Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4767
Peer-review started: February 9, 2017
First decision: February 23, 2017
Revised: March 1, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: July 14, 2017
To report early imaging assessment of ablated area post electrochemotherapy (ECT) in patients with locally advanced pancreatic cancer (LAPC).
ECT was performed in 19 LAPC patients enrolled in an approved ongoing clinical phase I/II study. Before and after ECT, 18 patients underwent computed tomography (CT) scan, 11 patients underwent morphological and functional magnetic resonance (MR) scan (dynamic contrast enhanced-MRI) calculating wash-in slope (WIS) and wash-out slope (WOS); diffusion weighted imaging calculating pseudo-diffusivity (Dp), perfusion fraction (fp) and tissue diffusivity (Dt); 10 patients underwent positron emission tomography (PET). Response evaluation criteria in solid tumour (RECIST) on MR and CT were used to assess tumour therapy response. Choi on CT, PET response criteria in solid tumors (PERCIST) on PET and functional parameters on MR were used to evaluate treatment response.
For each patient no significant reduction was measurable by CT and MR using RECIST. According Choi criteria a partial response was obtained in 18/18 (100.0%) patients. According PERCIST criteria 6/10 (60.0%) patients showed a partial response, 3/10 (30.0%) stable disease and 1/10 (10.0%) progression disease. Moreover, using functional MR parameters, a significant reduction of viable tumour after ECT can be observed. According ΔWIS and ΔWOS 9/11 (81.8%) patients exhibited a partial response and 2/11 (18.2%) stable disease; 8/11 (72.7%) patients were considered in partial response by ΔDp evaluation and 3/11 (27.3%) in stable disease; according ΔDt 7/11 (63.6%) patients showed a partial response, 1/11 (9.1%) showed progression of disease and 3/11 (27.3%) were stable. Perfusion fraction fp showed a significant reduction after ECT only in four patients. No significant difference was observed after ECT in signal intensity of T1-weighted images and T2-weighted images, and in equilibrium-phase of contrast study, according to χ2 test was observed. A good correlation was reported between ΔHounsfield unit and Δmaximum standardized uptake value and between Δfp and ΔWOS, with a significant statistically difference (P < 0.05) using Spearman correlation coefficient.
Perfusion and diffusion MR derived parameters, Choi, PERCIST criteria are more performant than morphological MR and CT criteria to assess ECT treatment response.
Core tip: Aim of this study was to assess and to report early imaging assessment of ablated area post electrochemotherapy in patients with locally advanced pancreatic cancer emphasizing the role of new functional imaging tools in magnetic resonance imaging compared to standard morphological response evaluation criteria in solid tumour, Choi criteria and positron emission tomography response evaluation criteria in solid tumour.
- Citation: Granata V, Fusco R, Setola SV, Piccirillo M, Leongito M, Palaia R, Granata F, Lastoria S, Izzo F, Petrillo A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J Gastroenterol 2017; 23(26): 4767-4778
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4767.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4767
Adenocarcinoma of pancreas is among the most aggressive forms of cancer. Surgical resection is the only potentially curative treatment for pancreatic cancer. Unfortunately, the majority of patients have grossly unresectable disease; over 80% of patients with pancreatic cancer have locally advanced or metastatic disease[1]. Current standard therapy is chemotherapy and/or radiotherapy. The most frequently used chemotherapy agent in LAPC was Gemcitabine; moreover some studies have shown the combination of Gemcitabine with other chemotherapy agents increases overall survival[1-4]. Because a limited group of patients responds to chemotherapy, additional therapies were explored in order to obtain tumor debulking or interstitial ablation[5-8]. A potential therapy was the electroporation that can be delivered in either an irreversible[9-11], as a direct ablation modality, or a reversible manner[12-15], as a physical delivery system, based on the strength and duration of the electrical field. Reversible electroporation has been performed to increase uptake of chemotherapy into tumor cells. Reversible electroporation combined with low doses of chemotherapeutic drugs was known as Electrochemotherapy (ECT)[15]. The delivering of an electrical field determines a transient increase of cell permeability with a consequent increase of intracellular dose of chemotherapeutic drugs, using low doses and reducing the chemotherapy cytotoxic effects[12-15]. Preclinical studies showed the effectiveness of ECT on pancreatic cancer[15,16]. Our previous studies investigated the safety and effectiveness of ECT in patients with locally advanced pancreatic cancer[17,18]. Recently, Tarantino et al[19] investigated the feasibility of percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis.
Until now oncologic therapy is principally based on different combinations of surgery, radiotherapy, and chemotherapy, however targeted therapies, hormonotherapy, immunotherapy, and interventional techniques, with the introduction a new promise ablation techniques to treat deeper tumors have emerged as alternative potential cancer treatments[17-23]. Currently, standard imaging techniques and morphologic response criteria do not provide the necessary information to evaluate tumor response. Magnetic resonance imaging (MRI) offers a combination of anatomic, physiologic, and molecular information, which may overcame these limitations, and is being increasingly used for therapy response assessment[23,24].
The purpose of our study is to report the early imaging assessment of treated area with ECT in locally advanced pancreatic cancer, emphasizing the role of new functional imaging tools in MRI compared to standard morphological response evaluation criteria in solid tumour (RECIST), Choi[25] and PERCIST criteria[26].
The patients were enrolled in a clinical phase I/II study approved by the Ethical Committee of the National Cancer Institute “G. Pascale Foundation - IRCCS” of Naples (deliberation n. 482 of 02/07/2014). The study endpoints were the feasibility and safety of ECT in the multimodal treatment of pancreatic cancer in patients with locally advanced disease and not suitable for radical surgery.
Nineteen patients (9 female and 10 male) from November 2011 to December 2016 were enrolled in this prospective study. Inclusion criteria were: age between 18-80 years; good mental health; life expectancy ≥ 3 mo; histologically confirmed diagnosis of pancreatic adenocarcinoma; locally advanced disease (stage III) confirmed with preoperative radiological assessment, unfit for curative surgery. Exclusion criteria were: pregnant women, significant heart disease, coagulation disturbances, allergy to bleomycin, lung and kidney dysfunction, implanted defibrillator or pacemaker, concomitant presence of distant metastases. All patient enrolled have signed the informed consensus.
All patients enrolled with diagnosis of locally advanced pancreatic adenocarcinoma received systemic chemotherapy before ECT treatment. Patient characteristics were summarized in Table 1. Two chemotherapy regimens were adopted: Gemcitabine + Oxaliplatin (GEMOX) or 5-FU/Leucovorin, Irinotecan, and Oxaliplatin (FOLFIRINOX). Details of chemotherapy regimens were reported in our previous publication[27].
| Patients (n = 19) | |
| Histotype, % | |
| Adenocarcinoma | 100 (19/19) |
| Location, % | |
| Head | 57.9 (11/19) |
| Body/tail | 42.1 (8/19) |
| Largest diameter lesion, cm (range) | 5.2 (2.2-9.9) |
| Venus involvement (SMV or PV), % | |
| Yes | 84.2 (16/19) |
| No | 15.7 (3/19) |
| Arterial encasement, % | |
| Yes | 57.9 (11/19) |
| No | 42.1 (8/19) |
Fourteen (14/19, 73.9%) patients were subjected to GEMOX and five patients (5/19, 26.3%) were treated with FOLFIRINOX before ECT treatment (mean time between the start of chemotherapy treatment and ECT was 118 d, range 115-136). The patients with stable disease or partial response after chemotherapy, proven by clinical and radiological examination, were suitable to receive ECT treatment.
ECT was delivered in open laparotomy through an adequate midline incision to allow both appropriate staging of the disease and appropriate mobilization of the pancreatic malignancy based on its tumor location and infiltration. In the case of pancreatic head lesions, an extensive Kocher maneuver was performed in order to mobilize the duodenum and the head of the pancreas over the area of local invasion to allow easier caudal and cranial needle placement. Similarly, mobilization of the transverse colon were done inferiorly, depending on the degree of infiltration. In this way, the surgeon who performed the treatment was able to decide whether to use needle electrodes with fixed geometry (hexagonal or linear) or with variable configuration using multiple insertion of single-needle; through the transverse mesocolon or, if the mobilization of the transverse mesocolon was impossible, the needle electrodes were inserted superior to the base of the transverse mesocolon.
Bleomycin was administrated intravenously (15000 IU/m2) before the application of electrical pulses to the target area. Electric pulses were applied by needle electrodes with linear, hexagonal configuration or variable geometry (IGEA S.p.A., Carpi, Italy) depending on the size and location of the tumors. Cliniporator™ (IGEA S.p.A., Italy) was used to deliver electric voltage with the following parameters: 8-96 pulses of 400-1000 V and 910-1000 V/cm, of 100 μs duration, at 1-5000 Hz repetition frequency in according to ESOPE (European Standard Operating procedure of Electrochemotherapy) protocol[28] or a single pulse for a single relived R-wave (ECG synchronization) for custom geometry. Electric impulses were synchronized with the ECG for a safe delivery of the electric impulses to pancreas. ECG synchronization was obtained with Accusync 42 (medical device provided by IGEA S.p.A., Carpi, Italy). Treatment was completed within the window from 8 to 28 min after the end of the bleomycin bolus.
According to the study protocol, the patients underwent baseline MRI and/or computer tomography (CT) and/or 18F-FDG positron emission tomographic (PET)/CT study 5-7 d before ECT and post-treatment at 1, 3, 6 and 12 mo. Long-term follow-up was carried out with radiological imaging obtained every three months in the time thereafter.
We considered, in this paper, only the morphological and functional results obtained in CT, PET/CT and MR imaging at one month as prognostic early indicator of therapy response. The gold standard to defining the assessment after therapy has been the consensus between two radiological modalities (Choi, PERCIST, Dynamic Contrast Enhanced-Magnetic Resonance Imaging and Diffusion Weighted Imaging; for two latter considering the consensus of response of two parameters).
MR and CT protocol: MR protocol consists of morphological and functional imaging including dynamic contrast enhanced-magnetic resonance imaging (DCE-MRI) and diffusion weighted imaging (DWI) sequences. Imaging was performed with a 1.5 T scanner (Magnetom Symphony, Siemens Medical System, Erlangen, Germany) equipped with a phased-array body coil. Patients were placed in a supine, head-first position.
Morphological pre-contrast axial T2-weigthed (T2-w) 2D Half-Fourier Acquisition Single-Shot Turbo Spin-Echo (HASTE), with and without fat suppression; morphological pre-contrast axial T1-weigthed (T1-w) Fast Low Angle Shot (FLASH) 2D in-out phase; morphological pre contrast axial T1-w fat suppression FLASH 2D out phase; morphological post contrast axial and coronal T1-w Volumetric Interpolated Breath-hold Examination (VIBE) fat suppression were acquired.
A free breathing axial single shot echo planar DWI pulse sequence parameters were obtained with multiple b value = 0, 50, 100, 150, 400, 800, 1000 s/mm2.
After, DCE-MRI sequences, we obtained 1 sequence before and 120 sequences, without any delay, after intravenous injection of 2 mL/kg of a positive, gadolinium-based paramagnetic contrast medium (Gadobutrol Gd-DTPA, Bayer Pharma AG, Berlin, Germany). The contrast medium was injected using Spectris Solaris® EP MR (MEDRAD Inc., Indianola, PA, United States), with a flow rate of 2 mL/s, followed by a 10-mL saline flush at the same rate. DCE-MRI T1-w were acquired using Time-Resolved Angiography with Stochastic Trajectories 3-D axial images in order to reduce temporal resolution (3 s).
Parameters details for each MR sequence were provided in Table 2.
| Sequence | Orientation | TR/TE/FA (ms/ms/deg.) | FOV (mm2) | Acquisition matrix | Slice thickness/gap (mm) |
| HASTE T2-W | Axial | 1500/90/180 | 380 × 380 | 320 × 320 | 5/0 |
| FLASH T1-W, in-out phase | Axial | 160/4.87/70 | 285 × 380 | 192 × 256 | 5/0 |
| FLASH T1-W, out phase | Axial | 178/2.3/80 | 325 × 400 | 416 × 412 | 3/0 |
| DWI | Axial | 7500/91/90 | 340 × 340 | 192 × 192 | 3/0 |
| VIBE T1-W | Axial | 4.89/2.38/10 | 325 × 400 | 320 × 260 | 3/0 |
| TWIST T1-W, Pre and post contrast agent injection | Axial | 3.01/1.09/25 | 300 × 300 | 256 × 256 | 2/0 |
Non contrast-enhanced phase and triple-phase contrast-enhanced MDCT was performed with a 64-detector row scanner (Optima 660, GE Healthcare, United States). MDCT scanning parameters were 120 kVp, 100-470 mAs (NI 16.36), 2.5-mm slice thickness and table speed 0.984/1 mm/rotation. Scans were carried out including a region encompassing the liver from diaphragm to iliac crests. Phases were as follows; hepatic arterial phase 30-40 s after injection of 120 mL of a nonionic contrast medium (iomeprol, Iomeron 400, Bracco, Milan, Italy) with a bolus-triggered technique [120 kVp; 40-60 mA; trigger threshold, 180 Hounsfield units in descending aorta], portal and equilibrium phase 90 s and 120 s after contrast injection. The contrast medium was administered at a rate of 4 mL/s through antecubital vein with an automated injector system (Empower CTA, E-Z-EM Inc., New York, United States).
MR and CT image analysis: Four blinded observers with at least 10 years’ experience in interpretation of MR and CT images of the pancreas independently and randomly reviewed the images acquired before and after ECT. The interval between reviews of the CT and MR images was at least 5 d. The response to ECT was evaluated according the RECIST 1.1 criteria[29]. Objective therapeutic responses according to RECIST 1.1 are as follows: complete remission (CR) is disappearance of target lesion for at least 4 wk; partial remission (PR) is a decline of at least 30% in tumor diameter; stable disease (SD) is neither PR nor progressive disease (PD); and PD is at least a 20% increase in tumor diameter and 5-mm absolute increase was required. Moreover for CT images, the response to ECT was evaluated according the Choi criteria[24]: CR is disappearance of target lesion; PR is a decrease in tumor size ≥ 10% or decrease in tumor density ≥ 15% on CT; SD is neither PR nor PD; and PD is an increase in tumor size ≥ 10% and does not meet PR criteria by tumor density.
For functional MR analysis tumor borders were manually segmented on transversal T1-W images VIBE fat suppression post contrast (equilibrium phase). In each slice a region of interest (ROI) was delineated according to the tumor geometry. The border of the ROI was placed in the tumor periphery close to the tumor margin, so that the ROI encompassed almost the whole tumor area. DW-MRI analysis[30,31] and DCE-MRI semi-quantitative analysis[32] was performed on the ROIs previously described. For each pixel Time Intensity Curves (TICs) were obtained and per each TIC, 2 shape descriptors were computed: the WI slope (WIS), the WO slope (WOS) as described in[31]. DW-MRI analysis was performed using Variable Projection Curve-Fitting algorithm (VarPro), as reported by[33], to estimate the IVIM-related parameters of bi-exponential model: pseudo-diffusivity (Dp), perfusion fraction (fp) and tissue diffusivity (Dt). Per each descriptor (dimensional parameters, density on CT images and perfusion and diffusion coefficients) median value was obtained and the percentage changes between pre and post treatment [ΔX = (Xpre - Xpost)/Xpre; X is the generic shape descriptor] were calculated. No image registration was applied to our data acquired. We take care to exclude from the analysis the slices where was visible motion artifacts. Moreover, a volumetric analysis for functional parameters measurements was performed thus minimizing errors due to voxel misalignments.
This data analysis was performed using in-house software written in Matlab R2007a (The MathWorks, Inc., Natick, MA, United States). The following parameters were also evaluated for each single target area in MRI before and after treatment: signal intensity respectively in T1-weighted images, in T2-weighted sequences and in equilibrium phase of contrast study.
18F-FDG PET Data acquisition and images analysis:18F-FDG PET/CT studies were acquired 60 min after the administration of 300-385 MBq of FDG either with a Siemens ECAT EXACT 47 or a General Electric DST 600 PET-CT scanner. All calibrations on the scanners to obtain accurate SUV readings were regularly performed. Patients fasted for at least 6 h, and blood glucose level was < 150 mg/dL. Each patient underwent the baseline and the pre-operative study on the same scanner.
Irregular volumes of interest (VOIs) were semi-automatically drawn by the expert investigator on orthogonal planes using a dedicated workstation and software using an arbitrary threshold, as reported previously[34]. For each patient both studies were analyzed at the same time in order to minimize discrepancies in VOI positioning. For each study maximum standardized uptake value (SUVmax) values of the pancreas lesion were recorded. The analysis of 18F-FDG PET/CT results was performed by comparing measurements obtained in the pancreatic lesion at baseline (SUV1) and after treatment (SUV2). This change was expressed as the percentage of SUV reduction [ΔSUV = (SUV1−SUV2)/SUV1 × 100]. Objective therapeutic responses was defined according to PERCIST 1.0 are as follows[26]: complete metabolic response (CMR) is complete resolution of 18F-FDG uptake within the measurable target lesion and indistinguishable from surrounding background blood-pool levels with no new 18F-FDG-avid lesions; partial metabolic response (PMR) is reduction of a minimum of 30% in the target tumor 18F-FDG SUVmax; stable metabolic disease is disease other than CMR, PMR or progressive metabolic disease; and progressive metabolic disease is a 30% increase in 18F-FDG PET/CT SUVmax or advent of new 18F-FDG-avid lesions that are typical of cancer.
Data were expressed in terms of median value ± SD. Spearman correlation coefficient for non-parametric variables was used to assess the correlation between percentage changes of tissue density of CT, and of perfusion and diffusion parameters. A P value < 0.05 was considered statistically significant. Percentage of objective response was reported for each modality. χ2 test was, also, used to compare pre- and post-ECT imaging findings. A P value < 0.05 was regarded as statistically significant.
All analyses were performed using Statistics Toolbox of Matlab R2007a (The Math-Works Inc., Natick, MA, United States).
Basal imaging involved CT, PET and MR scans. Mean time between basal imaging assessment and ECT was 9 d (range 7-14). Mean time between ECT and first follow-up radiological assessment was 36 d (range 31-43).
CT was performed for eighteen patients before and after ECT; morphological and functional MR was obtained for 11 patients before and after ECT and 10 patients were subjected to 18F-FDG PET/CT before and after treatment. One died to complication after treatment (24-48 h after ECT). Four patients rejected MR scan due to claustrophobia complications. Three patients were affected by allergy to Gadolinium chelates (MR contraindication). In 4 patients the patient clinical conditions did not allow to perform 18F-FDG PET/CT study in the range that would make the data comparable, before and after treatment; in other 4 patients the PET study was performed in a different hospital with low quality of the images.
In Table 3 we reported the measure of largest diameters obtained by CT and MR for each patients, before and after one month of treatment.
| Patient No. | Age | Sex | Tumor size | Tumor response after ECT treatment | ||
| CT (mm) | MR (mm) | 1st radiological evaluation after ECT (CT); size (mm) | 1st radiological evaluation after ECT (MR); size (mm) | |||
| 1 | 48 | M | 99 | 95 | 90 | 87 |
| 2 | 63 | F | 43 | 48 | 38 | 43 |
| 3 | 71 | F | 59 | 64 | 54 | 57 |
| 4 | 61 | F | 22 | 26 | 19 | 23 |
| 5 | 72 | F | 51 | 49 | 49 | - |
| 6 | 80 | F | 48 | - | 45 | - |
| 7 | 60 | F | 33 | - | 24 | - |
| 8 | 62 | F | 30 | - | 22 | - |
| 9 | 67 | M | 99 | - | - | - |
| 10 | 57 | M | 56 | - | 46 | - |
| 11 | 74 | M | 56 | 58 | 59 | 51 |
| 12 | 67 | M | 63 | 68 | 55 | 55 |
| 13 | 59 | M | 28 | 30 | 28 | 24 |
| 14 | 79 | M | 50 | 41 | 46 | 38 |
| 15 | 71 | M | 35 | 34 | 56 | - |
| 16 | 80 | M | 53 | - | 49 | - |
| 17 | 80 | M | 64 | 55 | 49 | 46 |
| 18 | 59 | F | 51 | 51 | 66 | 65 |
| 19 | 62 | F | 53 | 53 | 50 | 49 |
In Table 4 we showed the percentage change, between before and after ECT, of largest diameter by CT and MR and the median value percentage change of tissue density in ΔHU, of perfusion and diffusion quantitative parameters derived by MR imaging (WIS, WOS, Dt, fp and Dp) and of maximum SUV value. Moreover, median values ± SD of percentage changes, before and after ECT were reported in Table 4.
| No. | ΔCT largest diameter | ΔHU | ΔMR largest diameter | ΔWIS | ΔWOS | ΔDt | Δfp | ΔDp | ΔSUVmax | Response assessment |
| 1 | 11.6% | 22.7% | 8.4% | 35.4% | 40.0% | -78.1% | 12.1% | 61.9% | -177.8% | PR |
| 2 | 9.1% | 40.4% | 10.4% | 84.7% | 85.0% | -32.7% | 27.3% | 78.7% | PR | |
| 3 | 8.5% | 34.0% | 11.5% | 94.0% | 74.4% | -64.4% | 28.5% | 50.3% | 38.5% | PR |
| 4 | 13.6% | 7.8% | 2.0% | 88.0% | 76.0% | -16.8% | 32.7% | 36.6% | PR | |
| 5 | 3.9% | 48.7% | ||||||||
| 6 | 6.3% | 18.9% | ||||||||
| 7 | 27.3% | 49.5% | ||||||||
| 8 | 26.7% | 51.6% | ||||||||
| 9 | ||||||||||
| 10 | 17.9% | 42.6% | 100.0% | PR | ||||||
| 11 | -5.4% | 49.1% | 12.1% | 18.4% | 9.7% | -20.4% | -33.3% | 5.4% | 66.5% | SD |
| 12 | 12.7% | 6.8% | 19.1% | 57.9% | 98.0% | -34.2% | 11.7% | 12.0% | -17.9% | SD |
| 13 | 6.7% | 44.4% | 20.0% | 7.9% | -17.4% | 32.5 | 2.2% | 36.9% | 46.8% | PR |
| 14 | 8.0% | 44.8% | 7.3% | 67.6% | 110.0% | -32.9% | 44.3% | 92.0% | 44.4% | PR |
| 15 | -60.0% | 83.3% | ||||||||
| 16 | 7.5% | 23.4% | ||||||||
| 17 | 23.4% | 35.5% | 16.4% | 55.7% | 307.1% | -18.0% | 62.6% | 32.2% | 18.8% | PR |
| 18 | -29.4% | 40.0% | -9.8% | 34.9% | 58.7% | -16.3% | 46.7% | 66.4% | 17.0% | PR |
| 19 | 5.7% | 44.0% | 7.5% | 67.3% | -24.6% | -30.5% | -22.6% | -90.7% | 32.3% | PR |
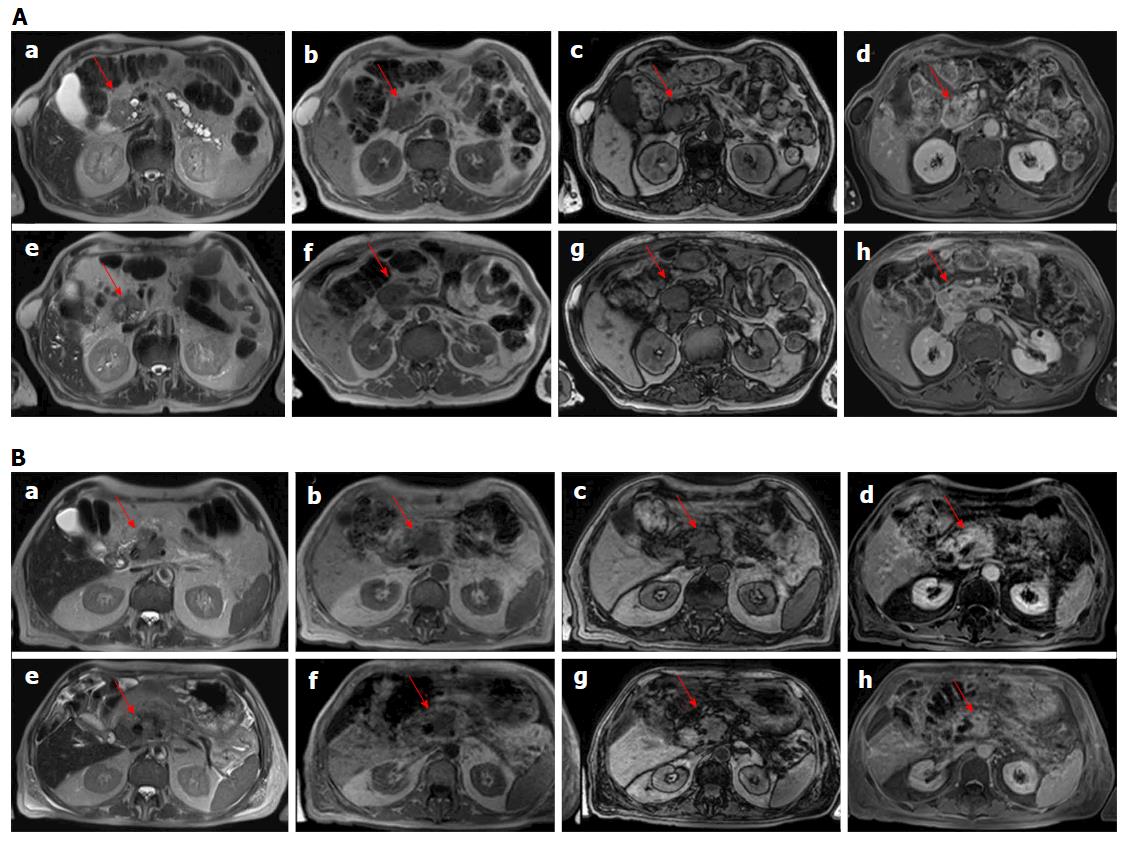
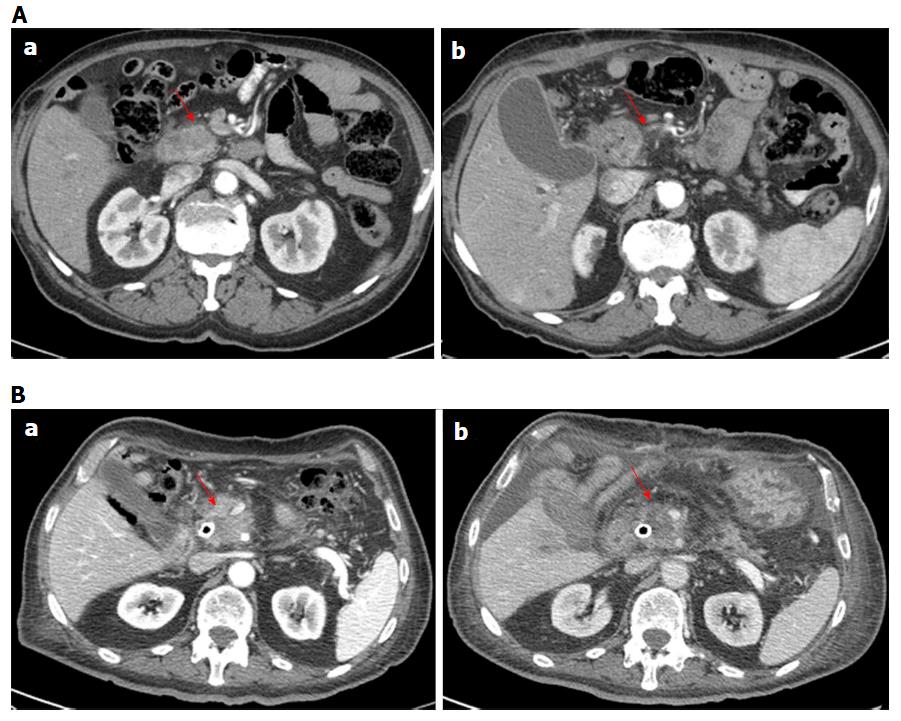
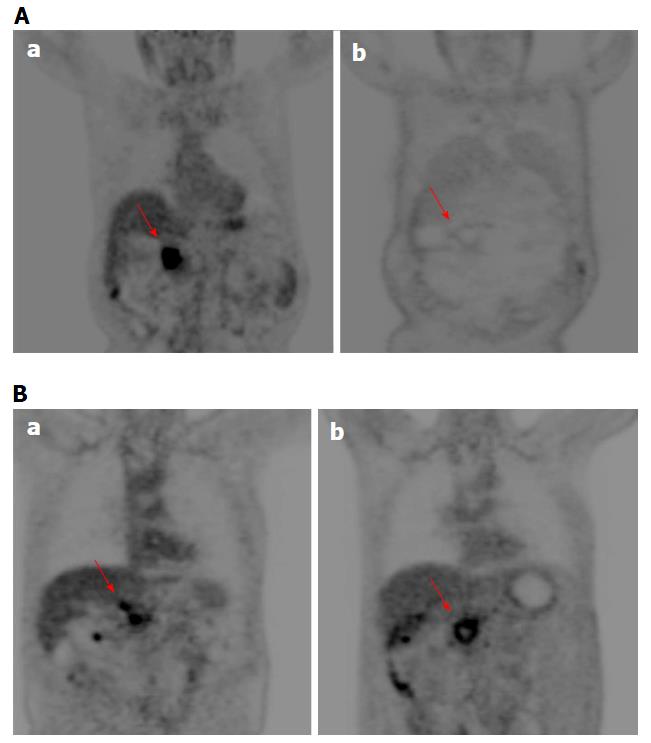
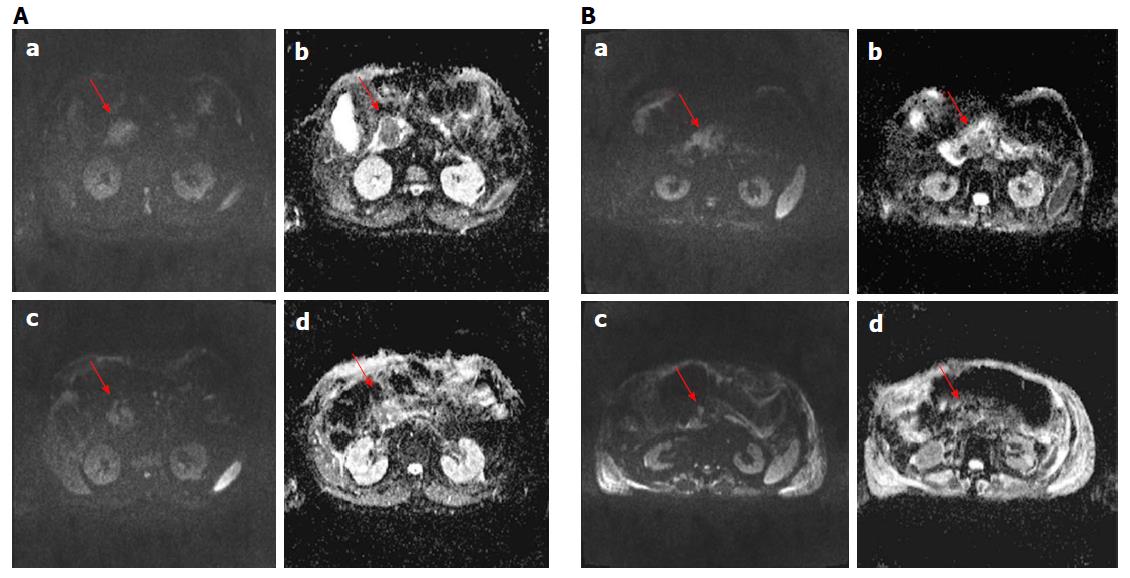
For each patient no significant reduction of largest diameter percentage change by CT and MR was observed. According to RECIST criteria, all patients had stable disease using MR imaging while using CT imaging one patient showed progression disease (Figure 1 for MRI and 2, 6 for CT imaging). According to Choi criteria 18/18 (100.0%) patients were considered in partial response (Figure 2). According to PERCIST criteria 6/10 (60.0%) exhibited partial response (Figure 3), 3/10 (30.0%) stable disease and 1/10 (10.0%) progression disease. Moreover, using functional MR derived parameters, significant reduction of viable tumor tissue were observed: a reduction of 30% of ΔWIS, ΔWOS, Δfp, ΔDp and an increase of 30% of ΔDt was considered as significant variation after treatment and was defined as partial response. For both ΔWIS and ΔWOS 9/11 (81.8%) patients showed partial response and 2/11 (18.2%) were considered stable. Eight elevenths (72.7%) patients were considered in partial response by ΔDp evaluation and 3/11 (27.3%) was considered in stable disease. According to ΔDt 7/11 (63.6%) patients showed partial response, 1/11 (9.1%) progression disease and 3/11 (27.3%) stable disease (Figure 4). Perfusion fraction fp showed a significant reduction after ECT only for four patients. Final decision on treatment response was taken considering the accordance with at least two imaging technique (see last column of Table 4).
We found no statistically significant difference of target area signal intensity obtained by T1-weighted images, T2-weighted images and equilibrium-phase of contrast study between before and after treatment, according to Chi-square test.
Spearman correlation coefficient was performed for each couple of parameters and was reported in Table 5. A good correlation was reported between ΔHU and ΔSUVmax and between Δfp and ΔWOS, with a significant statistically difference (P < 0.05).
| ΔCT maximum diameter (%) | ΔHU (%) | ΔMR maximum diameter (%) | ΔWash-in (%) | ΔWash-out (%) | ΔDt (%) | Δfp (%) | ΔDp (%) | ΔSUVmax | ||
| ΔCT maximum diameter (%) | Correlation coefficient | 1.000 | -0.183 | 0.227 | 0.418 | 0.655a | -0.273 | 0.364 | -0.036 | -0.127 |
| P value | 0.468 | 0.502 | 0.201 | 0.029 | 0.417 | 0.272 | 0.915 | 0.726 | ||
| n | 18 | 18 | 11 | 11 | 11 | 11 | 11 | 11 | 10 | |
| ΔHU (%) | Correlation coefficient | -0.183 | 1.000 | 0.027 | -0.373 | -0.373 | 0.318 | -0.291 | 0.064 | 0.758a |
| P value | 0.468 | 0.937 | 0.259 | 0.259 | 0.340 | 0.385 | 0.853 | 0.011 | ||
| n | 18 | 18 | 11 | 11 | 11 | 11 | 11 | 11 | 10 | |
| ΔMR maximum diameter (%) | Correlation coefficient | 0.227 | 0.027 | 1.000 | -0.345 | -0.082 | -0.036 | -0.409 | -0.391 | 0.183 |
| P value | 0.502 | 0.937 | 0.298 | 0.811 | 0.915 | 0.212 | 0.235 | 0.637 | ||
| n | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 9 | |
| ΔWash-in (%) | Correlation coefficient | 0.418 | -0.373 | -0.345 | 1.000 | 0.527 | -0.436 | 0.318 | 0.209 | -0.150 |
| P value | 0.201 | 0.259 | 0.298 | 0.096 | 0.180 | 0.340 | 0.537 | 0.700 | ||
| n | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 9 | |
| ΔWash-out (%) | Correlation coefficient | 0.655a | -0.373 | -0.082 | 0.527 | 1.000 | -0.264 | 0.709a | 0.291 | -0.383 |
| P value | 0.029 | 0.259 | 0.811 | 0.096 | 0.433 | 0.015 | 0.385 | 0.308 | ||
| n | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 9 | |
| ΔDt (%) | Correlation coefficient | -0.273 | 0.318 | -0.036 | -0.436 | -0.264 | 1.000 | 0.118 | -0.164 | 0.417 |
| P value | 0.417 | 0.340 | 0.915 | 0.180 | 0.433 | 0.729 | 0.631 | 0.265 | ||
| n | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 9 | |
| Δfp (%) | Correlation coefficient | 0.364 | -0.291 | -0.409 | 0.318 | 0.709a | 0.118 | 1.000 | 0.545 | -0.400 |
| P value | 0.272 | 0.385 | 0.212 | 0.340 | 0.015 | 0.729 | 0.083 | 0.286 | ||
| n | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 9 | |
| ΔDp (%) | Correlation coefficient | -0.036 | 0.064 | -0.391 | 0.209 | 0.291 | -0.164 | 0.545 | 1.000 | -0.167 |
| P value | 0.915 | 0.853 | 0.235 | 0.537 | 0.385 | 0.631 | 0.083 | 0.668 | ||
| n | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 9 | |
| ΔSUVmax | Correlation coefficient | -0.127 | 0.758a | 0.183 | -0.150 | -0.383 | 0.417 | -0.400 | -0.167 | 1.000 |
| P value | 0.726 | 0.011 | 0.637 | 0.700 | 0.308 | 0.265 | 0.286 | 0.668 | ||
| n | 10 | 10 | 9 | 9 | 9 | 9 | 9 | 9 | 10 | |
Although it has been shown that the ECT is a promising technique for cancer treatment[15-18,21,28], there is still the problem of how to assess treated tumor response. In our preliminary experience, we demonstrated that RECIST 1.1, using the variation of largest diameter, both on CT and MR images, do not provide a appropriate patients stratification in responders or non-responders after ECT. In fact, according to RECIST criteria, all patients were classified with stable disease by MR imaging while using CT scan one patient showed progression disease. The RECIST criteria restrictions are well known, as also reported by Lencioni et al[35] in the assessment of residual viable tumor of treated HCC and by Choi[36] in Gastrointestinal Stromal Tumor. ECT potentiates the cytotoxic effect of chemotherapy and, therefore, the CHOI or PERCIST criteria would appear to be more suitable for early treatment evaluation[25,26,36,37]. In fact, according to our results, using Choi criteria (tissue density percentage change) 18/18 (100%) patients were considered in partial response. For PERCIST criteria 6/10 (60.0%) showed partial response, 3/10 (30.0%) stable disease and 1/10 (10.0%) progression disease. A good correlation was reported between ΔHU and ΔSUVmax. The accuracy of Choi criteria is known in the evaluation of target therapies[38]; during imatinib treatment therapy, Choi criteria have proved to be very useful to differentiate responders by non-responders and offer an potent prognostic indicator in terms of progression-free survival[36]. A recent study of van der Veldt et al[38] found that the Choi criteria may be helpful in assessing early metastatic renal cell carcinoma treated with sunitinib while Stacchiotti et al[39] showed that the Choi criteria were superior to the RECIST criteria to evaluate soft-tissue sarcoma response after chemotherapy and radiation therapy. Because many newer cancer therapies may be more cytostatic than cytocidal, appreciable tumor response may be associated to a decrease in metabolism, without a reduction in tumor size. Then, metabolic response can be a hopeful early indicator of tumor response and may be even more predictive of outcome than morphologic criteria[37]. So, the PERCIST criteria were proposed in 2009 to define and validate quantitative approaches to evaluating PET tumor response[26]. In a study on evaluation of response to chemotherapy in non-small cell lung cancer, PERCIST is more sensitive in detecting complete remission and progression, and these criteria might be the significant predictor of outcomes[40]. Avallone et al[34] demonstrated that, after preoperative radio-chemotherapy in locally advanced rectal cancer, FDG-PET is both an early predictor of pathologic response and a valuable prognostic tool. So, according to literature, on the value of the functional data obtained in CT and PET, compared with only morphological data in CT and MRI, to evaluate the response to ECT, it appears more appropriate to use Choi or PERCIST Criteria, although it would be better to link the two data[22-26,34-40]. García-Figueiras et al[24] demonstrated that standard imaging modalities and current morphologic response criteria do not always offer the adequate information for early therapy assessment, especially when target or ablated therapy were considered. According to García-Figueiras et al[24], MRI is able to predict treatment success before size changes become evident, thanks its capability to integrate anatomic, physiologic, and functional tissue information, which may overcome these limitations. In our study the morphologic information obtained by MRI examination did not show a dimensional change of treated pancreas lesion neither no statistically-significant difference of signal intensity obtained from T1-weighted images, T2-weighted images and equilibrium-phase of contrast MR study. WIS, WOS, Dt and Dp values showed a significant reduction after ECT. Our results confirmed the data reported by Hjouj et al[41] that established as MRI might be used for brain electroporation treatment monitoring. Quantitative functional MR derived parameters such as WIS, WOS, Dt, Dp, fp, have allowed the identification of necrotic areas and of fibrotic tissue compared to any residual tumor[17] so as to overcome the limitations of RECIST 1.1. Considering the response evaluation accordance between at least 2 radiological modalities, 10 patients of our population, after one month from ECT, showed a significant reduction of viable tissue associated to a partial response, while two patients showed stable disease. We, also, demonstrated a good correlation between ΔHU and ΔSUVmax and between Δfp and ΔWOS, with a significant statistically difference (P < 0.05). Sakane et al[42] has demonstrated significant correlation between apparent diffusion coefficient and SUV in pancreatic cancer, and that leads us to think that in responder patients where the SUV is reduced significantly also Dt is reduced significantly as reported in our results.
The major limitations of this study are the small number of patients evaluated and the availability for all patients of the same diagnostic techniques, to compare all results obtained and to validate the potential, in term of efficacy, of perfusion and diffusion MR derived parameters, to differentiate responders by not responders after ECT with PET, CT and MR examination. The future goal is to increase the radiological data and to have a more homogeneous group in order to compare the results.
In conclusion, ECT is a promising technique for locally advanced pancreatic cancer, but there is still the issue of how to monitor the treatment response. Conventional morphologic data (RECIST criteria) obtained by CT or MR imaging were not able to differentiate partial, complete or incomplete response after ECT while the changes in functional parameters, obtained with PET (SUVmax), MR (wash-in and wash-out and for DCE and Dp, fp and Dt for DWI) and CT (tissue density) study could be more suitable to assess ECT response.
Adenocarcinoma of pancreas is among the most aggressive forms of cancer. Surgical resection is the only potentially curative treatment for pancreatic cancer. Unfortunately, the majority of patients have grossly unresectable disease; over 80% of patients with pancreatic cancer have locally advanced or metastatic disease. Current standard therapy is chemotherapy and/or radiotherapy. The most frequently used chemotherapy agent in LAPC was Gemcitabine; moreover some studies have shown the combination of Gemcitabine with other chemotherapy agents increases overall survival. Because a limited group of patients responds to chemotherapy, additional therapies were explored in order to obtain tumor debulking or interstitial ablation.
Reversible electroporation combined with low doses of chemotherapeutic drugs, known as electrochemotherapy (ECT), has been used to promote chemotherapy uptake into tumor cells reducing reducing its cytotoxic effect.
Currently, standard imaging modalities and response criteria do not always provide the adequate and necessary information to assess tumor ECT response. The innovation of the study is to report the early imaging assessment of treated area with ECT in locally advanced pancreatic cancer, emphasizing the role of new functional imaging tools in magnetic resonance imaging (MRI) compared to standard morphological response evaluation criteria in solid tumour (RECIST), Choi and positron emission tomography ERCIST criteria.
New functional imaging tools in MRI that allow diffusion and perfusion tissue assessment could be used for early ECT tumor response.
ECT consists of the concomitant administration of low doses of chemotherapeutic drugs and reversible electroporation by means the delivering of an external electrical field to a cell membrane that induces a transient and reversible orientation of its polar molecules, with an increased permeability.
This paper report a single center experience of Imaging assessment in locally advanced pancreatic cancer treated with electrochemotherapy. The topic is interesting, the weakness of the manuscipt is limited by the small number of patients and heterogeneous data since not all patients underwent the same radiologic examinations
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Luchini C, Sperti C S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9215] [Cited by in F6Publishing: 9719] [Article Influence: 883.5] [Reference Citation Analysis (3)] |
| 2. | Granata V, Fusco R, Catalano O, Setola SV, de Lutio di Castelguidone E, Piccirillo M, Palaia R, Grassi R, Granata F, Izzo F. Multidetector computer tomography in the pancreatic adenocarcinoma assessment: an update. Infect Agent Cancer. 2016;11:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4838] [Cited by in F6Publishing: 5099] [Article Influence: 392.2] [Reference Citation Analysis (1)] |
| 4. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4035] [Cited by in F6Publishing: 4330] [Article Influence: 393.6] [Reference Citation Analysis (0)] |
| 5. | Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Pai M, Yang J, Zhang X, Jin Z, Wang D, Senturk H, Lakhtakia S, Reddy N. Endoscopic ultrasound guided radiofrequency ablation (EUSRFA) for pancreatic ductal adenocarcinoma. Gut. 2013;62:A153. [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 167] [Cited by in F6Publishing: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Carrafiello G, Ierardi AM, Fontana F, Petrillo M, Floridi C, Lucchina N, Cuffari S, Dionigi G, Rotondo A, Fugazzola C. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24:1513-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Crowley JM. Electrical breakdown of bimolecular lipid membranes as an electromechanical instability. Biophys J. 1973;13:711-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 325] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Neumann E, Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol. 1972;10:279-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 365] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Zimmermann U, Pilwat G, Riemann F. Dielectric breakdown of cell membranes. Biophys J. 1974;14:881-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 582] [Cited by in F6Publishing: 401] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Sugar IP, Neumann E. Stochastic model for electric field-induced membrane pores. Electroporation. Biophys Chem. 1984;19:211-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 171] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Mir LM, Orlowski S. Mechanisms of electrochemotherapy. Adv Drug Deliv Rev. 1999;35:107-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 542] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 15. | Jaroszeski MJ, Dang V, Pottinger C, Hickey J, Gilbert R, Heller R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs. 2000;11:201-208. [PubMed] [Cited in This Article: ] |
| 16. | Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Granata V, Fusco R, Piccirillo M, Palaia R, Lastoria S, Petrillo A, Izzo F. Feasibility and safety of intraoperative electrochemotherapy in locally advanced pancreatic tumor: a preliminary experience. Eur J Inflamm. 2014;12:467-477. [Cited in This Article: ] |
| 18. | Granata V, Fusco R, Piccirillo M, Palaia R, Petrillo A, Lastoria S, Izzo F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int J Surg. 2015;18:230-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Tarantino L, Busto G, Nasto A, Fristachi R, Cacace L, Talamo M, Accardo C, Bortone S, Gallo P, Tarantino P. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J Gastroenterol. 2017;23:906-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 20. | Granata V, de Lutio di Castelguidone E, Fusco R, Catalano O, Piccirillo M, Palaia R, Izzo F, Gallipoli AD, Petrillo A. Irreversible electroporation of hepatocellular carcinoma: preliminary report on the diagnostic accuracy of magnetic resonance, computer tomography, and contrast-enhanced ultrasound in evaluation of the ablated area. Radiol Med. 2016;121:122-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Tafuto S, von Arx C, De Divitiis C, Maura CT, Palaia R, Albino V, Fusco R, Membrini M, Petrillo A, Granata V. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int J Surg. 2015;21 Suppl 1:S78-S82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Granata V, Fusco R, Catalano O, Piccirillo M, De Bellis M, Izzo F, Petrillo A. Percutaneous ablation therapy of hepatocellular carcinoma with irreversible electroporation: MRI findings. AJR Am J Roentgenol. 2015;204:1000-1007. [PubMed] [Cited in This Article: ] |
| 23. | Granata V, Fusco R, Catalano O, Filice S, Amato DM, Nasti G, Avallone A, Izzo F, Petrillo A. Early Assessment of Colorectal Cancer Patients with Liver Metastases Treated with Antiangiogenic Drugs: The Role of Intravoxel Incoherent Motion in Diffusion-Weighted Imaging. PLoS One. 2015;10:e0142876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | García-Figueiras R, Padhani AR, Baleato-González S. Therapy Monitoring with Functional and Molecular MR Imaging. Magn Reson Imaging Clin N Am. 2016;24:261-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Weng Z, Ertle J, Zheng S, Lauenstein T, Mueller S, Bockisch A, Gerken G, Yang D, Schlaak JF. Choi criteria are superior in evaluating tumor response in patients treated with transarterial radioembolization for hepatocellular carcinoma. Oncol Lett. 2013;6:1707-1712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S-150S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2417] [Cited by in F6Publishing: 2598] [Article Influence: 173.2] [Reference Citation Analysis (0)] |
| 27. | He J, Page AJ, Weiss M, Wolfgang CL, Herman JM, Pawlik TM. Management of borderline and locally advanced pancreatic cancer: where do we stand? World J Gastroenterol. 2014;20:2255-2266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, Billard V, Geertsen PF, Larkin JO, Miklavcic D. Electrochemotherapy - An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer Suppl. 2006;4:3-13. [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 29. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15860] [Cited by in F6Publishing: 18991] [Article Influence: 1266.1] [Reference Citation Analysis (1)] |
| 30. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [PubMed] [Cited in This Article: ] |
| 31. | Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401-407. [PubMed] [Cited in This Article: ] |
| 32. | Fusco R, Petrillo A, Petrillo M, Sansone M. Use of Tracer Kinetic Models for Selection of Semi-Quantitative Features for DCEMRI. Appl Magn Reson. 2013;44:1311-1324. [DOI] [Cited in This Article: ] |
| 33. | Fusco R, Sansone M, Petrillo A. The Use of the Levenberg-Marquardt and Variable Projection Curve-Fitting Algorithm in Intravoxel Incoherent Motion Method for DW-MRI Data Analysis. Appl Magn Reson. 2015;46:551-558. [DOI] [Cited in This Article: ] |
| 34. | Avallone A, Aloj L, Caracò C, Delrio P, Pecori B, Tatangelo F, Scott N, Casaretti R, Di Gennaro F, Montano M. Early FDG PET response assessment of preoperative radiochemotherapy in locally advanced rectal cancer: correlation with long-term outcome. Eur J Nucl Med Mol Imaging. 2012;39:1848-1857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2583] [Cited by in F6Publishing: 2903] [Article Influence: 207.4] [Reference Citation Analysis (36)] |
| 36. | Choi H. Response evaluation of gastrointestinal stromal tumors. Oncologist. 2008;13 Suppl 2:4-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K. Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics. 2013;33:1323-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 38. | van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Haanen JB, Boven E. Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer. 2010;102:803-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 39. | Stacchiotti S, Collini P, Messina A, Morosi C, Barisella M, Bertulli R, Piovesan C, Dileo P, Torri V, Gronchi A. High-grade soft-tissue sarcomas: tumor response assessment--pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009;251:447-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 40. | Ding Q, Cheng X, Yang L, Zhang Q, Chen J, Li T, Shi H. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST). J Thorac Dis. 2014;6:677-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 28] [Reference Citation Analysis (0)] |
| 41. | Hjouj M, Last D, Guez D, Daniels D, Sharabi S, Lavee J, Rubinsky B, Mardor Y. MRI study on reversible and irreversible electroporation induced blood brain barrier disruption. PLoS One. 2012;7:e42817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Sakane M, Tatsumi M, Kim T, Hori M, Onishi H, Nakamoto A, Eguchi H, Nagano H, Wakasa K, Hatazawa J. Correlation between apparent diffusion coefficients on diffusion-weighted MRI and standardized uptake value on FDG-PET/CT in pancreatic adenocarcinoma. Acta Radiol. 2015;56:1034-1041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |