished from many other cancer types by the excessive amount of scar tissue (“desmoplasia”) that surrounds the malignant cells and occupies up to 80% of the entire cancer nodule[35,36]. The desmoplastic stroma consists predominantly of cancer-associated fibroblasts (CAFs), inflammatory cells, small blood vessels, and extracellular matrix (ECM)[36] (Figure 1). Resistance to therapy is the most important clinical challenge in PC. The increased use of combination chemotherapy could benefit selected patients in both a palliative and neo-adjuvant setting, but this treatment strategy is non-specific and has no significant impact on long-term outcome. Numerous studies have now documented tumour-promoting functions of the key components of the tumour stroma, including CAFs, ECM, endothelial cells, and inflammatory cells. However, mouse studies focused on stromal depletion have illustrated a tumour-suppressing, rather than a tumour-promoting, role of the stromal compartment. Hence, the precise role of the tumour stroma is currently controversial, and the aim of this review is to assess the context-dependent role of the various stromal components in the development of PC, as well as their potential for the future development of new treatment strategies.
CAFs, originally known as carcinoma-associated fibroblasts[37], play a significant role in tumour growth and progression. These cells display a myofibroblast-like phenotype, characterized by a spindle shape and the expression of α-smooth muscle actin (α-SMA). Functionally, CAFs are characterized by their production of a wide variety of ECM molecules and cytokines.
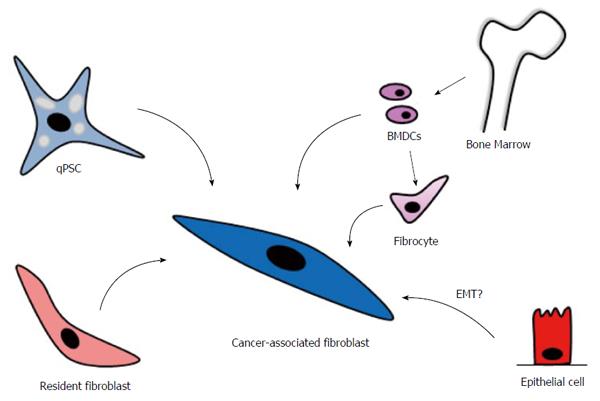
CAFs can originate from different cellular sources, including pancreatic stellate cells (PSCs), which are considered to be the most important source by far, and probably resident fibroblasts and bone marrow-derived cells (BMDCs) (Figure 2)[38,39]. Epithelial cells, through epithelial-mesenchymal transition, have also been proposed as a cellular source of CAFs, but this hypothesis is still under debate[40,41].
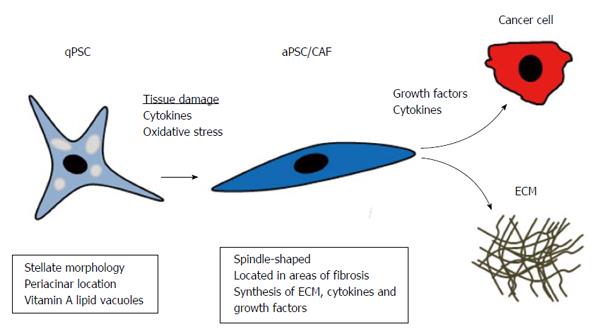
PSCs: PSCs are the most important cellular source of CAFs in PC[39]. Additionally, PSCs are the main effector cells in the fibrotic process of chronic pancreatitis[42-45]. PSCs have much in common with hepatic stellate cells (HSCs) because they both store vitamin A and are both characterized by their stellate morphology. PSCs came into focus when they were isolated and cultured by two independent research groups in 1998[46,47]. However, retrospectively, it was probably the same vitamin A-storing cell described by Watari in mice and by Ikejiri in humans[48,49]. In the normal pancreas, quiescent PSCs (qPSCs) are located in the periacinar space, and in the rat pancreas, they constitute approximately 4% of all parenchymal cells[46,47]. qPSCs function as a storage site for vitamin A and might be involved in ECM turnover because they have the capacity to produce matrix metalloproteinases (MMPs)[50]. Numerous biomarkers for qPSCs have been reported[39], including desmin[46,47], nestin[51], vimentin[47], synemin[52], and glial fibrillary acidic protein (GFAP)[46], but some of these markers have been identified in rats only, and most of them are not entirely specific for qPSCs. Hence, entirely specific immunohistochemical biomarkers for human qPSCs applicable in routine diagnostics have yet to be identified. During tissue injury or carcinogenesis, qPSCs become activated, attaining a state called activated PSCs (aPSCs), and they develop a myofibroblast-like phenotype[39] (Figure 3). There are several factors that activate qPSCs, such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGFβ)[53,54], tumour necrosis factor alpha (TNFα), and interleukins 1, 6, and 10 (IL-1, IL6 and IL10)[55]. PSCs express receptors for most of these cytokines in chronic pancreatitis, as well as in PDAC[44,45,56,57]. Activated PSCs, in turn, produce PDGF and TGFβ, which contribute to autocrine signalling[58,59]. Cultured rat qPSCs are activated by alcohol derivatives and oxidative stress, which could explain the fibrotic reaction in alcoholic chronic pancreatitis[60]. This activation is a reversible process because incubation of PSCs with trans-retinol retinoic acid induced quiescence in aPSCs in vitro[61]. Additionally, human aPSCs were deactivated when cultured on Matrigel and treated with N-acetylcysteine (NAC)[62]. NAC induced similar cell cycle arrest in activated HSCs through the mitogen-activated protein kinase (MAPK) kinase pathway[63].
Activated PSCs lose their vitamin A lipid vacuoles, develop a spindle-shaped morphology, and begin to express α-SMA[46,47]. They differ from their quiescent counterparts in that they proliferate and develop migratory and phagocytic properties[50,64]. The secreted proteomes of cultured qPSCs and aPSCs have been compared, utilizing an immortalized cell line derived from human PSCs[65]. Proteomic profiling identified 641 unique proteins secreted by aPSC, compared to only 46 unique proteins identified in qPSCs. These proteins were associated with proliferation, inflammation, ECM remodelling, cell motility, and invasion, supporting an active role for aPSCs in PC progression[65].
Bone marrow-derived cells: CAFs can arise from stem cells originating from the bone marrow[66-69]. Bone marrow-derived cells (BMDCs) were recruited to the pancreatic stroma in a rat model of acute pancreatitis induced by a choline-deficient/ethionine-supplemented diet[70]. Tagged BMDCs from female mice were transplanted into male rats and contributed to the aPSC pool, as indicated by their expression of desmin and α-SMA. In an experimental chronic pancreatitis mouse model, BMDCs were tracked with green fluorescent protein on the Y-chromosome in sex-mismatched transplanted mice[66]. Desmin-positive BMDCs engrafted to the pancreas and contributed to approximately 5% of all PSCs. A fraction of the bone marrow-derived cells expressed α-SMA, indicating that BMDCs had been activated and had contributed to tissue repair[66]. In another study, BMDCs were transplanted from male β-actin-EGFP mice into female C57/BL6 mice, which were divided into two groups. One group had caerulein-induced chronic pancreatitis and the other had dimethylbenzanthracene (DMBA)-induced PC. The engrafted BMDCs contributed to the population of aPSCs in fibrotic areas in both groups[67]. Similar observations had already been made in rats in a 2009 study[71]. Chronic pancreatitis is a risk factor for the development of PC[26], and based on the above studies, it is tempting to speculate that BMDCs might contribute to the CAF population in human PC.
It has been documented that BMDCs with a fibrocyte precursor phenotype (CD45+, collagen 1+) circulate in the peripheral blood before engrafting to the fibrotic pancreas[68,72]. In the pancreas, as well as other organs, mature fibrocytes transform into myofibroblasts and participate in fibrogenesis by producing ECM and MMPs[68,73,74]. When BMDCs from male mice were injected into the tail veins of female mice, followed by induction of pancreatic fibrosis with cerulein, CD45+ fibrocytes harbouring the Y-chromosome circulated in the peripheral blood, indicating that they represented transplanted BMDCs[68]. These circulating fibrocytes functionally contributed to pancreatic fibrosis by differentiating into myofibroblasts[68]. Using a similar approach with induced pancreatic insulinomas in mice, donor-derived fibrocytes were found in the stroma of these tumours[72].
Resident fibroblasts: Resident (local) fibroblasts are an important CAF source in many types of cancers[75]. Fibroblasts are abundant in connective tissue, producing ECM and contributing to tissue homeostasis. They are of mesodermal origin and characterized by their spindle shape and extended cell processes[76,77]. One challenge in studying fibroblasts is the lack of specific molecular markers. Fibroblasts from different body sites display a diverse transcriptional pattern, as shown in the gene expression profiles of 50 primary human fibroblast cultures obtained at 10 different sites from 16 donors[78]. Fibroblast-specific protein 1 (FSP-1) seems to be the most useful marker for fibroblasts in vivo[76,77,79]. In the mouse pancreas, FSP-1 expression has been identified in interlobular fibroblasts[80], as well as in in dendritic cells[81] and aPSCs[80]. In PDAC, FSP-1 has been documented in both human and mouse CAFs[82,83] as well as in human PC cells[84,85]. The above studies demonstrate that FSP-1 is not a totally specific marker for resident pancreatic fibroblasts. High FSP-1 expression in human PDAC has been associated with increased invasion and shorter survival[84-86].
Upon tissue damage, cell necrosis/apoptosis leads to the release of inflammatory cytokines (e.g., TGFβ1 and PDGF) and chemokines from local inflammatory cells, endothelial cells, or mesenchymal cells[87,88]. Subsequently, resident fibroblasts are activated, and they proliferate and differentiate into myofibroblasts, thereby contributing to the CAF pool. However, this process has not yet been documented in human PDAC, in which qPSCs seem to be by far the most important source of CAFs[39].
CAFs and PDAC cells mutually promote each other’s proliferation and differentiation. Supernatants from cultured PDAC cells induced the proliferation and production of ECM proteins in PSCs[89]. Conversely, CAF culture media induced the proliferation and migration of PDAC cells, and the growth rate of PDAC cells was markedly increased when PSCs were co-injected into nude mice[89-91].
To mimic the in vivo crosstalk between PC cells and stromal cells, 3D cell cultures of human PC cells and immortalized PSCs were cultured on Matrigel and collagen, simulating the in vivo PDAC microenvironment[92]. Under these conditions, PSCs modulated the expression of adhesion molecules on the cancer cells, increasing their invasiveness, in association with the downregulation of E-cadherin and the upregulation of β-catenin[92]. These findings suggest that PSCs could play an important role in PC metastasis. Another study co-cultured PSCs with PDAC cells using conventional cell cultures[93]. The epithelial markers E-cadherin, cytokeratin 19, and β-catenin were downregulated in PDAC; the mesenchymal markers vimentin and snail were upregulated; and cancer cell migration was increased[93]. In an orthotopic xenograft mouse model, in which male human PSCs were co-injected with female PDAC cells into the pancreases of female mice, PSCs followed the PC cells to the metastatic sites, suggesting that PSCs could play a role in the settlement of metastatic PDAC cells[94].
In addition to their role as promoters of PC cell proliferation and migration, CAFs have also been shown to protect PC cells from chemotherapy and radiation therapy (CRT). Hwang et al[90] isolated human PSCs from resected PC samples and developed an immortalized cell line. When the in vitro effects of PSC-conditioned media on PC cell survival were assessed in the presence of gemcitabine (100 μmol/L) or radiation therapy (100-Gy), components in the PSC media protected the PC cells against apoptosis[90]. Using monocultures or direct co-culture of PC cells with PSCs, PC cell survival during radiation was increased in the presence of PSCs[91,95]. This radioprotective effect of PSCs was significantly reduced using antibodies blocking β1-integrin signalling[91]. However, in this study, it was not confirmed that PSC-conditioned media displayed radioprotective properties, and it was suggested that direct contact between PSCs and the PDAC cells was necessary[91]. The ability of PSCs to radioprotect PC cells in vivo was demonstrated in xenograft models in which nude mice received subcutaneous injections of human PC cells alone or together with human PSCs[91,95]. Tumour growth was delayed as a response to radiation in both cases, but the response was less pronounced in tumours formed from both PC cells and PSCs than in tumours formed from PC cells alone[91,95]. Cultured human tumour-derived PSCs, obtained from fine-needle aspirates, had a more activated phenotype after exposure to CRT, compared to PSCs isolated prior to CRT[96].
Based upon the above observations, one would assume that a decrease in the CAF population would result in a concomitant decrease in PC cell proliferation/migration and an increased response to CRT. Data regarding the consequences of CAF depletion in the PDAC stroma have, however, been conflicting. The effect of gemcitabine was examined in KrasLSL.G12D/+;p53R172H/+;PdxCretg/+ (KPC) mice depleted of desmoplastic stroma and CAFs[97]. The KPC mouse is a frequently applied GEMM, expressing endogenous mutant Kras and p53 alleles in pancreatic cells and developing pancreatic tumours that morphologically closely resemble human PC[97]. Like their human counterparts, the KPC mouse tumours also have a poor response to gemcitabine. In control KPC mice, only two of 17 tumours responded to gemcitabine treatment, and this finding is similar to clinical results in human PDAC patients, in whom the response rate is 5% to 10%. When applying IPI-926, an inhibitor of the sonic hedgehog (shh) pathway, depletion of the desmoplastic stroma and of α-SMA positive CAFs was observed. This finding resulted in increased vascularization and more effective drug delivery, with improved overall survival. These data suggest that depletion of stromal tissue and CAFs in PDAC stimulated angiogenesis and enhanced drug delivery[97]. This finding could explain why inhibitors of angiogenesis have failed to significantly improve outcomes in PC, as approaches would potentially result in the decreased delivery of chemotherapeutic agents. Recently, IPI-926 was applied in a phase I study in combination with oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) (Table 1)[98]. The study was closed early because a separate phase II trial of IPI-926 plus gemcitabine indicated a shorter median survival in patients receiving this treatment.
| Clinical trial | Target | Drug | Results |
| Bramhall et al[138], 2002 | ECM | Marimastat: MMP inhibitor | No survival advantage was found in patients receiving marimastat in combination with gemcitabine compared to patients receiving gemcitabine alone |
| Moore et al[137], 2003 | ECM | Bay 12-9566: MMP inhibitor | Phase III trial was closed early because Bay12-9566 treatment was inferior to gemcitabine treatment |
| Strimpakos et al[141], 2013 | ECM | PEGPH20: degradation of hyaluronan | Phase Ib trial of PEGPH20 administration in combination with gemcitabine showed no significant toxicity. Phase II trials are warranted |
| Lutz et al[217], 2011; Laheru et al[218], 2008 | Immune cells | GVAX: GM-CSF-based immunotherapy | Phase II trial showed that GM-CSF-based immunotherapy was a safe immunotherapeutic approach in combination with chemoradiation therapy. Phase II, multicentre trials are warranted |
| Royal et al[192], 2010 | Immune cells | Ipilimumab: CTLA-4 inhibitor | Phase II trial of administration of ipilimumab did not show an acceptable response as a single-agent therapy |
| Le et al[219], 2013 | Immune cells | Ipilimumab + GVAX | Phase Ib trial of ipilimumab in combination with GVAX demonstrated an improvement in overall survival compared to patients receiving ipilimumab alone. Phase II trials are warranted |
| Brahmer et al[194], 2012 | Immune cells | BMS-936559: PDL1 inhibitor | Phase I trial found no objective response in PC patients receiving BMS-936559 treatment |
| Beatty et al[168], 2013 | Immune cells | CP-870893: CD40 agonist | Phase I trial showed that CP-870,893 was well-tolerated in combination with gemcitabine in patients with advanced PDAC, and immune activation was observed. Phase II trials are warranted |
| Ko et al[98], 2015 | Stromal depletion | IPI-926 (saridegib): hedgehog inhibitor | Phase Ib trial administered IPI-926 in combination with FOLFIRINOX. The study was closed early because a separate trial documented that the patients experienced a shorter median survival |
| Kindler et al[210], 2010 | Angiogenesis | Bevacizumab: VEGF-A inhibitor | Phase III trial of bevacizumab in combination with gemcitabine did not improve overall survival compared to patients receiving gemcitabine alone |
| Kindler et al[211], 2011 | Angiogenesis | Axitinib: inhibitor of VEGF receptors 1, 2 and 3 | Phase III trial of axitinib in combination with gemcitabine did not improve overall survival compared to patients receiving gemcitabine alone |
The desmoplastic stroma, however, does not only form a barrier that reduces tumour perfusion and hampers the effect of chemotherapeutic treatment. Recent data, based on studies on GEMMs, have indicated that the desmoplastic stroma, as a whole, might reduce the ability of PDAC cells to invade the surrounding tissue and metastasize[99-101]. When PKT (Ptf1acre/+; LSL-KrasG12D/+; Tgfbr2flox/flox) mice developing spontaneous PC were depleted of α-SMA-positive myofibroblasts, more invasive tumours developed and survival was reduced[99]. In agreement with these findings in mice, immunohistochemical scoring of interstitial α-SMA positive cells from 53 resected PDAC specimens revealed that small numbers of α-SMA positive cells were associated with shorter survival[99]. Stromal depletion by deleting shh was examined in the PKCY mouse model, resulting in earlier tumour development and decreased survival[100]. In subsequent experiments, shh deletion resulted in a significantly reduced stroma and the reduction of α-SMA positive myofibroblasts.
When primary human cancer-associated PSCs were treated with the Vitamin D analogue calcipotriol, lipid droplet formation increased and α-SMA expression decreased, resulting in the induction of quiescence in CAFs[102]. To examine the clinical potential of vitamin D receptor activation, calcipotriol was administered to KPC mice together with gemcitabine, resulting in significant reduction of tumour size in 70% of mice, while the intratumoral concentration of gemcitabine triphosphate, the active metabolite of gemcitabine, increased by 500%. The median survival was increased by 57%, supporting the possibility that stromal reprogramming to a more quiescent state, rather than its total ablation, is efficient in PC[102]. Notably, this study indicated that deactivation of CAFs increased the susceptibility of PC cells to chemotherapy. Based on these assumptions, Gundewar et al[103] identified a new agent that could potentially inhibit the anti-therapeutic effects of CAFs. These authors were able to inhibit the proliferation of immortalized human PSCs when cultured with L49H37, a synthetic analogue of curcumin. The L49H37-treated cells were retained in the G0/G1 phase, possibly due to elevated phosphorylation of ERK1/2 and the downregulation of p21.
Taken together, the above studies illustrate the complex role of the desmoplastic tumour stroma in the progression of PC. Most of the initial studies indicated that the desmoplasia and the cells producing it hampered the effect of chemotherapy and promoted PDAC cell proliferation and metastasis. More recent studies, however, have supported the idea that the desmoplastic stroma might form a barrier that reduces the invasion of PDAC cells. Hence, the role of the desmoplasia seems to be context-dependent. Induction of quiescence in CAFs, leading to modulation of the tumour stroma rather than ablation, could be the most promising approach for the future development of treatments targeting tumour desmoplasia in PC.
High “stromal activity”, i.e., elevated expression of α-SMA-positive CAFs in the stroma surrounding the cancer cells, had a negative prognostic impact in PDAC patients, as shown in a study of 233 resected PDAC specimens[104]. The significance of high α-SMA expression in the PDAC tumour stroma was later supported by a study of 162 patients[105]. However, as mentioned above, depletion of α-SMA-positive myofibroblasts in the PDAC stroma of PKT mice was associated with shorter survival[99]. Large amounts of secreted protein acidic and rich in cysteine (SPARC), an alternative CAF marker in PDAC, had negative prognostic value in PDAC[106-109]. Immunohistochemical characterization of 299 PDAC resection specimens revealed that patients labelled positive for stromal SPARC had a median survival of 15 mo, compared to 30 mo for patients with no stromal SPARC expression[109]. Immunohistochemical characterization of SPARC expression in PC biopsy specimens from 58 non-resectable patients prior to CRT revealed that patient survival was inversely correlated with stromal SPARC expression[108]. High SPARC expression, however, had a negative prognostic impact only in patients treated with adjuvant gemcitabine after resection but not in adjuvant treatment-naive patients[107].
The desmoplastic stroma in PDAC is not a homogeneous tissue. Different stromal compartments can be more or less active, and the distribution and frequency of different cell types vary in them. The desmoplastic stromal compartments were classified based on in situ hybridization experiments on human PDAC tissues[110]. Messenger RNA expression of 12 genes was scored in the entire tumour stroma, called the panstroma, compared to the stroma in the immediate vicinity of the cancer cells, called the juxtatumoral stroma. Three genes (apolipoprotein C-1, apolipoprotein D and MMP11) were observed in the juxtatumoral stroma but not in stromal cells located distantly from the cancer cells. Further, α-2 macroglobulin was localized to non-neoplastic endothelial cells and the juxtatumoral stroma but not to the peripheral stroma. It was suggested that the juxtatumoral stroma plays a more active role in the invasive process in PDAC[110]. The expression of the above-mentioned genes in PDAC was further evaluated in seven PCs associated with intraductal papillary mucinous neoplasms (IPMNs), of which two were colloid carcinomas, two were tubular carcinomas, and three were mixed colloid/tubular carcinomas[111]. The three genes were expressed in the juxtatumoral stroma in all areas with tubular carcinoma, but no expression was observed in the juxtatumoral stroma of the two colloid carcinomas. This study also included eight PDAC specimens, two of which had liver metastases. Apolipoprotein C-1 and apolipoprotein D, but not MMP11, were also expressed focally in one of the metastases[111]. These data indicate that the three juxtatumoral markers could be of specific importance for the invasion of pancreatobiliary-type adenocarcinomas but not necessarily for the implantation of metastatic PC cells in the liver.
Alpha-SMA is routinely used to identify aPSCs and CAFs in PDAC. However, different subsets of CAFs might be present in the PC stroma[82,112]. Mouse models of PC (Rip1Tag2 mice) and breast cancer (4T1 cells injected into BALB/c mice) harboured CAF subpopulations, in which FSP1-positive CAFs in particular showed only minimal overlap with other CAF markers, such as neuron-glial antigen 2 (NG2), α-SMA, and PDFGRβ[82]. A CD10-positive subpopulation of CAFs was identified in human PDAC specimens[112]. They were localized juxtatumorally, in close proximity to PDAC cells in 28 of 83 specimens, and these patients had significantly shorter survival. Additional studies focusing on the possible different roles of CAF subpopulations would be of great interest, as such studies have the potential to cast additional light on the suggested context-dependent functional roles of CAFs in PDAC.
The desmoplastic stroma in PDAC contains large amounts of ECM molecules (Figure 4). CAFs are responsible for ECM synthesis in PDAC. Many ECM proteins have been described in PDAC, including fibronectin[53,113], laminin[53,113,114], tenascin C[115], hyaluronan[116-119], collagen I[47,53,114,120,121], collagen III[53,114], collagen IV[114,122,123], and collagen XIA[124,125].
Increased PC cell death was observed in vitro when cancer cells were detached from the ECM, whereas survival was increased when cancer cells were cultured with laminin or fibronectin[126]. In PC resection specimens, samples with high stromal activity and low collagen deposition were associated with shorter patient survival, whereas the opposite was true for samples with low stromal activity and high collagen deposition[104].
Collagens I and IV induce PC cell migration and metastasis[127,128]. The human PC cell lines BxPC-3 and Panc-1 became more motile when cultured on collagen I-coated dishes, compared to non-, fibronectin-, or laminin-I-coated plates[127]. The migratory properties were accompanied by a more mesenchymal phenotype, due to upregulation of N-cadherin, vimentin, and snail[127,129]. Collagen I-induced PC cell migration was demolished after treatment with an inhibitor of c-Jun NH2-terminal kinase (JNK). Inhibition of other signalling pathways, however, did not influence collagen I-induced migration[129].
ECM breakdown is considered a mandatory step in the processes of tumour invasion and metastasis[130]. The family of matrix metalloproteinases (MMPs) are the enzymes that are most responsible for degrading ECM proteins[131]. ECM turnover is a highly dynamic process, and ECM components are constantly synthesized, predominantly by fibroblasts, and broken down by MMPs. The different MMPs share a similar domain structure, and their activity is regulated by tissue inhibitors of metalloproteinases (TIMPs)[131]. The expression of mRNAs encoding ECM-degrading MMPs was examined in human PC specimens[132]. MMP-2 and MMP-9 were elevated compared to the normal pancreas, and by in situ hybridization, they were particularly located in PC cells and spindle-shaped stromal cells (CAFs/aPSCs)[132]. Expression of these MMPs and of MMP13 was later observed in cultured rat PSCs. MMP2 expression was significantly increased when PSCs were culture-activated or stimulated with TGF-β1 and IL-6[50].
Koshiba et al[133] examined the role of MMP-2 in human PC resection specimens using gelatine zymography. The MMP-2 activation ratio was significantly higher than in the normal pancreas, and the ratio was significantly higher in high tumour stage specimens (pT3) and in patients with lymph node and distant metastases compared to pT1 tumours without metastasis. Hence, the upregulation of MMP-2 might play a significant role in tumour invasion and metastasis[133]. However, no correlation has been observed between MMP-2 expression and overall survival[134,135]. The prognostic role of MMP2 was not confirmed in a later study that examined the immunohistochemical expression of MMPs 1-3, 7-9, 11, 12, and 14 in resection specimens[136]. Instead, high expression of MMP7 in particular as well as MMP11 was found to be associated with a poor prognosis. Administration of MMP inhibitors, either alone or in combination with gemcitabine, to patients with advanced PDAC has not shown promising results (Table 1)[137,138].
The interstitial fluid pressure (IFP) in organs is typically lower than or at the same level as the intravascular pressure in terminal blood vessels, thereby allowing the delivery and diffusion of solutes and fluid. High IFP might explain the limited delivery of chemotherapeutic drugs into cancer nodules[117,139]. In KPC mice, the ECM compound hyaluronan was present in large amounts in the juxtatumoral stroma and was also deposited around early precursor lesions of PC[116]. The IFP was significantly increased, and hyaluronan contributed to the increase in IFP in PC tumours, as indicated after implanting matrices with PC cells and different concentrations of hyaluronan. Conversely, the IFP was decreased after treatment with PEGPH20, a hyaluronan-degrading enzyme[116]. The increase in IFP was associated with vascular collapses, further contributing to reduced tumour perfusion. PEGPH20 was able to restore functional perfusion of the collapsed vascular structures. Overall, survival improved after the treatment of tumour-bearing mice with a combination of PEGPH20 and gemcitabine[116,118]. Importantly, increased drug delivery to the tumour cells does not necessarily guarantee an increased drug response. When gemcitabine was administered to KPC mice in combination with 3,4,5,6-tetrahydrouridine (THU), an inhibitor of the gemcitabine-inactivating properties of cytidine deaminase, a significantly increased concentration of the active gemcitabine metabolite was observed in both plasma and tumour biopsies[140]. Surprisingly, higher concentrations of active gemcitabine did not significantly reduce tumour volumes in mice treated with gemcitabine + THU compared to gemcitabine only. Phase I trials of PEGPH20 administration in combination with gemcitabine have been performed, and no significant toxicity has been observed (Table 1)[141]. Therefore, phase II trials are currently planned[141].
Epithelial-mesenchymal transition (EMT), originally called epithelial-mesenchymal transformation[142], is characterized by the loss of epithelial properties and the acquisition of a mesenchymal phenotype. Epithelial cell markers, such as E-cadherin, are downregulated, while the expression of mesenchymal cell markers, such as vimentin, is increased[143,144]. Three types of EMT have been defined: type 1 is associated with implantation of the fertilized ovum, the following embryogenesis, and development of organs, type 2 with tissue regeneration and fibrotic processes, and type 3 with cancer progression and metastasis[145]. The importance of type 3 EMT in the development of metastases in human PC is still debated, and most studies have been performed on animals and cultured human cells[146]. Snail and slug, two transcription factors that repress E-cadherin, were expressed in human PC tissues but not in normal epithelial cells[147]. E-cadherin, fibronectin and vimentin expression was characterized in 34 PC resection specimens using immunohistochemistry[148]. In a subgroup of these specimens, PC cells expressed vimentin and fibronectin and showed downregulation of E-cadherin. These patients had decreased survival, indicating that EMT might be related to a more aggressive cancer type[148]. PKCY mice (Pdx1-Cre; KrasG12D; p53fl/+; RosaYFP) mimic human PDAC in that they develop PanINs, primary tumours, and metastases with a morphology similar to that in humans[144]. Further, the pancreatic epithelial cells in PKCY mice could be tracked (YFP+-labelled cells). PC cells that underwent EMT could be identified by their expression of zinc finger E-box-binding homeobox (Zeb1) and the loss of E-cadherin. Zeb1 is an activator of EMT, promoting tumorigenesis[149]. Of the YFP+-labelled cells in the PKCY tumours, 42% underwent EMT[144]. Interestingly, EMT could be observed in PanINs before the onset of tumour formation because YFP+ Zeb1+ cells were already present in 8- to 10-wk-old PKCY mice, when no histological evidence of PDAC was present. At the same stage, circulating pancreatic epithelial cells (YFP+ cells) could be observed in the blood by flow cytometry, and YFP+ cells had seeded to the liver in some PanIN mice. Circulating pancreatic tumour cells from both PanIN and PDAC mice maintained a mesenchymal phenotype, as indicated by the expression of Zeb1 and reduced expression of E-cad, CK19, and EpCAM[144].
The presence and function of specialized tumour-initiating cells, the so-called cancer stem cells (CSCs), have been controversial[150]. CSCs are defined as malignant cells with stem cell properties, that is, the ability to undergo self-renewal and the potential to differentiate into all of the cell types corresponding to the original tumour. It has been hypothesized that EMT precedes the acquisition of stem cells traits[151,152]. In PC, support for this hypothesis has been found in several studies[153-156]. Immunohistochemical aldehyde dehydrogenase (ALDH) expression was detected in 90 of 269 PC resection specimens and was related to reduced overall survival[153]. ALDH-positive PC cells were more tumorigenic than ALDH-negative cells in vitro as well as when injected subcutaneously into the flanks of athymic mice[153].
Well-described CSC markers in PC include CD24, CD44, and CD133[157,158]. Human PC cells with a CD24+, CD44+, epithelial-specific antigen (ESA)+ phenotype were 100 times more tumorigenic than CD24-, CD44-, ESA- PC cells when subcutaneously injected into NOD/SCID mice[157]. Similarly, 500 patient-derived CD133+ pancreatic CSCs were capable of inducing orthotopic tumours in athymic mice, whereas 106 CD133- tumour cells did not form any tumours[158]. The expression of CD24, CD44, and CD133 relative to EMT was examined in cell cultures, as well as PC resection specimens[154]. The expression of vimentin was correlated positively, and E-cadherin was correlated negatively with the expression of CD24, C44, and CD133, thus supporting the theory that type 3 EMT might be correlated with the initiation of a stem cell program in PC[154]. Vimentin and urokinase-type plasminogen activator receptor expression was correlated with CD24 and CD44 expression in PC resection specimens[155]. Given the upregulation of mesenchymal markers in PC cells, a functional role of CD133 in PC tumorigenesis was suggested[156]. In vitro knockdown of CD133 with shRNA in the highly migratory human PC cell line Capan1M9 (CD133high) generated the cell line shCD133M9, which showed reduced expression of mesenchymal factors, such as slug, N-cadherin, and fibronectin. Moreover, when injected orthotopically into nude mice, shCD133M9 cells were less invasive and produced fewer metastases[156].
Nodal and activin are secreted proteins that belong to the TGFβ superfamily, and they are strongly expressed in pancreatic CSCs and PSCs[159]. Inhibition of the nodal/activin pathway reversed CSC self-renewal and tumorigenesis[159]. In a later study, CSCs from human PDAC xenografts were isolated by sphere formation assay, and conditioned media from the cancer cells promoted nodal and activin A expression in PSCs[160]. Conversely, conditioned media from PSCs resulted in only a small increase in activin expression in PC cells. However, conditioned media from PSCs resulted in increased invasiveness of CSCs through nodal/activin signalling[160]. It was proposed that PSCs might form a niche for CSCs, promoting invasiveness and self-renewal through nodal/activin signalling.
In 3D indirect co-cultures with different human PC cell lines, PSCs enhanced the CSC phenotype, as indicated by increased sphere formation and CSC markers[95]. In this model, each sphere was believed to derive from one single cell. In a xenograft model, human PC cells were transplanted into mice alone or with human PSCs, inducing CD24 and CD326 (EpCAM). The stemness-promoting effect of PSCs was in part attributed to TGFβ signalling because TGFβ neutralizing antibodies inhibited CSC sphere formation ability and downregulated EMT markers[95].
Macrophages might also potentiate CSC features in PC[161]. When human sphere-derived CSC-enriched PC cells were injected into nude mice, tumour growth accelerated when co-injected with macrophages. In subsequent co-culture studies in trans-wells, microarray analyses showed upregulation of human cationic antimicrobial protein 18 (hCAP-18) and its cleavage product leucine leucine-37 (LL-37) in macrophages. The expression of hCAP-18/LL-37 in human PDAC was increased compared to the normal pancreas. Serial staining showed that hCAP-18/LL-37 was mainly expressed in CD68+ macrophages. When CRAMP (the murine homologue of hCAP-18/LL-37)-knockout bone marrow mononuclear cells were transplanted into irradiated KPC mice, tumour formation was less pronounced than in bone marrow cells from wild-type mice[161]. Further, when CSCs were transplanted into wild-type or CRAMP-knockdown mice, tumour formation and CD133 expression were significantly reduced. Chemoresistance in CSCs increased following rLL-37 treatment, in addition to gemcitabine or abraxane, and increased CD133+ cells were observed. Exposure of M1 macrophages to CSC-conditioned media increased polarization towards the M2 phenotype and upregulation of LL37. This effect could be abolished by blocking nodal/activin/TGF-β1 signalling[161]. Inhibition of the LL37 receptors FPR2 and P2X7R reduced the ability of LL37 treated sphere-derived PDAC cells to form colonies and to invade in vitro, and CD133+ cells were reduced in number, suggesting that LL37 inhibition could have therapeutic potential. In KPC mice, LL37 receptor inhibitors resulted in decreases in circulating tumour cell numbers and liver metastases[161].
The desmoplastic stroma in PDAC contains large numbers of inflammatory cells - mainly macrophages, T cells, mast cells, and neutrophilic granulocytes (Figure 4). Inflammatory cells play multiple opposing roles in the progression of PDAC, and as a whole, they represent a double-edged sword in the biology of PC. Some inflammatory cells, particularly cytotoxic CD8+ T cells, act anti-carcinogenically by eliminating PC cells[162]. Others, particularly M2 macrophages, contribute to tumour progression by the synthesis of angiogenic and proliferation-promoting cytokines and chemokines[163,164].
The evolving PC-related immune reaction was characterized in KrasG12D mice[165]. These mice express a single mutant Kras allele in progenitor cells and develop early PanIN lesions, followed by the progression through all of the PanIN stages and finally invasive lesions that metastasize. Morphologically, these lesions are similar to their counterparts in the human pancreas[16,165]. The pre-invasive PanIN lesions in KrasG12D mice were accompanied by the upregulation of CD45-positive leukocytes in the surrounding stroma[165]. In the early PanIN lesions, the immune response was dominated by immunosuppressive cells, such as macrophages (CD11b+), regulatory T cells (Tregs, Foxp3+), and myeloid-derived suppressor cells (MDSCs, Gr1+, CD11b+), but no effector T cell activity was observed, and CD8+ T cells were scarce. Increased CD8+ T cells were only found in a subset of advanced cancers[165]. CD40 belongs to the superfamily of TNF receptors, and it is expressed in a wide variety of cells, including monocytes, macrophages, B cells, dendritic cells, fibroblasts, endothelial cells, and epithelial cells[166]. CD40 activation is involved in the development of a T cell-dependent antitumour response[167]. In human PDAC and GEMMs of PC, activation of CD40 by agonist antibodies increased the effect of gemcitabine[167], mediated through CD40-activated macrophages (F4/80+) in the tumour stroma. In a subsequent phase I study, CP-870,893, a monoclonal antibody specific for the agonist CD40, was well tolerated in combination with gemcitabine in patients with advanced PDAC, and immune activation was observed (Table 1)[168]. This promising finding calls for phase II studies.
Immunohistochemistry of 137 PC resection specimens revealed an increase in macrophage and mast cell frequencies in the PC stroma compared to the normal pancreas[169]. A systematic immunohistochemical analysis of infiltrating immune cells in the PC stroma was performed in 212 human resection specimens[170]. In 78% of the specimens, CD163+ or CD204+ M2 macrophages dominated over HLA-DR+ and CD68+ M1 macrophages, in correlation with the infiltration of neutrophils (CD66b+), and both were negatively correlated with M1 macrophages. Univariate and multivariate survival analyses demonstrated that high levels of pan macrophages (CD68+), M2 macrophages, and neutrophils were associated with shorter survival, and a high ratio of M1 macrophages to pan-macrophages was associated with longer survival[170]. High levels of CD4+ T cells or CD8+ T cells were associated with longer survival, whereas a high ratio of Tregs (FOXP3+ and CD4+) to CD4+ T cells was associated with shorter survival. Infiltration of M2 phenotype macrophages (CD163+ and CD204+) had a stronger correlation with lymph node metastasis than pan-macrophages in pancreatic cancer resections[171,172]. Similarly, a large number of M2 macrophages was correlated with increased lymphatic vessel density and poor prognosis. Hence, M2 macrophages might indicate poor prognosis, in part due to increased lymph node metastases[171,172]. This connection could be partially explained by the production of vascular endothelial growth factor (VEGF)-C by macrophages, leading to an increase in the number of peritumoral lymph vessels[169,173,174].
Macrophage-stellate cell crosstalk plays an important role in PC fibrogenesis. Lipopolysaccharide (LPS)-activated macrophages induced ECM synthesis in cultured rat and human PSCs through TGF-β signalling[175]. Quiescent PSCs became activated when co-cultured with macrophages[176]. Conversely, macrophages increased their cytokine production in the presence of PSCs. PSCs isolated from patients (normal pancreas and PC) predominantly expressed Th2 cytokines (IL4 and IL13) and promoted macrophage polarization towards M2[177]. Macrophages induced EMT in PC cells in co-culture experiments of PC cells with M2 macrophages, through Toll-like receptor 4 (TLR4)/IL-10 signalling[178]. Modulation of macrophage polarization from the immunosuppressive M2 phenotype to the tumour-inhibiting M1 phenotype could represent a novel strategy in the treatment of PC. Furthermore, the expression of histidine-rich glycoprotein (HRG) was increased in Panc02 mouse pancreatic tumour cells when transduced with a lentiviral vector encoding human HRG[179]. When implanted into WT mice, HRG+ orthotopic Panc02 pancreatic tumours grew slower and had fewer metastases, and these effects were mediated through the induction of macrophage polarization from M2 towards M1[179]. Macrophages might induce gemcitabine resistance in PC[180]. In an in vitro study in which PC cells were co-cultured with macrophages, PC cell apoptosis and caspase-3 pathway activation were reduced during gemcitabine treatment in the presence of macrophages. Further, increased response to gemcitabine was observed in CCR2-/- mice, characterized by reduced macrophage infiltration and activation. Macrophages could induce gemcitabine resistance in a paracrine manner because they were associated with increased cytidine deaminase in PC cells[180].
Mast cells have mainly been assigned roles in allergy and autoimmunity, but they might also play a role in PC biology. Mast cell infiltration has been observed in PC, and their numbers are correlated with the number of lymph node metastases[169]. Mast cells are increased in human PC specimens compared to the normal pancreas, and this increase is most pronounced in advanced grade tumours[181]. High mast cell infiltration held unfavourable prognostic value with reduced survival[181,182]. Mast cell distribution has been examined in two main PC compartments: the intratumoral compartment and peritumoral, non-neoplastic tissue. Each of these compartments was further subdivided into central and border regions[183]. High mast cell counts in the intratumoral border zone only were associated with worse overall survival[183].
In mast cell cultures, the addition of conditioned media from PC cell cultures increased migratory mast cell activity, and mast cell-conditioned media induced PC cell proliferation and migration[181]. Using the genetically engineered K-rasG12V mouse model, which spontaneously develops chronic pancreatitis, PanINs, and invasive PC, mast cell infiltration was found to be an early event, occurring in chronic pancreatitis and early PanINs. Injection of PC cells into Kitw-sh/w-sh mast cell-depleted mice reduced tumour growth and increased survival. “Normal” tumour growth was restored when bone marrow-derived cultured mast cells were injected[182]. When mast cells were incubated with conditioned media from PC cells, PSCs, and ductal epithelial cells, only the medium from PC cells induced mast cell migration, and mast cell-conditioned media increased PC cell and PSC proliferation[184]. Additionally, co-cultures of mast cells with either PC cells or PSCs resulted in mast cell activation, and neutralization of IL-13 and tryptase suppressed PSC activation. Further, tumour-bearing mice treated with AMD3100, a CXCR4 antagonist that mediates mast cell migration, resulted in a 50% decrease in tumour volume and increased overall survival[184].
These findings illustrate the potential importance of mast cells in PC progression and the possible benefit of the future development of mast cell-targeted therapies in PC. To date, however, such therapies do not play a role in clinical practice. Moreover, it has been reported in GEMMs in which mast cells were absent (Pdx1-Flp; FSF-KrasG12D/+; Cpa3Cre mice) that the formation, number, and stage of PanINs at 9 mo of age were not different from Pdx1-Flp-FSF-KrasG12D/+ controls, which frequently showed mast cells; at 12 mo, PDAC formation was observed in both groups[185].
In PC, regulatory T cells (Tregs, CD4+ and CD25+) are increased in peripheral blood, as well as in the tumour microenvironment[186]. Treg increment in the stroma induces immune invasion in PC because Tregs suppress the anticancer immune response through inhibitory cytokines, such as IL-10 and TGF-β, thereby influencing cytotoxic CD8+ T cell activity[186]. When the TGF-β-expressing PC cell line PAN02 was subcutaneously injected into C57BL/6 mice, CD4+ CD25+ Tregs in tumour-draining lymph nodes were increased[187]. When Rag-1-/- mice lacking CD4 and CD8 T cells were injected with CD4+ CD25- cells from normal mice, with or without a subsequent injection of PAN02 cells, elevation of Foxp3 signals (a specific marker for Tregs) was only observed in the tumour-draining lymph nodes of PAN02 challenged mice. This outcome was inhibited by TGF-β neutralizing antibodies, suggesting that PC cells promote the upregulation of Tregs through TGF-β[187]. Furthermore, upregulation of addressins in intratumoral endothelial cells might selectively recruit Tregs, and Treg migration could be suppressed by selective addressin antagonism[188].
Lymphocyte infiltration of distinct stromal PC compartments varies: the frequencies of CD8+ T cells, FoxP3+ regulatory T cells, CD20+ B cells, and CD56+ natural killer cells in the juxtatumoral compartments were all very low compared to the frequencies in the panstroma[189]. In this tissue micro-array (TMA) study of PC resection specimens and other pancreatobiliary diseases, CD68+ macrophages, in contrast, were more frequent juxtatumorally. No significant variation was observed in the distribution of CD4+ T cells or neutrophils. Patients with more extensive juxtatumoral CD8+ T cell infiltration had improved survival[189]. PSCs, through cytokine and chemokine signalling, were shown to reduce the migration of CD8+ T cells to the juxtatumoral stromal compartment in PC, suggesting an immunosuppressive role of PSCs in PC.
Luminex multiplex immunoassays were used to examine the expression of cytokines and chemokines in cultured PC-derived PSC lines[190]. Compared to human foetal primary pancreatic fibroblasts, PSCs expressed high levels of the MDSC-promoting cytokines IL-6, VEGF, macrophage colony-stimulating factor (M-CSF), stromal cell-derived factor 1 (SDF-1), and monocyte chemoattractant protein-1 (MCP-1). In agreement, culture of peripheral blood mononuclear cells (PBMCs) with PSC supernatant promoted MDSC differentiation, which in turn reduced T cell proliferation. MDSCs expressed CD11b and CD33 and inhibited tumour-specific immune responses by suppressing CD8+ T cells[190].
As indicated in the above sections, the PC tumour environment displays several immunosuppressive properties, which makes immunotherapeutic approaches challenging. Immune checkpoint therapy is a promising strategy that targets the endogenous, immunosuppressive regulatory pathways in T cells described above. Promising results have been obtained, for example, in the treatment of malignant melanoma, and three immune checkpoint agents have been approved for this disease by the United States Food and Drug Administration[191]. Similar strategies are under investigation for PC treatment, but so far have achieved only limited success, for example, in a phase II trial of the cytotoxic T lymphocyte antigen 4 (CTLA4)-blocking antibody ipilimumab (Table 1)[192]. PC cells express colony-stimulating factor 1 (CSF1), whereas the CSF1 receptor (CSF1R) is predominantly expressed in the tumour stroma, and inhibition of CSF1R in a mouse model of PDAC resulted in the depletion of tumour-associated macrophages, enhancement of cytotoxic T cell infiltration, and reduced tumour progression[193]. However, the effects of ipilimumab therapy in the phase II trial were rather limited, which could be attributed to the synchronous induction of the T-cell checkpoint molecules CTLA4 and programmed death-ligand 1 (PDL1). When PDAC mice were treated with a combination of CSF1R inhibitors and PDL1 receptor or CTLA4 antagonists, stronger tumour regression was observed when these drugs were used alone. Hence, CSF1/CSF1R inhibition might have the potential to improve the effects of checkpoint-based immunotherapy in PC[193]. A phase I trial examined the effect of the PDL1-inhibiting antibody BMS-936559 in patients with selected advanced cancers, including PDAC[194]. No objective response in patients with pancreatic cancer was observed compared to patients with ovarian cancer, renal-cell cancer, melanoma, and non-small-cell lung cancer.
In PC, as in many other cancers, the need for oxygen and nutrients increases during tumour growth, leading to the synthesis of new blood vessels through the proliferation of endothelial cells in the pre-existing blood vessels, a process called angiogenesis[195]. Simultaneously, proteolytic enzymes break down the ECM[196]. Despite this angiogenesis, direct intratumoral measurement of oxygenation has revealed significant intratumoral hypoxia in PC[197]. Hypoxic conditions have also been described in the tumour stroma, as evidenced by the upregulation of hypoxia-inducible factors in CAFs[198,199].
Angiogenesis in PC is regulated by a complex interplay between different cell types in the tumour stroma. Under hypoxic (0.75%-1% O2), compared to normoxic (21% O2), conditions, cultured PSCs increased their production of collagen I and the proangiogenic vascular endothelial growth factor (VEGF)[200,201]. VEGF-synthesis in PSCs was also demonstrated by immunofluorescence in PC specimens[200]. Conditioned media of hypoxia-cultured PSCs induced the proliferation and migration of endothelial cells and induced angiogenesis in vitro as well as in vivo in mice[200]. The proliferation of endothelial cells in cell cultures was increased by up to 47% after treatment with PSC supernatant, whereas PC cell supernatant reduced endothelial cell growth[201]. Furthermore, CD31 was upregulated in primary tumours of BALB/c mice co-injected with human PC cells and human PSCs compared to mice injected with PC cells only[94]. In addition to PSCs, VEGF is predominantly expressed in PC cells and endothelial cells, as well as in tumour-associated macrophages[202,203]. VEGF is an important unfavourable prognostic marker in PC[204]. Multivariate analyses have revealed a significant association between high VEGF and PC recurrence, and it has been suggested that VEGF-promoted metastasis might in part be the cause of this early recurrence[202,205].
TNP-470 is a synthetic analogue of the fungus-derived bioactive agent fumagillin, which inhibits endothelial cell proliferation[206]. TNP-470 significantly reduced tumour size and spread in orthotopic xenograft models of PC in mice[207]. This early study indicated that therapies targeting angiogenesis have potential in PC. Administration of small doses of TNP-470 (30 mg/kg) or gemcitabine (50 mg/kg) alone had no significant effect, but a combination of these therapies reduced tumour growth and metastases and improved median survival[208]. In a phase II clinical trial, the recombinant, humanized monoclonal VEGF antibody bevacizumab was administered in combination with gemcitabine to 52 advanced-stage PC patients[209]. A response rate of 21% and a median survival of 8.8 mo were observed, which were considered superior to gemcitabine alone. The following phase III trial, using gemcitabine plus bevacizumab compared to gemcitabine plus placebo in 602 patients with advanced PC, unfortunately did not show any significant efficacy of this regimen, however (Table 1)[210]. Exploiting a similar strategy, inhibition of VEGF receptors with axitinib in combination with gemcitabine did not improve overall survival in a phase III trial in patients with advanced PDAC[211]. In all, targeting VEGF signalling alone appears to be an ineffective strategy in the treatment of PDAC.
The median survival after the diagnosis of PC is only 8 mo[212]. Surgical resection offers the only hope for significantly prolonged survival, but even after surgery, the median survival is only 21 mo. Surgical resection can only be offered to approximately 20% of patients because the remaining patients present with advanced disease at the time of diagnosis. The current standard of care for PC patients is the nucleoside analogue gemcitabine[213]. However, the survival benefit of gemcitabine treatment is minimal, and better therapeutic strategies are needed. FOLFIRINOX was found to be associated with a survival advantage compared to gemcitabine in a clinical trial of metastatic PC[214]. Pre-clinical studies have indicated that therapies targeting the pancreatic cancer stroma could offer hope for better prognoses for PC patients, but so far, it has not been possible to translate these promising results to a clinical setting[215,216]. In Table 1, we summarize important clinical trials describing drugs that target components of the pancreatic cancer stroma.
PC is one of the most aggressive known cancer types, and its management remains highly challenging. Gemcitabine, the standard chemotherapeutic drug used in PC, has only a limited effect on patient survival[213]. Therefore, new treatment strategies, targeting for example the PC stroma, are highly warranted. Because of the complex interplay of the central components of the tumour stroma - cancer-associated fibroblasts (CAFs), extracellular matrix, and inflammatory and endothelial cells - it is often ineffective to use a treatment strategy that blocks single isolated factors. Early studies suggested that large numbers of peritumoral CAFs indicated a poor prognosis in PC[104], whereas more recent data have indicated that the depletion of CAFs promotes tumour aggressiveness[99,100]. The significance of the desmoplastic stroma might be context-dependent during PC progression. Stromal reprogramming, such as the induction of quiescence in aPSCs/CAFs or the antagonism of stromal growth factors, rather than stromal depletion, could represent a more appropriate treatment strategy in PC. Such strategies might be effective only at certain stages of PC development and progression, and they might have to be combined with other approaches such as antiangiogenic or immunotherapeutic approaches.
P- Reviewer: Kleeff J, Sperti C, Talukdar R S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8789] [Cited by in F6Publishing: 9415] [Article Influence: 941.5] [Reference Citation Analysis (0)] |
| 2. | Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199-1210; discussion 1210-1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1125] [Cited by in F6Publishing: 1087] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 3. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1500] [Cited by in F6Publishing: 1541] [Article Influence: 154.1] [Reference Citation Analysis (0)] |
| 4. | Aichler M, Seiler C, Tost M, Siveke J, Mazur PK, Da Silva-Buttkus P, Bartsch DK, Langer P, Chiblak S, Dürr A. Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: a comparative study in transgenic mice and human tissues. J Pathol. 2012;226:723-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969-2972. [PubMed] [Cited in This Article: ] |
| 6. | Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 855] [Cited by in F6Publishing: 758] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 7. | Maitra A, Adsay NV, Argani P, Iacobuzio-Donahue C, De Marzo A, Cameron JL, Yeo CJ, Hruban RH. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Murphy SJ, Hart SN, Lima JF, Kipp BR, Klebig M, Winters JL, Szabo C, Zhang L, Eckloff BW, Petersen GM. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology. 2013;145:1098-1109.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Jimenez RE, Z’graggen K, Hartwig W, Graeme-Cook F, Warshaw AL, Fernandez-del Castillo C. Immunohistochemical characterization of pancreatic tumors induced by dimethylbenzanthracene in rats. Am J Pathol. 1999;154:1223-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Schmid RM, Klöppel G, Adler G, Wagner M. Acinar-ductal-carcinoma sequence in transforming growth factor-alpha transgenic mice. Ann N Y Acad Sci. 1999;880:219-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Wagner M, Lührs H, Klöppel G, Adler G, Schmid RM. Malignant transformation of duct-like cells originating from acini in transforming growth factor transgenic mice. Gastroenterology. 1998;115:1254-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 141] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Esposito I, Seiler C, Bergmann F, Kleeff J, Friess H, Schirmacher P. Hypothetical progression model of pancreatic cancer with origin in the centroacinar-acinar compartment. Pancreas. 2007;35:212-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Ijichi H. Genetically-engineered mouse models for pancreatic cancer: Advances and current limitations. World J Clin Oncol. 2011;2:195-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Lett. 2009;279:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1827] [Cited by in F6Publishing: 1773] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 17. | Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1150] [Cited by in F6Publishing: 1340] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 18. | Coleman SJ, Watt J, Arumugam P, Solaini L, Carapuca E, Ghallab M, Grose RP, Kocher HM. Pancreatic cancer organotypics: High throughput, preclinical models for pharmacological agent evaluation. World J Gastroenterol. 2014;20:8471-8481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Porta M, Fabregat X, Malats N, Guarner L, Carrato A, de Miguel A, Ruiz L, Jariod M, Costafreda S, Coll S. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7:189-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 636] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 21. | Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28:645-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, Negri E, Li D, Risch HA, Olson SH. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012;23:1880-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 246] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 23. | Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076-2083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 737] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 24. | Lucenteforte E, La Vecchia C, Silverman D, Petersen GM, Bracci PM, Ji BT, Bosetti C, Li D, Gallinger S, Miller AB. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23:374-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 25. | Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA, Silverman DT, Ji BT, Gallinger S, Holly EA. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23:2964-2970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 26. | Lowenfels AB, Maisonneuve P, Lankisch PG. Chronic pancreatitis and other risk factors for pancreatic cancer. Gastroenterol Clin North Am. 1999;28:673-685, x. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Bang UC, Benfield T, Hyldstrup L, Bendtsen F, Beck Jensen JE. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology. 2014;146:989-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 28. | Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer. 2009;8:109-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Esposito I, Konukiewitz B, Schlitter AM, Klöppel G. Pathology of pancreatic ductal adenocarcinoma: facts, challenges and future developments. World J Gastroenterol. 2014;20:13833-13841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 63] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 30. | Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, Gerdes B, Kress R, Ziegler A, Raeburn JA. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 325] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 31. | Brand RE, Lerch MM, Rubinstein WS, Neoptolemos JP, Whitcomb DC, Hruban RH, Brentnall TA, Lynch HT, Canto MI. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460-1469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 32. | Bartsch DK, Gress TM, Langer P. Familial pancreatic cancer--current knowledge. Nat Rev Gastroenterol Hepatol. 2012;9:445-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Meckler KA, Brentnall TA, Haggitt RC, Crispin D, Byrd DR, Kimmey MB, Bronner MP. Familial fibrocystic pancreatic atrophy with endocrine cell hyperplasia and pancreatic carcinoma. Am J Surg Pathol. 2001;25:1047-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Amann ST, Toskes PP. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1123] [Cited by in F6Publishing: 994] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 35. | Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 596] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 36. | Kadaba R, Birke H, Wang J, Hooper S, Andl CD, Di Maggio F, Soylu E, Ghallab M, Bor D, Froeling FE. Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J Pathol. 2013;230:107-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 37. | Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002-5011. [PubMed] [Cited in This Article: ] |
| 38. | Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1455] [Cited by in F6Publishing: 1520] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 39. | Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 315] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 40. | Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1319] [Cited by in F6Publishing: 1308] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 41. | Pinzani M. Epithelial-mesenchymal transition in chronic liver disease: fibrogenesis or escape from death? J Hepatol. 2011;55:459-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grünert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Casini A, Galli A, Pignalosa P, Frulloni L, Grappone C, Milani S, Pederzoli P, Cavallini G, Surrenti C. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J Pathol. 2000;192:81-89. [PubMed] [Cited in This Article: ] |
| 44. | Detlefsen S, Sipos B, Feyerabend B, Klöppel G. Fibrogenesis in alcoholic chronic pancreatitis: the role of tissue necrosis, macrophages, myofibroblasts and cytokines. Mod Pathol. 2006;19:1019-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Detlefsen S, Sipos B, Zhao J, Drewes AM, Klöppel G. Autoimmune pancreatitis: expression and cellular source of profibrotic cytokines and their receptors. Am J Surg Pathol. 2008;32:986-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in F6Publishing: 678] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 47. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 744] [Cited by in F6Publishing: 766] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 48. | Watari N, Hotta Y, Mabuchi Y. Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin A administration. Okajimas Folia Anat Jpn. 1982;58:837-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Ikejiri N. The vitamin A-storing cells in the human and rat pancreas. Kurume Med J. 1990;37:67-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Phillips PA, McCarroll JA, Park S, Wu MJ, Pirola R, Korsten M, Wilson JS, Apte MV. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut. 2003;52:275-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Lardon J, Rooman I, Bouwens L. Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem Cell Biol. 2002;117:535-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Zhao L, Burt AD. The diffuse stellate cell system. J Mol Histol. 2007;38:53-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 464] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 54. | Vonlaufen A, Phillips PA, Yang L, Xu Z, Fiala-Beer E, Zhang X, Pirola RC, Wilson JS, Apte MV. Isolation of quiescent human pancreatic stellate cells: a promising in vitro tool for studies of human pancreatic stellate cell biology. Pancreatology. 2010;10:434-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 280] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 56. | Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 319] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 57. | Yuzawa S, Kano MR, Einama T, Nishihara H. PDGFRβ expression in tumor stroma of pancreatic adenocarcinoma as a reliable prognostic marker. Med Oncol. 2012;29:2824-2830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, Johnson PA, Johnson CD, Bateman AC, Fine DR. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787-1798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 59. | Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 429] [Cited by in F6Publishing: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 60. | Apte MV, Phillips PA, Fahmy RG, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Naidoo D, Wilson JS. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 61. | Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, Clevers H, Hart IR, Kocher HM. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology. 2011;141:1486-1497, 1497.e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 314] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 62. | Jesnowski R, Fürst D, Ringel J, Chen Y, Schrödel A, Kleeff J, Kolb A, Schareck WD, Löhr M. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine. Lab Invest. 2005;85:1276-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 63. | Kim KY, Rhim T, Choi I, Kim SS. N-acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J Biol Chem. 2001;276:40591-40598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 64. | Shimizu K, Kobayashi M, Tahara J, Shiratori K. Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology. 2005;128:2105-2118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Wehr AY, Furth EE, Sangar V, Blair IA, Yu KH. Analysis of the human pancreatic stellate cell secreted proteome. Pancreas. 2011;40:557-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | Marrache F, Pendyala S, Bhagat G, Betz KS, Song Z, Wang TC. Role of bone marrow-derived cells in experimental chronic pancreatitis. Gut. 2008;57:1113-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Scarlett CJ, Colvin EK, Pinese M, Chang DK, Morey AL, Musgrove EA, Pajic M, Apte M, Henshall SM, Sutherland RL. Recruitment and activation of pancreatic stellate cells from the bone marrow in pancreatic cancer: a model of tumor-host interaction. PLoS One. 2011;6:e26088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Lin WR, Inatomi O, Lee CY, Kallis YN, Otto WR, Jeffery R, Poulsom R, Alison MR. Bone marrow-derived cells contribute to cerulein-induced pancreatic fibrosis in the mouse. Int J Exp Pathol. 2012;93:130-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Scarlett CJ. Contribution of bone marrow derived cells to the pancreatic tumor microenvironment. Front Physiol. 2013;4:56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Akita S, Kubota K, Kobayashi A, Misawa R, Shimizu A, Nakata T, Yokoyama T, Takahashi M, Miyagawa S. Role of bone marrow cells in the development of pancreatic fibrosis in a rat model of pancreatitis induced by a choline-deficient/ethionine-supplemented diet. Biochem Biophys Res Commun. 2012;420:743-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Pan JJ, Oh SH, Lee WC, Petersen BE. Bone marrow-derived progenitor cells could modulate pancreatic cancer tumorigenesis via peritumoral microenvironment in a rat model. Oncol Res. 2009;17:339-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492-8495. [PubMed] [Cited in This Article: ] |
| 73. | Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 74. | Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 386] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 75. | Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol. 2014;4:62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 316] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 76. | Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3347] [Cited by in F6Publishing: 3361] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 77. | Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 602] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 78. | Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877-12882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 844] [Cited by in F6Publishing: 803] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 79. | Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 786] [Cited by in F6Publishing: 851] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 80. | Zechner D, Knapp N, Bobrowski A, Radecke T, Genz B, Vollmar B. Diabetes increases pancreatic fibrosis during chronic inflammation. Exp Biol Med (Maywood). 2014;239:670-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Boomershine CS, Chamberlain A, Kendall P, Afshar-Sharif AR, Huang H, Washington MK, Lawson WE, Thomas JW, Blackwell TS, Bhowmick NA. Autoimmune pancreatitis results from loss of TGFbeta signalling in S100A4-positive dendritic cells. Gut. 2009;58:1267-1274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 551] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 83. | Farrow B, Rowley D, Dang T, Berger DH. Characterization of tumor-derived pancreatic stellate cells. J Surg Res. 2009;157:96-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Ikenaga N, Ohuchida K, Mizumoto K, Yu J, Fujita H, Nakata K, Ueda J, Sato N, Nagai E, Tanaka M. S100A4 mRNA is a diagnostic and prognostic marker in pancreatic carcinoma. J Gastrointest Surg. 2009;13:1852-1858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Tsukamoto N, Egawa S, Akada M, Abe K, Saiki Y, Kaneko N, Yokoyama S, Shima K, Yamamura A, Motoi F. The expression of S100A4 in human pancreatic cancer is associated with invasion. Pancreas. 2013;42:1027-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Mahon PC, Baril P, Bhakta V, Chelala C, Caulee K, Harada T, Lemoine NR. S100A4 contributes to the suppression of BNIP3 expression, chemoresistance, and inhibition of apoptosis in pancreatic cancer. Cancer Res. 2007;67:6786-6795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 87. | Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1080] [Cited by in F6Publishing: 1107] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 88. | Klöppel G, Detlefsen S, Feyerabend B. Fibrosis of the pancreas: the initial tissue damage and the resulting pattern. Virchows Arch. 2004;445:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 89. | Bachem MG, Schünemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 90. | Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 794] [Cited by in F6Publishing: 878] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 91. | Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 2011;71:3453-3458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 92. | Froeling FE, Mirza TA, Feakins RM, Seedhar A, Elia G, Hart IR, Kocher HM. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am J Pathol. 2009;175:636-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 93. | Kikuta K, Masamune A, Watanabe T, Ariga H, Itoh H, Hamada S, Satoh K, Egawa S, Unno M, Shimosegawa T. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403:380-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 94. | Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, Biankin AV, Goldstein D, Pirola RC, Wilson JS. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585-2596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 95. | Al-Assar O, Demiciorglu F, Lunardi S, Gaspar-Carvalho MM, McKenna WG, Muschel RM, Brunner TB. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother Oncol. 2014;111:243-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 96. | Cabrera MC, Tilahun E, Nakles R, Diaz-Cruz ES, Charabaty A, Suy S, Jackson P, Ley L, Slack R, Jha R. Human Pancreatic Cancer-Associated Stellate Cells Remain Activated after in vivo Chemoradiation. Front Oncol. 2014;4:102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 97. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2272] [Cited by in F6Publishing: 2392] [Article Influence: 159.5] [Reference Citation Analysis (0)] |
| 98. | Ko AH, LoConte N, Tempero MA, Walker EJ, Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci DV. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas. 2016;45:370-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 99. | Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1517] [Cited by in F6Publishing: 1665] [Article Influence: 166.5] [Reference Citation Analysis (0)] |
| 100. | Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1359] [Cited by in F6Publishing: 1469] [Article Influence: 146.9] [Reference Citation Analysis (0)] |
| 101. | Gore J, Korc M. Pancreatic cancer stroma: friend or foe? Cancer Cell. 2014;25:711-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 102. | Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 681] [Cited by in F6Publishing: 762] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 103. | Gundewar C, Ansari D, Tang L, Wang Y, Liang G, Rosendahl AH, Saleem MA, Andersson R. Antiproliferative effects of curcumin analog L49H37 in pancreatic stellate cells: a comparative study. Ann Gastroenterol. 2015;28:391-398. [PubMed] [Cited in This Article: ] |
| 104. | Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 334] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 105. | Sinn M, Denkert C, Striefler JK, Pelzer U, Stieler JM, Bahra M, Lohneis P, Dörken B, Oettle H, Riess H. α-Smooth muscle actin expression and desmoplastic stromal reaction in pancreatic cancer: results from the CONKO-001 study. Br J Cancer. 2014;111:1917-1923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 106. | Guweidhi A, Kleeff J, Adwan H, Giese NA, Wente MN, Giese T, Büchler MW, Berger MR, Friess H. Osteonectin influences growth and invasion of pancreatic cancer cells. Ann Surg. 2005;242:224-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Sinn M, Sinn BV, Striefler JK, Lindner JL, Stieler JM, Lohneis P, Bischoff S, Bläker H, Pelzer U, Bahra M. SPARC expression in resected pancreatic cancer patients treated with gemcitabine: results from the CONKO-001 study. Ann Oncol. 2014;25:1025-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 108. | Mantoni TS, Schendel RR, Rödel F, Niedobitek G, Al-Assar O, Masamune A, Brunner TB. Stromal SPARC expression and patient survival after chemoradiation for non-resectable pancreatic adenocarcinoma. Cancer Biol Ther. 2008;7:1806-1815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 109. | Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C, Goggins M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 305] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 110. | Iacobuzio-Donahue CA, Ryu B, Hruban RH, Kern SE. Exploring the host desmoplastic response to pancreatic carcinoma: gene expression of stromal and neoplastic cells at the site of primary invasion. Am J Pathol. 2002;160:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 111. | Ricci F, Kern SE, Hruban RH, Iacobuzio-Donahue CA. Stromal responses to carcinomas of the pancreas: juxtatumoral gene expression conforms to the infiltrating pattern and not the biologic subtype. Cancer Biol Ther. 2005;4:302-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Ikenaga N, Ohuchida K, Mizumoto K, Cui L, Kayashima T, Morimatsu K, Moriyama T, Nakata K, Fujita H, Tanaka M. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology. 2010;139:1041-1051, 1051.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 113. | Mollenhauer J, Roether I, Kern HF. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2:14-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 114. | Linder S, Castaños-Velez E, von Rosen A, Biberfeld P. Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology. 2001;48:1321-1327. [PubMed] [Cited in This Article: ] |
| 115. | Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, Kayed H, Giese N, Kleeff J, Friess H. Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol. 2006;208:673-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 116. | Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1408] [Cited by in F6Publishing: 1468] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 117. | Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer. 2013;108:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 118. | Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 775] [Cited by in F6Publishing: 779] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 119. | Theocharis AD, Tsara ME, Papageorgacopoulou N, Karavias DD, Theocharis DA. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim Biophys Acta. 2000;1502:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 120. | Long D, Lu J, Luo L, Guo Y, Li C, Wu W, Shan J, Li L, Li S, Li Y. Effects of octreotide on activated pancreatic stellate cell-induced pancreas graft fibrosis in rats. J Surg Res. 2012;176:248-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 121. | Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 457] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 122. | Paulo JA, Urrutia R, Kadiyala V, Banks P, Conwell DL, Steen H. Cross-species analysis of nicotine-induced proteomic alterations in pancreatic cells. Proteomics. 2013;13:1499-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Ko SH, Hong OK, Kim JW, Ahn YB, Song KH, Cha BY, Son HY, Kim MJ, Jeong IK, Yoon KH. High glucose increases extracellular matrix production in pancreatic stellate cells by activating the renin-angiotensin system. J Cell Biochem. 2006;98:343-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 124. | Erkan M, Weis N, Pan Z, Schwager C, Samkharadze T, Jiang X, Wirkner U, Giese NA, Ansorge W, Debus J. Organ-, inflammation- and cancer specific transcriptional fingerprints of pancreatic and hepatic stellate cells. Mol Cancer. 2010;9:88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 125. | García-Pravia C, Galván JA, Gutiérrez-Corral N, Solar-García L, García-Pérez E, García-Ocaña M, Del Amo-Iribarren J, Menéndez-Rodríguez P, García-García J, de Los Toyos JR. Overexpression of COL11A1 by cancer-associated fibroblasts: clinical relevance of a stromal marker in pancreatic cancer. PLoS One. 2013;8:e78327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 126. | Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology. 2003;125:1188-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 127. | Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745-11753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 128. | Öhlund D, Franklin O, Lundberg E, Lundin C, Sund M. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer. 2013;13:154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 129. | Shields MA, Dangi-Garimella S, Krantz SB, Bentrem DJ, Munshi HG. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. J Biol Chem. 2011;286:10495-10504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 130. | Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1247] [Cited by in F6Publishing: 1332] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 131. | Stetler-Stevenson WG, Liotta LA, Kleiner DE. Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993;7:1434-1441. [PubMed] [Cited in This Article: ] |
| 132. | Gress TM, Müller-Pillasch F, Lerch MM, Friess H, Büchler M, Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int J Cancer. 1995;62:407-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 183] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 133. | Koshiba T, Hosotani R, Wada M, Miyamoto Y, Fujimoto K, Lee JU, Doi R, Arii S, Imamura M. Involvement of matrix metalloproteinase-2 activity in invasion and metastasis of pancreatic carcinoma. Cancer. 1998;82:642-650. [PubMed] [Cited in This Article: ] |
| 134. | Singh N, Das P, Datta Gupta S, Sahni P, Pandey RM, Gupta S, Chauhan SS, Saraya A. Prognostic significance of extracellular matrix degrading enzymes-cathepsin L and matrix metalloproteases-2 [MMP-2] in human pancreatic cancer. Cancer Invest. 2013;31:461-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 135. | Määttä M, Soini Y, Liakka A, Autio-Harmainen H. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumor progression and clinical prognosis. Clin Cancer Res. 2000;6:2726-2734. [PubMed] [Cited in This Article: ] |
| 136. | Jones LE, Humphreys MJ, Campbell F, Neoptolemos JP, Boyd MT. Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer: increased expression of matrix metalloproteinase-7 predicts poor survival. Clin Cancer Res. 2004;10:2832-2845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 137. | Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296-3302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 138. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 388] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 139. | Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1526] [Cited by in F6Publishing: 1457] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 140. | Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci USA. 2013;110:12325-12330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 141. | Strimpakos AS, Saif MW. Update on phase I studies in advanced pancreatic adenocarcinoma. Hunting in darkness? JOP. 2013;14:354-358. [PubMed] [Cited in This Article: ] |
| 142. | Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 456] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 143. | Morris HT, Machesky LM. Actin cytoskeletal control during epithelial to mesenchymal transition: focus on the pancreas and intestinal tract. Br J Cancer. 2015;112:613-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 144. | Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1400] [Cited by in F6Publishing: 1520] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 145. | Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6575] [Cited by in F6Publishing: 7354] [Article Influence: 490.3] [Reference Citation Analysis (0)] |
| 146. | Ledford H. Cancer theory faces doubts. Nature. 2011;472:273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 147. | Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769-4776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 327] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 148. | Javle MM, Gibbs JF, Iwata KK, Pak Y, Rutledge P, Yu J, Black JD, Tan D, Khoury T. Epithelial-mesenchymal transition (EMT) and activated extracellular signal-regulated kinase (p-Erk) in surgically resected pancreatic cancer. Ann Surg Oncol. 2007;14:3527-3533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 149. | Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1262] [Cited by in F6Publishing: 1339] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 150. | Jordan CT. Cancer stem cells: controversial or just misunderstood? Cell Stem Cell. 2009;4:203-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 151. | Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2456] [Cited by in F6Publishing: 2486] [Article Influence: 165.7] [Reference Citation Analysis (0)] |
| 152. | Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6211] [Cited by in F6Publishing: 6535] [Article Influence: 408.4] [Reference Citation Analysis (0)] |
| 153. | Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 154. | Zhang Y, Wei J, Wang H, Xue X, An Y, Tang D, Yuan Z, Wang F, Wu J, Zhang J. Epithelial mesenchymal transition correlates with CD24+CD44+ and CD133+ cells in pancreatic cancer. Oncol Rep. 2012;27:1599-1605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 155. | Lee SH, Kim H, Hwang JH, Shin E, Lee HS, Hwang DW, Cho JY, Yoon YS, Han HS, Cha BH. CD24 and S100A4 expression in resectable pancreatic cancers with earlier disease recurrence and poor survival. Pancreas. 2014;43:380-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 156. | Ding Q, Miyazaki Y, Tsukasa K, Matsubara S, Yoshimitsu M, Takao S. CD133 facilitates epithelial-mesenchymal transition through interaction with the ERK pathway in pancreatic cancer metastasis. Mol Cancer. 2014;13:15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 157. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2377] [Cited by in F6Publishing: 2352] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 158. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1987] [Cited by in F6Publishing: 1789] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 159. | Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433-446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 160. | Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 161. | Sainz B, Alcala S, Garcia E, Sanchez-Ripoll Y, Azevedo MM, Cioffi M, Tatari M, Miranda-Lorenzo I, Hidalgo M, Gomez-Lopez G. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut. 2015;64:1921-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 162. | Wahab ZA, Metzgar RS. Human cytotoxic lymphocytes reactive with pancreatic adenocarcinoma cells. Pancreas. 1991;6:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 163. | Yoshikawa K, Mitsunaga S, Kinoshita T, Konishi M, Takahashi S, Gotohda N, Kato Y, Aizawa M, Ochiai A. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci. 2012;103:2012-2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 164. | Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 499] [Cited by in F6Publishing: 628] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 165. | Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518-9527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 653] [Cited by in F6Publishing: 701] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 166. | van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2-17. [PubMed] [Cited in This Article: ] |
| 167. | Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612-1616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1152] [Cited by in F6Publishing: 1204] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 168. | Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286-6295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 169. | Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, Bevilacqua G, Campani D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57:630-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 170. | Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 611] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 171. | Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211-e219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 426] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 172. | Gardian K, Janczewska S, Olszewski WL, Durlik M. Analysis of pancreatic cancer microenvironment: role of macrophage infiltrates and growth factors expression. J Cancer. 2012;3:285-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 173. | Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947-956. [PubMed] [Cited in This Article: ] |
| 174. | Tang RF, Itakura J, Aikawa T, Matsuda K, Fujii H, Korc M, Matsumoto Y. Overexpression of lymphangiogenic growth factor VEGF-C in human pancreatic cancer. Pancreas. 2001;22:285-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 175. | Schmid-Kotsas A, Gross HJ, Menke A, Weidenbach H, Adler G, Siech M, Beger H, Grünert A, Bachem MG. Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells. Am J Pathol. 1999;155:1749-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 176. | Shi C, Washington MK, Chaturvedi R, Drosos Y, Revetta FL, Weaver CJ, Buzhardt E, Yull FE, Blackwell TS, Sosa-Pineda B. Fibrogenesis in pancreatic cancer is a dynamic process regulated by macrophage-stellate cell interaction. Lab Invest. 2014;94:409-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 177. | Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, Habtezion A. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun. 2015;6:7158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 178. | Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93:844-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 316] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 179. | Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 529] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 180. | Weizman N, Krelin Y, Shabtay-Orbach A, Amit M, Binenbaum Y, Wong RJ, Gil Z. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2014;33:3812-3819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 181. | Strouch MJ, Cheon EC, Salabat MR, Krantz SB, Gounaris E, Melstrom LG, Dangi-Garimella S, Wang E, Munshi HG, Khazaie K. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res. 2010;16:2257-2265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 182. | Chang DZ, Ma Y, Ji B, Wang H, Deng D, Liu Y, Abbruzzese JL, Liu YJ, Logsdon CD, Hwu P. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17:7015-7023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 183. | Cai SW, Yang SZ, Gao J, Pan K, Chen JY, Wang YL, Wei LX, Dong JH. Prognostic significance of mast cell count following curative resection for pancreatic ductal adenocarcinoma. Surgery. 2011;149:576-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 184. | Ma Y, Hwang RF, Logsdon CD, Ullrich SE. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res. 2013;73:3927-3937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 185. | Schönhuber N, Seidler B, Schuck K, Veltkamp C, Schachtler C, Zukowska M, Eser S, Feyerabend TB, Paul MC, Eser P. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat Med. 2014;20:1340-1347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 186. | Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756-2761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1093] [Cited by in F6Publishing: 1059] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 187. | Liyanage UK, Goedegebuure PS, Moore TT, Viehl CT, Moo-Young TA, Larson JW, Frey DM, Ehlers JP, Eberlein TJ, Linehan DC. Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother. 2006;29:416-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 188. | Nummer D, Suri-Payer E, Schmitz-Winnenthal H, Bonertz A, Galindo L, Antolovich D, Koch M, Büchler M, Weitz J, Schirrmacher V. Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. J Natl Cancer Inst. 2007;99:1188-1199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 189. | Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C, Gribben JG. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 384] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 190. | Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007-3018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 191. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2870] [Cited by in F6Publishing: 3217] [Article Influence: 357.4] [Reference Citation Analysis (0)] |
| 192. | Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, Rosenberg SA. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 701] [Cited by in F6Publishing: 859] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 193. | Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057-5069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 768] [Cited by in F6Publishing: 897] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 194. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5599] [Cited by in F6Publishing: 5881] [Article Influence: 490.1] [Reference Citation Analysis (0)] |
| 195. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5115] [Cited by in F6Publishing: 5645] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 196. | Whipple C, Korc M. Targeting angiogenesis in pancreatic cancer: rationale and pitfalls. Langenbecks Arch Surg. 2008;393:901-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 197. | Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 423] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 198. | Ide T, Kitajima Y, Miyoshi A, Ohtsuka T, Mitsuno M, Ohtaka K, Koga Y, Miyazaki K. Tumor-stromal cell interaction under hypoxia increases the invasiveness of pancreatic cancer cells through the hepatocyte growth factor/c-Met pathway. Int J Cancer. 2006;119:2750-2759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 199. | Couvelard A, O’Toole D, Leek R, Turley H, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K. Expression of hypoxia-inducible factors is correlated with the presence of a fibrotic focus and angiogenesis in pancreatic ductal adenocarcinomas. Histopathology. 2005;46:668-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 200. | Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008;295:G709-G717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 201. | Erkan M, Reiser-Erkan C, Michalski CW, Deucker S, Sauliunaite D, Streit S, Esposito I, Friess H, Kleeff J. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 202. | Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 203. | Büchler P, Reber HA, Büchler M, Shrinkante S, Büchler MW, Friess H, Semenza GL, Hines OJ. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26:56-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 204. | Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553-1563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 232] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 205. | Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer. 2003;2:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 206. | Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348:555-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 940] [Cited by in F6Publishing: 898] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 207. | Hotz HG, Reber HA, Hotz B, Sanghavi PC, Yu T, Foitzik T, Buhr HJ, Hines OJ. Angiogenesis inhibitor TNP-470 reduces human pancreatic cancer growth. J Gastrointest Surg. 2001;5:131-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 208. | Jia L, Zhang MH, Yuan SZ, Huang WG. Antiangiogenic therapy for human pancreatic carcinoma xenografts in nude mice. World J Gastroenterol. 2005;11:447-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 209. | Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033-8040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 379] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 210. | Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 645] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 211. | Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 212. | Danish Pancreatic Cancer Database (DPCD). Landsdækkende database for patienter med kræft i bugspytkirtlen 2014-2015 [In Danish]. Cited 2015.12.17. Available from: http://www.dpcg.gicancer.dk/. [Cited in This Article: ] |
| 213. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] [Cited in This Article: ] |
| 214. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4838] [Cited by in F6Publishing: 5099] [Article Influence: 392.2] [Reference Citation Analysis (1)] |
| 215. | Liss AS, Thayer SP. Therapeutic targeting of pancreatic stroma. Source: Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India): Transworld Research Network 2012; Chapter 9. [PubMed] [Cited in This Article: ] |
| 216. | Rucki AA, Zheng L. Pancreatic cancer stroma: understanding biology leads to new therapeutic strategies. World J Gastroenterol. 2014;20:2237-2246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 87] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 217. | Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 218. | Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 219. | Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Donehower RC, Jaffee EM. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |