INTRODUCTION
Endoscopic submucosal dissection (ESD) is an advanced endoscopic technique that allows for en-bloc resection of early gastrointestinal tract cancers[1-3]. By achieving en-bloc resection, ESD has shown higher curative resection rates and lower recurrence rates than endoscopic mucosal resection (EMR)[4].
ESD was developed in Japan and has become standard of care for managing early cancers and tumors of the esophagus, stomach and colon. Its adoption in the Western world has been slow due to lower volume of gastric cancer, longer procedure times, and slow learning curve[5-7]. ESD also carries a significant risk of complications. The risk of immediate and delayed bleeding in gastric ESD is 7% and 5.5% respectively[8]. The risk of bleeding in colorectal ESD is 1.5%[1] and is rare in esophageal ESD[9].
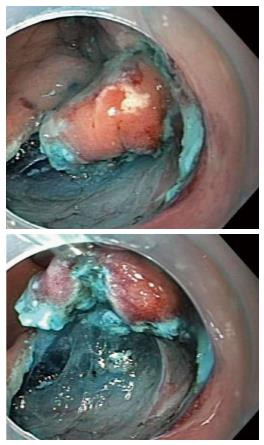
During conventional surgery, dissection is performed with one hand or device cutting and a second hand or device retracting the tissue so that the appropriate plane of dissection is easily seen. In ESD, the entire procedure is done through a single endoscope, and there is no second hand to help as in conventional surgery[10-12]. This limits visualization of the dissection plane increasing the technical challenge of the procedure (Figure 1). Adequate tissue tension and clear visibility of the dissection plane are important for effective and safe dissection[12-14]. Inability to visualize the dissection plane can lead to inadvertent cutting of blood vessels resulting in bleeding, and difficulty in stopping bleeding as the bleeding point is poorly visualized. It has been estimated that approximately 70% of procedure time during ESD is spent handling bleeding by inexperienced operators[15]. If the submucosal plane of dissection is not well visualized, inadvertent cutting of the muscle layer may lead to perforation. The risk of perforation in gastric, esophageal and colonic ESD is 1.2%-5.2%, 0%-6% and 2.4% respectively[1,8,16].
Figure 1 Endoscopic submucosal dissection with and without retraction.
Endoscopic submucosal dissection (ESD) without retraction on the left demonstrates the mucosa impeding the visual field. ESD with retraction using the clip-line method on the right allows for better visualization of the surgical field with the mucosa pulled away.
Several methods have been developed to help provide traction during ESD procedures[17]. Examples are the clip-with-line method[14], percutaneous traction method[18], sinker-assisted method[19], magnetic anchor method[12], external forceps method[20], internal-traction methods (clip band method[21] and medical ring method[22]), double-channel-scope method[23], outer-route method[24], double-scope method[25], endoscopic surgical platform method[26], robot-assisted method[27] and the Sakamoto and Osada clip (SO-Clip) method[28].
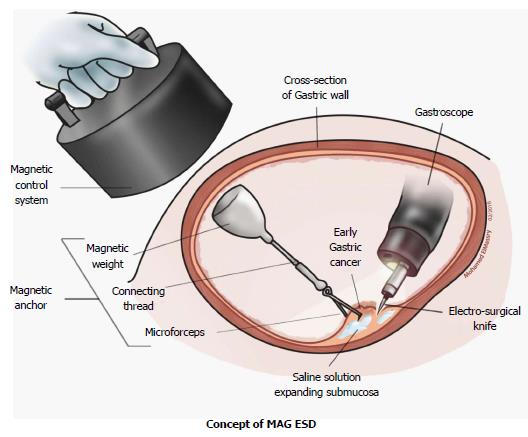
Magnetic anchor guided - endoscopic submucosal dissection (MAG-ESD) is an attractive method of traction as it has potential benefits compared to other current traction methods. It delivers traction independent of the endoscope by creating an invisible second hand for the operator. Unlike the double channel and outer-route methods, MAG-ESD does not interfere with the complex endoscopic movements needed to perform ESD. Unlike the clip-with-line, percutaneous traction, and sinker-assisted methods, MAG-ESD provides dynamic traction in a sense that the external magnet can be moved to change the direction of retraction. Permanent and electromagnets are the only two types of magnets available[29].
ELECTROMAGNETS
Electromagnets are made of a coil of an electric conductor which acts as a magnet only when an electric current passes through. The main advantage of electromagnets is that the magnetic field can be quickly changed by controlling the amount of electric current used. Their main disadvantage is being large and cumbersome. Another significant disadvantage of electric magnets is being electric current dependent. Without a current, no force is produced, making anchoring impossible. The only current which can be used is direct current, necessitating the need for an external power source and wired control system, because voltage and capacity of modern batteries are too low to supply the necessary energy. Moreover, every current pass generates thermal energy, generating heat close to the endoscopist.
USE OF ELECTROMAGNETS IN MAG-ESD
Initial concept of MAG-ESD
The concept of MAG-ESD was first postulated and experimented by Kobayashi et al[12]. They proposed that if a magnetic field is properly controlled and powered, it could generate sufficient force to use a micro-forceps for traction of the mucosa during EMR and thereby developed a magnetic anchor system to prove their theory. The magnetic anchor consisted of three parts: a handmade magnetic weight composed of magnetic stainless steel (SUS420F), a micro-forceps and a connecting thread. This system was tested in an ex-vivo porcine model. First, an incision was made by standard EMR technique in the mucosa. Then, a magnetic anchor was attached to the edge of the incised mucosa. The tip of mucosa was lifted by increasing the electric current through the electromagnet of the magnetic control system. Direction of traction for magnetic anchor could be simply manipulated by changing the position of the electromagnet over the animal or by changing the position of the animal itself. Hemorrhage was rare because blood vessels could be clearly visualized endoscopically and hemostatic procedures were conducted before cutting blood vessels. However, separation of the magnetic weight from the forceps and slipping of the micro-forceps from the mucosa were observed. In these cases, new magnetic anchors could be inserted without any problems and the malfunctioned anchors could be easily retrieved. One main drawback of this model was that the electromagnets used were large and cumbersome and not practical for most endoscopy suites.
First human experience
The feasibility of MAG-ESD in the resection of early gastric cancer (EGC) in humans was first looked at by Gotoda et al[30]. Twenty-five patients with EGC > 20 millimeters (mm) in diameter located in the gastric body were enrolled. The structure of the magnetic anchor was the same as that used in Kobayashi’s study and the magnet used was electromagnet as well. All tumors were resected en-bloc without any perforations or significant bleeding. All magnetic anchors were safely retrieved. The magnetic anchor system was reported to be supportive in 23 out of 25 operations by the performing endoscopists. All ESD scars were completely healed after 8 wk. Neither recurrent cancer nor distant metastases were observed in any of the patients during follow up. The authors concluded that MAG-ESD is a feasible and safe method that enables excellent visualization of the tissue and facilitates gastric ESD in patients with early gastric cancer.
PERMANENT MAGNETS
Permanent magnets are made from a material that stays magnetized (ferromagnetic). Permanent magnets have the advantage of being small in size. Neodymium-iron-boron rare earth magnets are the strongest permanent magnets available. They are highly resistant to demagnetization because of their atomic structure[31]. They are utilized in a variety of commercial applications including MRI machines. However, Neodymium magnets have a low resistance to corrosion, necessitating a protective coating to prevent potential release of harmful products. Thus, all neodymium magnets should be isolated from contact with tissues and fluids inside the body. The shielding material must be inert to the human body and non-obstructive to the magnetic field (e.g., gold or epoxy)[29].
USE OF PERMANENT MAGNETS IN MAG-ESD
Live animal trial
The Feasibility of counter-traction with a simplified MAG-ESD technique was evaluated in a live animal experiment in two beagle dogs[32]. The external magnet was a hand-held permanent magnet that can be locked by a flexible arm, so there was no need for an assistant to keep holding it in position during the procedure (Figure 2). Ten MAG-ESDs using neodymium permanent magnets were successfully performed in all cases without perforation in the stomach. Adequate counter-traction with good visualization was obtained using the external magnet except in two cases. None of the magnetic anchors became displaced during the procedure and all were retrieved after endoscopic resection.
Figure 2 Magnetic anchor system using neodymium permanent magnet.
ESD: Endoscopic submucosal dissection.
STANDARD ESD VS MAG ESD
The first comparative study between standard ESD and MAG-ESD was done on simulated gastric lesions in ex-vivo animal model[33]. Ten ESDs were performed using a MAG-ESD device and ten were performed by the standard ESD technique. The MAG-ESD device consisted of two neodymium magnets (one internal and one external), with sutures, and standard endoscopic clips. The magnet could be freely manipulated using a three dimensional hemisphere allowing the assistant to adjust the direction of counter-traction in accordance with the operator’s preference and providing the endoscopist with the broadest view of the dissection line. The MAG-ESD device provided dynamic multidirectional counter-traction during ESD. Endoscopists reported increased visualization in MAG-ESD compared to standard ESD. The total procedure time and the lesion dissection time for the MAG-ESD were significantly lower than the control group. No perforations or other adverse events occurred in either group. The authors concluded that this device reduced procedure time for an operator who was experienced in ESD and enhanced exposure of dissection line compared to standard ESD.
MAGNETIC ANCHOR GUIDANCE IN NATURAL ORIFICE TRANSLUMINAL ENDOSCOPIC SURGERY
Magnetic anchor guidance (MAG) has also been used in natural orifice transluminal endoscopic surgery (NOTES). In NOTES, operative instruments are passed through an opening created in a natural orifice such as the stomach, colon or vagina leaving the patient with no visible scars. NOTES faces similar challenges to ESD. The entire procedure is performed through a single endoscope and there is loss of triangulation and the second hand that is normally present in surgical procedures. Moreover, the instruments working area is reduced resulting in frequent collision of instruments known as “Sword fighting”.
Triangulation is the ability of the surgeon to manipulate tissue with traction and counter-traction permitting appropriate dissection and suturing. Flexible endoscopic surgery need to apply the same principles of triangulation to be an effective surgical approach[34,35]. Multiple endoscopic prototypes have been experimented in NOTES, but they failed for multiple reasons[36]. First, additional angulations at the tip of a flexible endoscope sacrificed stability which is needed for safe dissection and suturing. Second, angulation at the tip of flexible endoscope makes tactile feedback less reliable. Third, adding more functions to flexible endoscopes increases the complexity of the procedure leading to its impracticality by the operator. A single operator is only able to control a limited number of buttons and wheels. Adding more functions to the flexible endoscope allowing more angulations, more control buttons, wheels and levers will lead to practical limitations due to increased complexity. It has been concluded that current instruments are not suitable for effective surgery in the NOTES environment[37].
The magnetic anchor guidance system (MAGS) concept was tested in a laparoscopic prototype with a system of external neodymium magnetic anchors, tissue retractors and an internal camera[38]. This system aims at controlling an intra-abdominal laparoscope and multiple instruments introduced through a single trocar into the abdominal cavity. All instruments had neodymium permanent magnets. Surgeons were able to position the retractors under the liver or the spleen to elevate them by manipulating external magnetic anchors in a porcine model. The MAGS platform was sufficient to securely anchor the tissue retractors and the camera. These instruments could be moved within the abdomen without loss of magnetic coupling. Two non-survival porcine laparoscopic nephrectomies were successfully completed without complications using this system.
In another study with the same MAG platform, four non-survival porcine transvaginal NOTES cholecystectomy using magnetically anchored instruments were successfully performed[39,40]. This platform was also used successfully in the manipulation and stabilization of mesh for transcolonic endoscopic ventral hernia repair[41].
MAG has also been used in 40 patients undergoing single port laparoscopic cholecystectomy[42]. This MAG system consisted of two magnetic forceps and two external magnets. By moving the first external magnet, the first forceps grasping the gallbladder fundus was pulled upwards lifting the liver and exposing the gallbladder. By moving the second magnet, the second forceps grasping the Hartmann’s pouch was pulled towards the right lower quadrant or towards the epigastrium yielding the best exposure of Calot’s triangle. Then, the gallbladder was dissected laparoscopically. However, in 23 of the 40 patients, assistance was required using a 1-mm needle inserted through the abdominal wall to delicately separate the liver or help in the Calot triangle’s dissection.
MAGNETIC NON-CONTACT RETRACTION BY SURFACE FERROMAGNETIZATION
Wang et al[43] postulated that magnetic non-contact retraction has potential advantages over the existing direct physical retraction means such as forceps. Non-contact retraction provides complete atraumatic retraction and avoids tumor cell exfoliation. They conducted a pilot study using surface magnetic retraction of the gastric mucosa to facilitate mucosal resection in sixteen porcine stomach specimens. They prepared magnetic media of ferromagnetic micro-particles (Stainless steel 410) dispersed in cyanoacrylate liquid. The media was surface-glued to the target mucosa. A 3-mm neodymium permanent magnet was placed in contact with the media, and the mucosa was magnetically retracted by 10 mm. An in-vitro surgical resection of the retracted mucosa was then performed with the use of electrosurgical snares. The magnet was capable of maintaining retraction of the mucosa, via the medium, throughout the procedure. The data obtained showed that the force required to retract and lift the mucosa by 10 mm was 0.135 Newtons (N). There is a linear relationship between the retraction force and distance. The estimated total retracting force during cutting with Snares was 0.324 ± 0.154 N. They concluded that non-contact magnetic grasping is feasible with the development of the necessary sensor and control technology. The main limitation of this idea is that the magnetic force decreases sharply as the air gap increases. Hence, more powerful magnets would be needed for non-contact retraction at a safe operating distance.
LIMITATIONS OF MAG-ESD
In its current form, the most significant limitation of MAG-ESD is the coupling strength of magnets. Magnetic force decays over distance exponentially. So, the strength of magnetic force varies based on the patient’s abdominal thickness which depends mainly on the amount of abdominal fat. Experimental studies have proven that the maximal thickness of the abdominal wall where MAG-ESD is practical varies from 1.5 centimeters (cm) up to 4 cm[44,45]. So, MAG-ESD in its current form can’t be adopted for obese patients and is limited to thin and pediatric patients only[43]. More studies need to be done to be able to determine the appropriate relation between coupling force of magnets, weight of anchored instruments and abdominal wall thickness.
In non-obese women, the abdominal wall measured 1.7 ± 0.7 cm at the umbilicus and 2.0 ± 0.7 cm in the left upper quadrant. For obese women (BMI ≥ 30), thickness was 3.0 ± 1.2 cm at the umbilicus and 4.4 ± 1.0 in the left upper quadrant[46].
If we want to use more than one external magnet to control more than one intraabdominal/intraluminal tool as what is done in MAGS that is used in NOTES, we will be faced by another limitation. The maximum number of external magnets in the operating area is unknown. The minimum separation distance between external magnets in current external platforms is 3 cm before magnet attraction could be problematic. Such problems could be magnet-magnet interference and also operator hand-magnet collisions[38].
Some authors point out to the possible effects of magnetic fields on human tissues[29]. An example is the possible effect on blood flow which might cause clotting. Another problem is the interaction between magnets and other instruments used during procedures. Moreover, a ferromagnetic foreign body in human tissue can interact with the magnetic field. This technology is contraindicated with pacemakers[47], metal foreign body or recently implanted metal orthopedic prosthesis.
To test possible damages that might be caused by magnets on tissues, a study was conducted on three pigs by placing magnetic devices laparoscopically in the four abdominal quadrants[48]. The devices were left in place for 2 to 4 h. Full-thickness abdominal wall sections were harvested from these quadrants plus control at 0, 2, or 14 d after surgery. Histologic assessment was then performed. No gross or microscopic tissue damage was seen. Moreover, magnets are used in other applications in medicine, such as MRI, that are considered to be safe.
In summary, the main limitations of MAG-ESD technique are the coupling strength of magnets, abdominal wall thickness and possible harmful effects of magnets on human tissues. Moreover, the large size of the magnets and the high cost are significant problems that need to be overcome.
FUTURE
MAG-ESD has the potential to act as a surgeon’s “second hand” to make complex endoscopic procedures like ESD easier to perform. However, to achieve this, it has to overcome a number of challenges and limitations. Techniques like the clip-with-line method are able to offer basic retraction simply and affordably with equipment already available in an endoscopy suite. For the more complex MAG-ESD to be appealing, it has to offer a significant advantage during complex endoscopic procedures such as ESD, over its competitors. More recently, a trial by Rivas et al[49] used the Magnetic Surgical System developed by Levita Magnetics Corp. (San Mateo, CA) to successfully perform fifty reduced-port laparoscopic cholecystectomies with no serious adverse events and excellent exposure of the surgical site. If the safety, efficacy, and ease of use of this system could be translated to ESD, magnetic endoscopy could be more appealing.
In summary, several major limitations that need to be overcome include the sheer size of the magnets and its high costs. As magnetic attraction decays over distance, its effect may vary in patients with different abdominal wall thickness. Moreover, air insufflated during endoscopy will increase the distance between magnets. A magnetic control system that could adapt magnetic strength to distance would reduce the variability of MAG-ESD. The more precise the control of the internal magnet, the more it might act like the desired second hand. With increased precision, magnets may be used for indications other than retraction, such as tissue dissection, improve visualization, and ultimately enhance the field of endoscopic surgery.
ACKNOWLEDGMENTS
We would like to thank Mohamed Elmassry, MD for his significant contribution to this article by providing the artwork.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amornyotin S, Fiori E, Tepes B S- Editor: Qi Y L- Editor: A E- Editor: Wang CH