Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5422
Peer-review started: February 4, 2017
First decision: March 16, 2017
Revised: March 31, 2017
Accepted: June 19, 2017
Article in press: June 19, 2017
Published online: August 7, 2017
To investigated the hemostatic ability of the S and F1-10 methods in clinical and ex vivo studies.
The hemostatic abilities of the two methods were analyzed retrospectively in all six gastric endoscopic submucosal dissection cases. The treated vessel diameter, compressed vessel frequency, and bleeding frequency after cutting the vessels were noted by the recorded videos. The coagulation mechanism of the two power settings was evaluated using the data recording program and histological examination on macro- and microscopic levels in the ex vivo experiments using porcine tissues.
F1-10 method showed a significantly better hemostatic ability for vessels ≥ 2 mm in diameter and a trend of overall better coagulation effect, evaluated by the bleeding rate after cutting the vessels. F1-10 method could sustain electrical current longer and effectively coagulate the tissue wider and deeper than the S method in the porcine model.
F1-10 method is suggested to achieve a stronger hemostatic effect than the S method in clinical procedures and ex vivo models.
Core tip: The prevention of bleeding during endoscopic submucosal dissection (ESD) is one of the most important factors in safe and successful tumor removal. We investigated the difference in bleeding rates between S method and F1-10 method in stomach ESD. The investigation suggests that F1-10 method can achieve a stronger hemostatic effect than S method for large vessels. In addition, we investigated the difference of the hemostatic strength and mechanism using ex vivo model. F1-10 method could sustain electrical current longer and effectively coagulate wider and deeper areas of tissue than S method, resulting in a strong hemostatic effect.
- Citation: Ishida T, Toyonaga T, Ohara Y, Nakashige T, Kitamura Y, Ariyoshi R, Takihara H, Baba S, Yoshizaki T, Kawara F, Tanaka S, Morita Y, Umegaki E, Hoshi N, Azuma T. Efficacy of forced coagulation with low high-frequency power setting during endoscopic submucosal dissection. World J Gastroenterol 2017; 23(29): 5422-5430
- URL: https://www.wjgnet.com/1007-9327/full/v23/i29/5422.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i29.5422
Electrosurgery is used in many fields of medicine and is one of the most commonly used energy system in surgery. Basic understanding of electricity is required for the safe use of electrosurgical technology for patient care[1-6]. In the endoscopic field of gastroenterology, endoscopic mucosal resection and endoscopic submucosal dissection (ESD) are popular therapeutic strategies to resect benign and early cancerous lesions that require the use of an electrosurgical generator[7]. Preventing bleeding during operation, especially in ESD, is one of the most important factors to accomplish safe and successful tumor removal[8].
The modern electrosurgery has various coagulation modes. The forced coagulation utilizes a high output voltage > 200 Vp with an interrupted waveform. The forced coagulation creates sparks, which coagulate on superficial tissues. At the same time, a high output voltage coagulation mode, such as the forced coagulation, generally has a cutting ability[9]. In ESD, it can create appropriate coagulation for small vessels, such as the capillaries; however, its effect for non-small vessels larger than the capillaries is insufficient, because the non-small vessels are cut before being dehydrated adequately and have a risk of bleeding. Therefore, the forced coagulation mode is thought to be unsuitable for precoagulation of large vessels.
On the other hand, the peak voltage between the active electrode and the target tissue in the soft coagulation mode is limited to < 200 Vp. This mode does not cause any sparks, only uses Joule’s heat, and avoids rupturing the vessels[10,11].
We previously reported the effectiveness of vessel-sealing with endoknife compression using the soft coagulation mode (S method) in ESD[12]. This method is useful for coagulation without bleeding before cutting relatively large vessels, such as those penetrating between the muscularis propria. However, an important limitation of this mode is that the current cannot flow to the tissue when its resistance becomes too high because of the limited voltage below 200 Vp. Therefore, it has a risk of inadequate coagulation when the vessels are too large and the tissue resistance becomes too high while processing. Owing to this, hemostatic forceps were generally used to precoagulate large vessels. Therefore, we required a more effective setting for large vessels. The forced coagulation mode is usually not used for precoagulation; however, we found when the HF (high-frequency) power is reduced to a quite low level, it can avoid generating sparks. It was suggested that the forced coagulation mode with a low HF power setting be used for precoagulation to process larger vessels. Based on these, vessel-sealing with endoknife compression using the forced coagulation mode with low HF power setting (Effect 1, 10 W: F1-10 method) was also used in our facility (The peak voltage in the forced coagulation is adjusted from Effect 1 to 4; Effect 1 is the lowest peak voltage setting).
Accordingly, this setting hardly causes vessel rupture in spite of using the forced coagulation mode. Therefore, we retrospectively analyzed the effectiveness of bleeding control by the S and F1-10 methods to find the optimum power setting. Moreover, the mechanism of coagulation was analyzed by ex vivo experiments using porcine tissues to further evaluate the coagulation ability of these two settings.
Between April and December 2014, a single expert endoscopist (Toyonaga T) with an experience of > 4000 cases of ESD performed ESD to treat 36 gastric lesions at Kobe University Hospital and Kishiwada Tokushukai Hospital. Based on the clinical records and recorded videos of the ESD sessions, 22 cases were treated with only the S method and 14 cases with only the F1-10 method. Of these, the patients and lesions that met the following conditions, which can influence the bleeding tendency of the patients, and those lesions typically require no precoagulation, were excluded from this study: (1) patients under medication with any antiplatelet or anticoagulation effect; (2) patients undergoing dialysis; (3) lesions in the antrum; (4) lesions with ulcer; and (5) resected specimen < 30 mm. As a result, six cases each with the S and F1-10 methods were included in the study.
Informed consent for the procedure was obtained from all patients; this retrospective study was approved by both the ethics committees of Kobe University Hospital and Kishiwada Tokushukai Hospital.
ESD was carried out with a FlushKnife-BT 2.5 mm (DK-2618JB; Fujifilm, Tokyo, Japan) through a conventional single-channel endoscope (GIF-Q240 Olympus, Tokyo, Japan) with a transparent hood (Top Co., Ltd. Tokyo, Japan) at the tip of the endoscope. VIO 300D (ERBE Elektromedizin, Tübingen, DEU) was used as the electrical surgical generator.
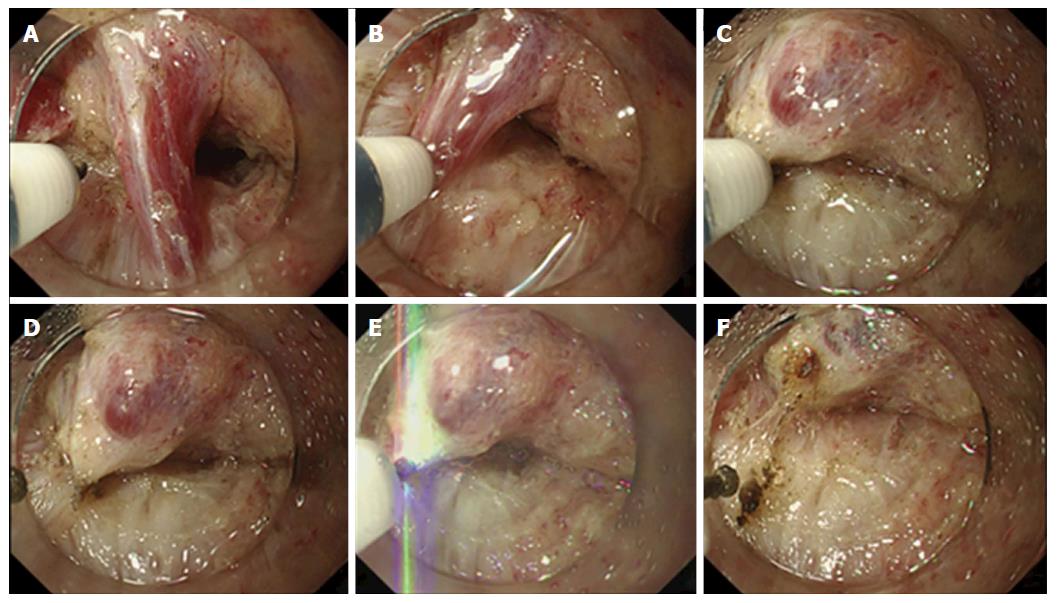
Vessel-sealing with endoknife compression was carried out as follows: (1) exposure and isolation of the target vessel in the submucosal layer; (2) compression of the vessel with the FlushKnife-BT and coagulating it with the S method (Effect 7, 100 W) or F1-10 method (Effect 1, 10 W) until the vessel turned white, which is a sign of coagulation completion; and (3) cutting of the vessel using the forced coagulation mode (Effect 3, 50 W) (Figure 1A-F)[12]. This technique was initially applied to process the vessel; however, when sufficient coagulation was not achieved, hemostatic forceps were used. Thereafter, when the same or larger-sized vessel was found in the same patient, hemostatic forceps were sometimes used without the trial of vessel-sealing because successful sealing was not expected.
A total of 12 recorded ESD sessions, including six each of the S and F1-10 method, were viewed by two endoscopists (Ishida T and Ohara Y). The treatment time was counted from the beginning of the local injection to the end of the treatment. The diameter of the processed vessels, frequency of the compressed vessel by the FlushKnife-BT, and time to complete the coagulation were evaluated in a blind manner. The diameter of the vessels was calculated by comparing it with the ball tip (0.9 mm) or the shaft (0.5 mm) of the FlushKnife, sheath (2.7 mm), and length between the sheath and the ball tip (2.5 mm)[13]. Bleeding after procession was defined when it required the use of hemostatic forceps or more than thrice the additional hemostatic procedure using the FlushKnife-BT. The bleeding rates after endoknife precoagulation were compared between the S and F1-10 methods.
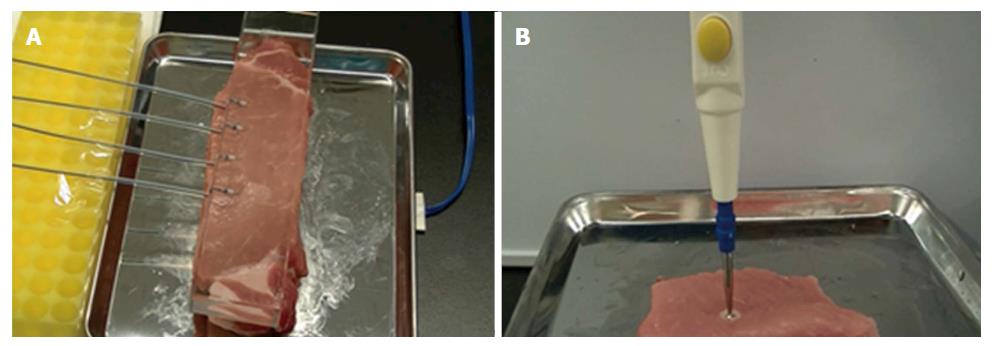
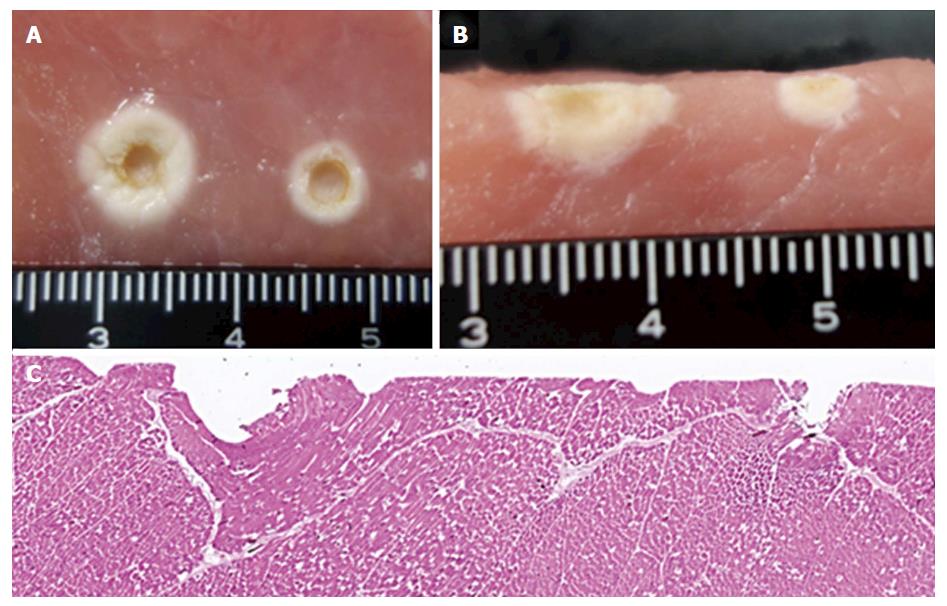
A 2.5-cm thick fresh pork block with a return electrode underneath was prepared in the metal vat. The unused FlushKnife-BT 2.5 mm was placed on the block and lightly pressed by the transparent glass to apply even pressure to each tip (Figure 2A). The VIO 300D was used as the electrosurgical generator, and electrical current was applied using the two different power settings, the S method (Effect 7, 100 W) and F1-10 method (Effect 1, 10 W), five times each with an unused FlushKnife-BT every time. The peak voltage (Vp), current elapsed time (s), and electric energy (W) were measured with the lapse of time by a data recording program (VIO DOKU, ERBE Elektromedizin, Tübingen, DEU). The current elapsed time was defined as the time from the start of the electric current until it went off. The 3-mm ball tip electrodes were used to investigate the depth of coagulation (Figure 2B) because the tip of the FlushKnife-BT was too small to evaluate the deference of the coagulated area. Paraffin-embedded sections were made, and hematoxylin-eosin (H and E) staining was performed to microscopically confirm the tissue denaturation in the examination using the FlushKnife-BT.
Categorical variables were compared using the two-sided Fisher's exact test and χ2 test. Continuous variables were compared using the Wilcoxon's rank-sum test. Statistical significances were defined as P < 0.05. All statistical analyses were performed using the JMP® 11 (SAS Institute Inc., Cary, NC, United States).
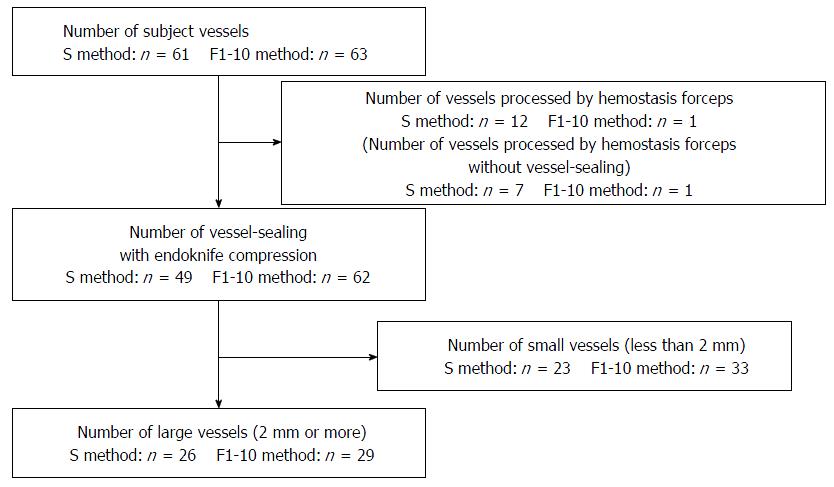
Patient and treated lesion characteristics are shown in Table 1. No significant difference was found between the S and F1-10 method groups. The total number of vessels that required coagulation was 61 and 63 in the S and F1-10 method groups, respectively. Notably, 12 vessels in the S method and one in the F1-10 method were treated by hemostatic forceps, without any trial of the endoknife precoagulation. Among these, seven vessels in the S method and one in the F1-10 method were treated with forceps because the vessels were larger than the vessel with failed precoagulation in the same patient. The rest of the 49 and 61 vessels in the S and F1-10 methods, respectively, processed by endoknife precoagulation were compared for bleeding control efficacy of the electrical output settings (Figure 3). The details of the processed vessels are shown in Table 2. The median vessel diameter was 2 mm in the S method and 1.5 mm in the F1-10 method. The median frequency of the compressed vessel was twice in both methods, and the median coagulation time was 9 s and 10 s in the S and F1-10 methods, respectively. No significant difference was found between the S and F1-10 methods. The bleeding rate after vessel processing was 18.4% (8/49) and 4.8% (3/62) in the S and F1-10 methods, respectively (P = 0.058, OR = 3.84, 95%CI: 0.96-15.34). No statistically significant differences were found; however, the F1-10 method strongly showed a trend in preventing bleeding after precoagulation compared with the S method (P = 0.058). We further investigated the bleeding rates of the large vessels, defined as ≥ 2 mm in diameter. There were 26 and 29 large vessels in the S and F1-10 methods, respectively. The bleeding rates of the large vessels were 26.9% (7/26) and 3.4% (1/29) in the S and F1-10 methods, respectively, which were significantly different (P = 0.021, OR = 10.32, 95%CI: 1.17-90.78) (Table 3).
| S method (n = 6) | F1-10 method (n = 6) | P3 value | |
| Age, median (yr) (range) | 70 (64-87) | 71 (53-79) | 0.81 |
| Sex (male/female) | 5:1 | 4:2 | 0.59 |
| Lesion location, U/M/L1 | 3/3/0 | 4/2/0 | 0.64 |
| Histology, well/mod/poor | 6/0/0 | 6/0/0 | 1.00 |
| Tumor size, median (mm) (range) | 13.5 (8-36) | 16 (6-23) | 0.60 |
| Resected size, median (mm) (range) | 50.5 (33-64) | 44.5 (31-56) | 0.63 |
| Depth of tumor invasion, M/SM1/SM22 | 4/2/0 | 3/1/2 | 0.42 |
| Median time of treatment (min) | 65 | 52.5 | 0.69 |
| Postoperative bleeding rate | 0 | 0 | 1.00 |
| En bloc resection rate | 100% | 100% | 1.00 |
| S method | F1-10 method | P1 value | |
| Number of vessels processed by vessel-sealing | 49 | 62 | |
| with endoknife compression | |||
| Median vessel diameter (mm) (range) | 2 (1-3) | 1.5 (1-3) | 0.68 |
| Median frequency of compressed vessel (times) (range) | 2 (1-8) | 2 (1-5) | 0.11 |
| Median time of coagulation (s) (range) | 9 (2-48) | 10 (1-58) | 0.11 |
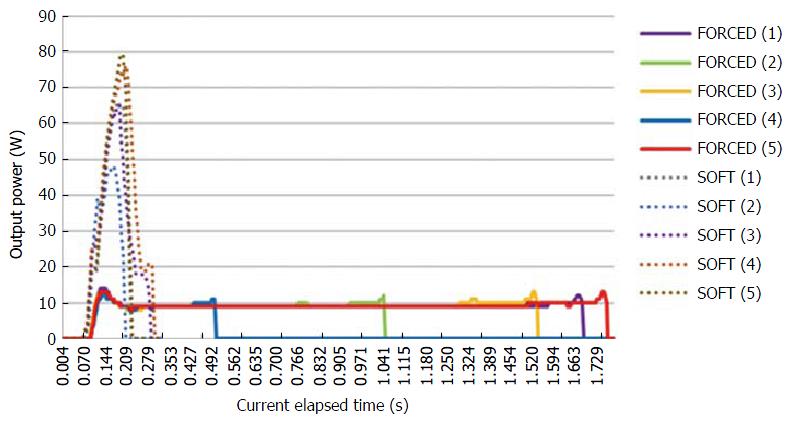
The peak voltage, current elapsed time, and electric energy were measured by the VIO DOKU using the FlushKnife-BT in a porcine block. The median peak voltage was 172 Vp and 560 Vp in the S and F1-10 methods, respectively. The time-dependent output power is shown in Figure 4. The median current elapsed time was 0.22 s and 1.54 s in the S and F1-10 methods, respectively; the F1-10 method could keep the electric current longer than the S method. The mean total electrical energy was 6.34 W and 12.5 W in the S and F1-10 methods, respectively (Table 4).
| FlushKnife-BT 2.5 mm | ||
| S method | F1-10 method | |
| Median peak voltage (Vp) | 172 (170-173) | 560 (538-587) |
| Median current elapse time (s) | 0.22 (0.20-0.30) | 1.54 (0.50-1.77) |
| Median total electric energy (W) | 6.34 (3.9-8.67) | 12.5 (3.47-13.8) |
The width and depth of coagulation using the 3-mm ball tip electrode were wider (8 mm vs 5 mm) and deeper (7 mm vs 3 mm) in the F1-10 method compared with the S method in the macroscopic views (Figure 5A and B). The H&E-stained section of the porcine block coagulated with the FlushKnife-BT showed that coagulation with the F1-10 method spread wider and deeper to the surrounding tissues compared to that with the S method (Figure 5C).
ESD has enabled an endoscopic en bloc resection of large tumors in the esophagus, stomach, and colorectum. However, intraoperative bleeding remains one of the major complications during ESD, although bleeding improved to some degree by the development of devices, such as endoknives, hemostatic forceps, and electrical surgical units[14]. In actual practice, it sometimes takes a lot of time to stop bleeding in ESD; once the lesion is bleeding, it is difficult to recognize the submucosal layers as these are blood-stained, making the procedure more difficult to perform[15]. The prevention of intraoperative bleeding is important to achieve a safe ESD. The FlushKnife-BT, which has a ball tip on top of the needle, improved the hemostasis effect compared with the conventional needle-type FlushKnife because its round ball tip compressed the vessels widely and decreased the current density[13]. However, the FlushKnife-BT still pose a bleeding risk as the vessels are cut before adequate coagulation.
It was suggested that the S method is useful for precoagulation. Soft coagulation regulates the peak voltage below 200 Vp, and it can dehydrate the vessels without any spark or rupture. Therefore, this mode was thought to be appropriate for this technique. However, its efficacy was insufficient, especially for large vessels in the upper and middle thirds of the stomach[16]. We introduced this technique using the F1-10 setting as aforementioned by modifying the peak voltage and power setting, and the usefulness of the S and F1-10 methods were compared in this study.
In the clinical study, the bleeding rates after vessel processing for large vessels were 26.9% and 3.4% in the S and F1-10 methods, respectively, which were significantly different. The median current elapsed time was longer, and the width and depth of coagulation were wider and deeper in the F1-10 method than in the S method in the ex vivo study.
Forced coagulation mode usually generates the sparks and fulguration accompanying the cutting function added to the coagulation. It was known that this mode was unsuitable for the endoknife precoagulation. However, when the peak voltage and electrical power in the forced coagulation were decreased to the level of the F1-10 setting, the current elapsed time became much longer than that of the S method and sparks were not observed in both ESD and experiment with the porcine tissue.
While the electrode heat dehydrates the tissues and increases tissue resistance, the electric current gradually decreased and turned off. This mechanism can be explained by Ohm's law, in which voltage (U) = resistance (R) × electric current (I). Forced coagulation creates a higher voltage than that of soft coagulation and could keep the current flow much longer even after the tissue resistance increased, as shown in the experiment. It can imply that under the generation of Joule's heat without sparks, the coagulation range is decided by the current elapsed time. For this mechanism, the F1-10 method achieved a wider and deeper coagulation area than the S method.
Our study showed the usefulness of the F1-10 method from the point of superior coagulation ability during ESD. On the other hand, this method may have the risk of perforation due to deep coagulation. However, we did not experience intraoperative perforation using the F1-10 method even during ESD in the esophagus and colorectum, which have thinner walls than the stomach. Further, no obvious increase in the postoperative perforation rate has been observed since the induction of the F1-10 method in our facility until the present. Such finding may be explained by the vessel-sealing with endoknife compression technique, which applies electrical energy after compressing and pulling the vessel apart from the muscle layer. Its concept is essentially the same as that of coagulation using hemostatic forceps. However, it must be emphasized that too much coagulation on the muscularis propria should be avoided to prevent deep coagulation. This caution is similar as with the hemostatic forceps should be used[17].
Recently, one research reported that the bleeding rate after endoknife precoagulation was not significantly different between the use of the F1-10 method and the hemostatic forceps with the soft coagulation during gastric ESD; further, the use of F1-10 did not result in serious intraoperative bleeding and reduced the frequency of using hemostatic forceps[18]. The F1-10 method was demonstrated to be as safe and effective for bleeding prevention as hemostatic forceps.
However, there were some limitations in our study. First, the vessels processed by hemostatic forceps were excluded from the analysis (12 in the S method and one in the F1-10 method); it has a potential to be a selection bias, however, those included were only the vessels larger than the ones for which precoagulation was not accomplished. Therefore, even if endoknife precoagulation was attempted, it was highly expected that it would fail. Although there is no research that compares the precoagulation effectiveness between the S-method and hemostatic forceps method yet, it is empirically known that the coagulation ability of hemostatic forceps is stronger than the S method; further, precoagulation using hemostatic forceps can reduce the bleeding risk[19,20]. If there was a selection bias, it would be for the advantage of the S method in this study. However, the result still suggested that the F1-10 method was superior to the S method. Second, the clinical data shown are achieved under the use of the FlushKnife-BT, which has a ball tip and is capable of firm vessel compression. This FlushKnife-BT method should not be simply applied when precoagulation with different types of ESD knives is performed. The ideal generator setting should be investigated for each bleeding control strategy and for different types of devices. Further, only a skilled endoscopist performed the ESD in the present study. The generalizability of the result in this study remains uncertain; however, we believe that if proper precoagulation procedure is conducted, similar results could be obtained.
In conclusion, the vessel-sealing with endoknife compression technique using the F1-10 method is better than that using the S method, especially for large vessels. Thus, the F1-10 method is considered to promote a safer and better quality ESD.
We would like to thank Masaki Yamazaki from AMCO Incorporated (Tokyo, Japan) for the review of terminology matters, and AMCO Incorporated (Tokyo, Japan) for supporting the VIO DOKU.
Bleeding control is one of the most important factors to successful and safe endoscopic submucosal dissection (ESD). The authors reported that the endoscopic precoagulation technique using the soft coagulation mode (S-method) is effective. However, the S method is insufficient for large vessels, and they usually have used hemostatic forceps for preventing bleeding. They found that the forced coagulation mode with low high-frequency power setting (F1-10 method) can exhibit a precoagulation function. F1-10 method is suggested to be useful for large vessel.
This studies first reported that the usefulness of the forced coagulation mode with low high-frequency power setting for the endoscopic vessel-sealing. This mode setting suggested a strong coagulation effect without rupturing the vessels compared with the conventional soft coagulation method in clinical procedure and ex vivo model.
The use of F1-10 method can lead to not only the prevention of bleeding but also cost savings and shortening ESD procedure.
This study suggested that F1-10 method achieve a stronger hemostatic effect than the S method in clinical procedures and ex vivo models.
The soft coagulation mode and forced coagulation mode are functions of ERBE VIO system. The Soft coagulation regulates the peak voltage below 200 Vp. The forced coagulation utilizes a high output voltage > 200 Vp with an interrupted waveform, and usually generates the sparks and fulguration accompanying the cutting function added to the coagulation. However, the High-frequency power is reduced to a quite low level, it can avoid generating sparks. Therefore, the authors suggested the usefulness of the forced coagulation mode with low high-frequency power setting in clinical procedures and ex vivo models.
This is a paper on the timely topic, comparing S method and F1-10 method. Authors indicated that F1-10 method is suggested to be useful for large vessel.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Xu MD S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Wu MP, Ou CS, Chen SL, Yen EY, Rowbotham R. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73. [PubMed] [Cited in This Article: ] |
| 2. | Alkatout I, Schollmeyer T, Hawaldar NA, Sharma N, Mettler L. Principles and safety measures of electrosurgery in laparoscopy. JSLS. 2012;16:130-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Odell RC. Surgical complications specific to monopolar electrosurgical energy: engineering changes that have made electrosurgery safer. J Minim Invasive Gynecol. 2013;20:288-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Sutton C, Abbott J. History of power sources in endoscopic surgery. J Minim Invasive Gynecol. 2013;20:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Feldman LS, Fuchshuber PR, Jones DB. The SAGES manual on the fundamental use of surgical energy (FUSE). Springer, New York. 2012;. [Cited in This Article: ] |
| 6. | Taheri A, Mansoori P, Sandoval LF, Feldman SR, Pearce D, Williford PM. Electrosurgery: part I. Basics and principles. J Am Acad Dermatol. 2014;70:591.e1-e14; quiz 605-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Morris ML, Tucker RD, Baron TH, Song LM. Electrosurgery in gastrointestinal endoscopy: principles to practice. Am J Gastroenterol. 2009;104:1563-1574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] [Cited in This Article: ] |
| 9. | Reidenbach HD, Buess G. Ancillary technology: electrocautery, thermocoagulation and laser. Springer Berlin Heidelberg, Berlin, 1992.. [Cited in This Article: ] |
| 10. | Sakuragi T, Okazaki Y, Mitsuoka M, Itoh T. Dramatic hemostasis of the transected pulmonary artery model using SOFT COAG electrosurgical output. Interact Cardiovasc Thorac Surg. 2008;7:764-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Sakuragi T, Ohma H, Ohteki H. Efficacy of SOFT COAG for intraoperative bleeding in thoracic surgery. Interact Cardiovasc Thorac Surg. 2009;9:767-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Tanaka S, Toyonaga T, Morita Y. Endoscopic vessel sealing: a novel endoscopic precoagulation technique for blood vessels during endoscopic submucosal dissection. Dig Endosc. 2013;25:341-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Toyonaga T, Man-I M, Fujita T, Nishino E, Ono W, Morita Y, Sanuki T, Masuda A, Yoshida M, Kutsumi H. The performance of a novel ball-tipped Flush knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther. 2010;32:908-915. [PubMed] [Cited in This Article: ] |
| 14. | ASGE Technology Committee. Maple JT, Abu Dayyeh BK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Konda V, Murad FM, Siddiqui UD, Banerjee S. Endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:1311-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 15. | Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Toyonaga T, Nishino E, Hirooka T, Ueda C, Noda K. Intraoperative bleeding in endoscopic submucosal dissection in the stomach and strategy for prevention and treatment. Dig Endosc. 2006;18; S123-127. [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Mann NS, Mann SK, Alam I. The safety of hot biopsy forceps in the removal of small colonic polyps. Digestion. 1999;60:74-76. [PubMed] [Cited in This Article: ] |
| 18. | Horikawa Y, Toyonaga T, Mizutamari H, Mimori N, Kato Y, Fushimi S, Okubo S. Feasibility of Knife-Coagulated Cut in Gastric Endoscopic Submucosal Dissection: A Case-Control Study. Digestion. 2016;94:192-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Toyonaga T, Nishino E, Man-I M, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc. 2012;45:362-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Park CH, Lee SK. Preventing and controlling bleeding in gastric endoscopic submucosal dissection. Clin Endosc. 2013;46:456-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |