Published online Aug 14, 2017. doi: 10.3748/wjg.v23.i30.5610
Peer-review started: April 15, 2017
First decision: June 1, 2017
Revised: June 12, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: August 14, 2017
To evaluate the diagnostic value and safety mainly regarding incidents of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for pancreatic cystic lesions (PCLs).
A total of 150 consecutive patients with suspected PCLs were prospectively enrolled from April 2015 to November 2016. We finally enrolled 140 patients undergoing EUS-FNA. We compared the diagnostic accuracy of EUS-FNA and pathological diagnosis, which is regarded as the gold standard, for PCLs. Patients undergoing EUS-FNA at least 1 wk preoperatively were monitored for incidents and adverse events to evaluate its safety.
There were 88 (62.9%) women and 52 (37.1%) men among 140 patients, with a mean age of 50.1 (± 15.4) years. There were 67 cysts located in the head/uncinate of the pancreas and 67 in the body/tail, and 6 patients had at least 1 cyst in the pancreas. There were 75 patients undergoing surgery and 55 undergoing EUS-FNA with interval at least 1 wk before other operations, with 3 patients undergoing the procedure twice. The accuracy of EUS-FNA in differentiating benign and malignant lesions was 97.3% (73/75), while the accuracy of characterizing PCL subtype was 84.0% (63/75). The incident rate was 37.9% (22/58), whereas only 1 AE was observed in 58 cases.
EUS-FNA is effective and safe for diagnosis of PCLs, however procedure-related incidents are common. Caution should be taken in patients undergoing EUS-FNA.
Core tip: Incidents are self-limiting and do not change therapy. Adverse events (AEs) of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) have attracted attention, whereas incidents are almost ignored. Although incidents do not interfere with procedures and treatment, documenting them might improve procedural quality and prediction of AEs. Our study was designed to evaluate the diagnostic value and safety mainly regarding incidents of EUS-FNA. We found the accuracy of EUS-FNA in differentiating benign and malignant lesions and characterizing pancreatic cystic lesions subtype was high. The AE rate was low, however procedure-related incidents are common and should be paid attention to.
- Citation: Du C, Chai NL, Linghu EQ, Li HK, Sun YF, Xu W, Wang XD, Tang P, Yang J. Incidents and adverse events of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions. World J Gastroenterol 2017; 23(30): 5610-5618
- URL: https://www.wjgnet.com/1007-9327/full/v23/i30/5610.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i30.5610
Pancreatic cystic lesions (PCLs) are becoming increasingly prevalent, with increased diagnosis related to the wide use of abdominal cross-sectional imaging. The incidence of asymptomatic cysts ranges from 0.7% to 24.3%[1-5]. With a broad differential diagnosis, PCLs are mainly divided into benign non-neoplastic cysts and neoplastic cysts, some of which have malignant potential or are of low malignancy. The frequency of malignancy among mucinous cystic neoplasms (MCNs) and intraductal papilla mucinous neoplasms (IPMNs) which are subtypes of neoplastic cysts, ranges from 3.9% to 81%[6], while the 2-year survival rate of malignant PCLs is as low as 10%[7].
Correct diagnosis and accurate classification of PCLs are important for making treatment decision. Endoscopic ultrasound (EUS) has high spatial resolution, and EUS-guided fine-needle aspiration (EUS-FNA) contributes to diagnosis by providing cystic fluid examination, cytology and biopsy[8]. EUS-FNA is the predominant method for diagnosis of PCLs[9,10]. However, compared with computed tomography (CT) and magnetic resonance imaging (MRI), EUS-FNA is an invasive operation. It is the top priority to ensure the safety of EUS-FNA.
Incidents are unplanned events that have no influence on completion of an operation and postoperative treatment, and adverse events (AEs) are defined as events that prevent completion of or change to the planned procedure[11]. Incidents are self-limiting and do not change therapy. AEs have attracted attention, whereas incidents are almost ignored. Although incidents do not interfere with procedures and treatment, documenting them might improve procedural quality and predict AEs.
There have been many studies on the safety and diagnostic accuracy of EUS-FNA for PCLs, but there have been few studies regarding the incidents related to this procedure. Our study was designed to evaluate the diagnostic value and safety mainly regarding incidents of EUS-FNA.
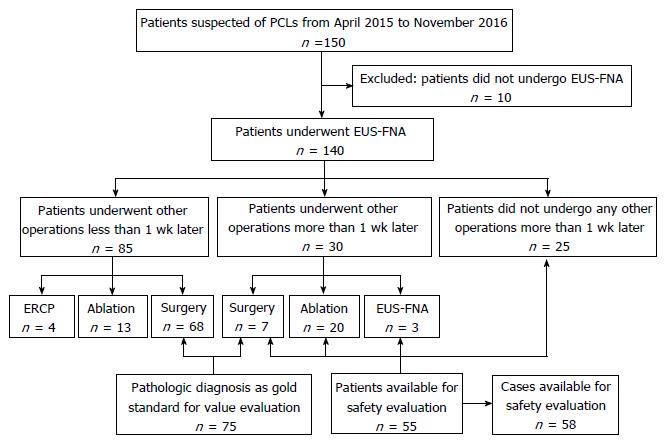
We prospectively enrolled 150 consecutive patients with suspected PCLs from April 2015 to November 2016. Excluding 10 patients who did not undergo EUS-FNA, we finally enrolled 140 patients. The indications for EUS-FNA were: (1) indeterminate PCLs in radiological imaging studies; (2) easier and safer access to the cyst; (3) age ≥ 18 years; and (4) signed informed consent. The following exclusion criteria were used: (1) reluctance to receive EUS-FNA or inability to sign informed consent independently; (2) high risk for operation, or pregnancy; (3) evidence of active acute pancreatitis, pancreatic necrosis or pseudocyst; and (4) coagulopathy (international normalized ratio > 1.5, platelets < 50000). When evaluating the diagnostic value, only the patients who underwent surgery were enrolled. When evaluating the safety of EUS-FNA, patients who did not undergo any other operation > 1 wk after EUS-FNA were studied.
Patients with suspected PCLs by imaging examination were requested to undergo EUS examination and EUS-FNA. The EUS and EUS-FNA procedures were performed by experts with > 10 years’ experience. Some patients underwent other operations, like surgery, EUS-guided ablation and endoscopic retrograde cholangiopancreatography, after the EUS-FNA. The presumed endoscopic diagnosis was made after taking EUS and cystic fluid examination findings into consideration. The diagnostic accuracy of EUS-FNA was compared with pathological diagnosis, which is regarded as the gold standard for diagnosis of PCL.
Patients undergoing EUS-FNA ≥ 1 wk before other operations were monitored for incidents and AEs to evaluate safety; therefore, patients who underwent other operations < 1 wk after EUS-FNA were excluded when evaluating the incident and AE rates. Any symptoms and signs of abdominal pain, fever, bleeding, nausea, infection, acute pancreatitis, perforation and hyperamylasemia, were recorded. Patients were monitored on the ward for ≥ 3 d and discharged when they did not feel any discomfort. If they were hospitalized for < 7 d, we followed them up by telephone to document incidents and AEs that might have arisen.
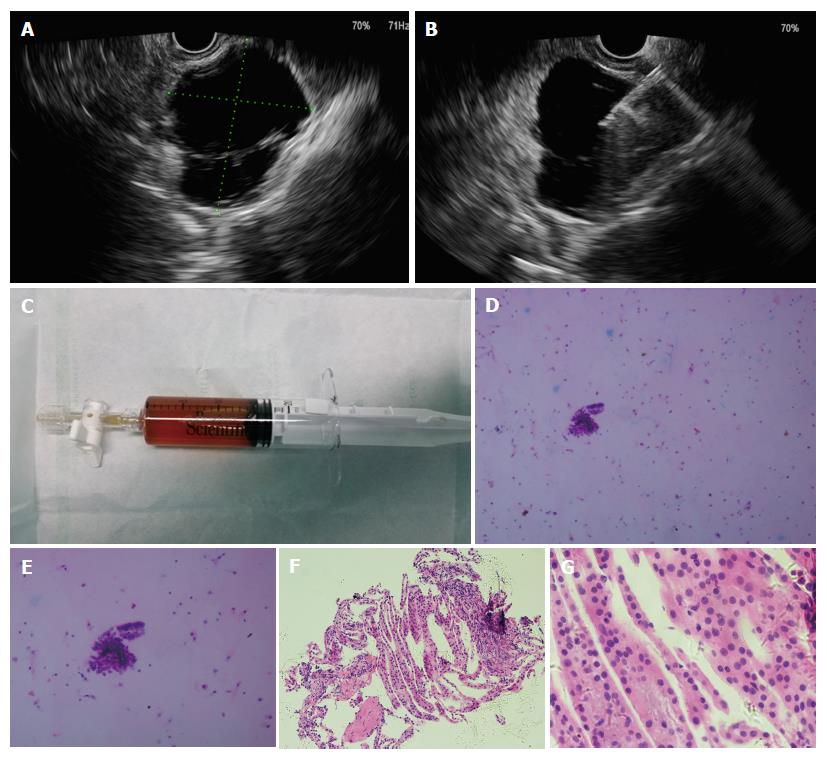
All patients with suspected PCLs underwent EUS evaluation with a liner-array echoendoscope (Prosound F75; Aloka, Tokyo, Japan, and GF-UCT260; Olympus, Tokyo, Japan) under intravenous anesthesia. The lesions were characterized by size, location, wall thickness, number of septa, morphology of the pancreatic duct, and presence of papilla or associated mass. Transgastric or transduodenal puncture of the cyst was done using a 22-gauge or 19-gauge needle (Echotip; Cook, Limerick, Ireland) and cystic fluid was aspirated. If the cystic fluid was too viscous for aspiration, 0.9% normal saline solution was used to decrease the viscosity of the cyst. The cyst fluid was sent for cytology and biochemical analysis. Biopsy of the cystic wall through a fine needle was done if necessary. The procedures are shown in Figure 1.
After EUS-FNA, patients were intravenously administered one dose of an intravenous antibiotic for 3 d and octreotide for 1 d. An intravenous proton pump inhibitor (PPI) for 1 d and an additional 3 d of an oral PPI were required. Six hours and the morning after the procedure, the patients were assessed for serum amylase and lipase levels. If these results were abnormal, rechecking was required once daily before they returned to normal. Oral intake of food was allowed 1 d after EUS-FNA if there was no severe AE.
Incidents were different from AEs. Incidents were defined as symptoms or signs that did not interfere with the planned treatment. AEs were defined as events that prevented completion of or change to the planned procedure. Moderate to severe abdominal pain that needed additional treatment was regarded as an AE, while mild abdominal pain was regarded as an incident. The size of PCLs was determined by their largest diameter. If EUS-FNA was performed on several cysts in one patient, the diameter was calculated as the sum of the largest diameters of these cysts. All of the patients were given a presumed diagnosis on the basis of EUS, cystic fluid analysis and cystic wall biopsy before surgery.
All calculations were performed using SPSS version 17.0. Quantitative data, including cystic size and patients’ age, were expressed by the mean or median and tested by t-test or nonparametric test. Enumeration data, like diagnostic accuracy rate and incident rate, were tested using χ2 or Fisher’s exact test. A P value < 0.05 was considered significant.
Basic characteristics are summarized in Table 1. There were 88 (62.9%) women and 52 (37.1%) men among 140 patients, with a mean age of 50.1 (± 15.4) years. There were 67 cysts located in the head/uncinate of the pancreas and 67 in the body/tail, and 6 patients had at least one cyst in the pancreas. Cystic fluid analysis was available for 89 patients and 1 patient had aspiration of two cysts. The levels of carcinoembryonic antigen, amylase, lipase and carbohydrate antigen 19-9 were 4.89 ng/mL (range: 0.20-19 636.5 ng/mL), 316.75 U/L (range: 1.2-275 020 U/L), 1713.60 U/L (range: 4.4-1 594 160 U/L) and 640.75 ng/mL (range: 1.07- > 20000 ng/mL), respectively. There were 75 patients undergoing surgery and 55 undergoing EUS-FNA with interval at least 1 wk before other operations, with 3 patients undergoing the procedure twice. Seventy pancreatic neoplastic cysts and five non-neoplastic cysts were found in pathological diagnosis. There were 25 MCNs, 27 serous cystic neoplasms, 7 solid pancreatic neoplasms, 8 IPMNs, 1 neuroendocrine neoplasm and 2 cystadenocarcinomas among the neoplastic cysts, and 2 pseudocysts, 2 true cysts and 1 case of cystic tuberculosis among the non-neoplastic cysts. There were 75 patients undergoing surgery after EUS-FNA and 58 were available for safety evaluation. The study flowchart is shown in Figure 2.
| Characteristic | Result1 |
| Age, yr | 50.1 ± 15.4 |
| Sex | |
| Female | 88 (62.9) |
| Male | 52 (37.1) |
| Cyst location | |
| Head/uncinate | 67 (47.9) |
| Body/tail | 67 (47.8) |
| Multiple cysts | 6 (4.3) |
| Pathological diagnosis | |
| Neoplastic cyst | 70 |
| MCN | 25 |
| SCN | 27 |
| SPN | 7 |
| IPMN | 8 |
| NEN | 1 |
| Cystadenocarcinoma | 2 |
| Non-neoplastic cyst | 5 |
| Pseudo cyst | 2 |
| True cyst | 2 |
| Cystic tuberculosis | 1 |
A total of 75 patients underwent surgery after EUS and pathological diagnosis was regarded as the gold standard. There were two malignant cysts by pathology and one was misdiagnosed by EUS-FNA. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of EUS-FNA in differentiating benign and malignant lesions were 98.6% (72/73), 50.0% (1/2), 98.6% (72/73), 50% (1/2) and 97.3% (73/75), respectively. When evaluating the capacity of characterizing subtype of PCLs, the accuracy of EUS-FNA was 84.0% (63/75).
Fifty-eight patients were available for safety evaluation and monitored for ≥ 7 d. Only 1 patient with moderate abdominal pain received additional treatment with anisodamine and the pain was relieved. No other AE occurred, which resulted in an AE rate of 1.7%.
Incidents were reported in 22 patients, with a rate of 37.9% (22/58). Seven patients developed abdominal pain; nine hyperamylasemia; four both abdominal pain and hyperamylasemia; one abdominal pain, low-grade fever and hyperamylasemia simultaneously; and one low-grade fever and hyperamylasemia simultaneously (Table 2).
| Incident | n |
| Abdominal pain | 7 |
| Hyperamylasemia | 9 |
| Abdominal pain + hyperamylasemia | 4 |
| Abdominal pain + low-grade fever + hyperamylasemia | 1 |
| Low-grade fever + hyperamylasemia | 1 |
The characteristics of the incidents/AEs and non-complaints groups are described in Table 3. We performed univariate analysis of the baseline patient and cystic characteristics to predict safety related to EUS-FNA. Among the variables, no significance was shown for age, sex, location and size of the lesions.
| Incidents/AEs group, n = 23 | Non-complaints group, n = 35 | P value | |
| Age, yr | 52.6 ± 19.0 | 52.6 ± 13.5 | > 0.05 (NS) |
| Sex | > 0.05 (NS) | ||
| Female | 12 | 18 | |
| Male | 11 | 17 | |
| Location | > 0.05 (NS) | ||
| Head/uncinate | 15 | 18 | |
| Body/tail | 5 | 15 | |
| Multiple cysts | 3 | 2 | |
| Size by EUS mm | 34.5 ± 20.1 | 41.2 ± 21.5 | > 0.05 (NS) |
PCLs are composed of true cysts, pseudocysts and cystic neoplasms. About 60% of PCLs are cystic tumors, followed by inflammation and trauma-related pseudocysts accounting for 30%[12]. PCLs have a wide range of lesions ranging from benign to malignant[13]. Although imaging modalities have made great advances, the accurate diagnosis of PCLs and differentiation of PCL subtypes remain challenging[13]. EUS and EUS-FNA contribute much to the diagnosis of PCLs because of their high resolution and the aid of cystic fluid and cytological analysis[14-18]. EUS-FNA can offer incremental diagnostic sensitivity with its ability to obtain cystic fluid and cytology from worrisome areas[19]. The American Gastroenterological Association Institute suggests that EUS-FNA should be used to examine PCLs with at least two high-risk features[20]. EUS-FNA might affect the management of 72% of incidental pancreatic cysts[21]. When referring to EUS-FNA, its diagnostic value and safety are the most important features for evaluating its feasibility.
Many studies have shown that the accuracy of EUS-FNA in diagnosis of PCLs ranged from 66.7% to 97%[22-25]. Under EUS-FNA, cystic fluid and cystic tissue can be collected for biochemical, cytological, genetic and pathological examination, which may help to diagnose and classify PCLs[26-28]. The diagnostic yield from combined EUS-FNA imaging is better than from EUS alone[29]. EUS-FNA contributes much to differentiation between benign and malignant PCLs[30] and between mucinous and non-mucinous cystic lesions[31,32]. Cytologic diagnosis with EUS-FNA is helpful to arrive at a more definitive diagnosis[5]. EUS with or without FNA is superior to CT and MRI in accurately classifying a cyst as neoplastic[33].
In our current study, EUS-FNA had a high sensitivity for differentiation of malignant cystic carcinoma from benign or malignant potential PCLs, but its specificity was only 50%. There were two cystic carcinomas diagnosed by EUS-FNA in our study and one was misdiagnosed. When EUS-FNA reveals malignance, we should accept its diagnosis with caution. Additional information, like age, clinical symptoms, history of present illness, blood test results and other imaging examinations, should be taken into account. However, pancreatic cancer has high malignancy with short survival time, we would rather misdiagnose than miss it. It is important to differentiate between mucinous and non-mucinous PCLs because their treatments are different.
In our research, EUS-FNA did well in classifying PCLs into different subtypes, with an accuracy of 84.0%. An earlier study suggested that diagnostic accuracy in distinguishing mucinous and non-mucinous PCLs increased up to 90% when taking cystic fluid tumor marker level, amylase level, mucin staining and cytology into consideration to make a presumed diagnosis[31]. Our result seemed lower, which may be because previous studies just made a distinction between mucinous and non-mucinous PCLs. The cystic wall puncture might increase the sensitivity of EUS-FNA[5]. A systematic review showed k-ras mutational analysis used as an individual screening test has a poor diagnostic accuracy and the combined test of cytology and k-ras benefited the diagnostic value[34].
When evaluating the safety of EUS-FNA, AEs have attracted a lot of attention, with AE rates ranging from 1.14% to 14%[35-39]. A large prospective multicenter study reported a complication rate of 6%[40]. In our study, the AE rate was 1.7%. A study enrolled 414 patients showed the AEs all occurred during the first day[41]. In accordance with a previous study, pancreatitis, infection, perforation, tumor seeding and clinically significant bleeding are the most common AEs of EUS-FNA[42]. The incidence of acute pancreatitis varies from 0% to 2.6% and bacteremia can be observed in ≤ 6% of EUS procedures and EUS-FNA[18,38,40,43,44]. The incidence of abdominal pain is 0%-3.6%, while fever is reported in 0%-4.1% of cases[41,43,45-47]. No protective effect was observed from periprocedural prophylactic antibiotic administration[48]. Debate remains about whether the complications of EUS-FNA for PCLs are more frequent than for pancreatic solid lesions[39,49].
The incident rate in our study was higher than in a previous study reporting three incidents among 73 patients with PCLs and 73 with solid lesions[39]. However, the AE rate in our study was lower compared with 5.5% (4/73) in PCLs of the previous study. There are several reasons for this difference. Although the definitions of incidents and AEs are both based on an American Society for Gastrointestinal Endoscopy (ASGE) workshop, the postoperative treatment may differ. The definitions are related to planned therapy so differences in therapy will affect the discrimination between AEs and incidents. There are no clear guidelines for post-EUS-FNA treatment. Serum amylase and lipase levels were detected only when patients complained of abdominal pain in the previous study. Hyperamylasemia alone was common in our study and not necessarily accompanied by abdominal pain. Therefore, the number of incidents might have been underestimated in the previous study. Although incidents have no effect on completion of the planned procedure, paying attention to them may help optimize our treatment. For example, hyperamylasemia was reported 6 h after EUS-FNA and amylase level returned to normal the morning after the procedure. Therefore, one dose of octreotide might be enough for most patients. Noticing incidents can help operators take more care before, during and after an operation. Incidents may predict AEs, and giving attention to incidents might decrease AEs.
To predict the incidents/AEs, we carried out univariate analysis to identify factors that might affect incidents/AEs. Incidents/AEs were similar in patients of different age and sex and with lesions of different location and size. A previous study also demonstrated that location cannot predict AEs[38]. Eloubeidi et al[50] reported that the type and size of the pancreatic lesion affected AEs. We speculated that factors predicting incidents and AEs were similar. However, factors that may predict incidents deserve further investigation.
Our study prospectively revealed incidents related to EUS-FNA that may help to reduce AEs. However, there were several limitations. First, although there were 140 patients enrolled, we evaluated the accuracy of EUS-FNA in 75 patients (group 1) and safety of EUS-FNA in 55 patients (group 2). They were different groups and 17 patients among enrolled patients were neither in group 1 nor in group 2. Second, although the ASGE workshop defines incidents and AEs, there is no clear guideline for post-EUS-FNA treatment. The discrimination of incidents and AEs may vary with planned treatment. The incidents in our study may be different when changes are made to postoperative treatment. The final limitation was our small number of participants. Nearly half of the EUS-FNA procedures were done followed by surgery immediately, which made the sample for safety evaluation small.
In conclusion, EUS-FNA is effective and safe for diagnosis of PCLs, and has a high diagnostic accuracy and low AE rate. However, incidents related to EUS-FNA are common. Caution should be taken in patients undergoing EUS-FNA to prevent incidents from evolving into AEs. Incidents are similar in patients of different ages and sex and with lesions of different location and size.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is the predominant method for diagnosis of pancreatic cystic lesions (PCLs). Compared with computed tomography and magnetic resonance imaging, EUS-FNA is an invasive operation. It is the top priority to ensure the safety of EUS-FNA.
There have been many studies on the safety and diagnostic accuracy of EUS-FNA for PCLs, but there have been few studies regarding the incidents related to this procedure. Their study was designed to evaluate the diagnostic value and safety mainly regarding incidents of EUS-FNA.
The current study noted the incidents related to EUS-FNA, which have often been ignored. EUS-FNA is safe with low incidence of adverse events (AEs). However, incidents related to EUS-FNA are common. This study also analyzed the factors that predict safety related to EUS-FNA.
Noticing incidents can help operators take more care before, during and after an operation. Incidents may predict AEs, and giving attention to incidents might decrease AEs.
Incidents were different from AEs. Incidents were defined as symptoms or signs that did not interfere with the planned treatment. AEs were defined as events that prevented completion of or change in the planned procedure.
This manuscript describes an interesting investigation about the incidents and AEs of EUS-FNA for PCLs. In this study, the authors evaluated the diagnostic value and safety mainly regarding incidents of EUS-FNA for PCLs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Casper S, Sunanda K S- Editor: Wang JL L- Editor: Filipodia E- Editor: Li D
| 1. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 617] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 2. | Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, Wilson SD, Pitt HA. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651-657; discussion 657-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 3. | Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M; American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796-804; quiz e14-e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 4. | Yoon WJ, Brugge WR. The safety of endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions. Endosc Ultrasound. 2015;4:289-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Martin AK, Zhou Z. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic cysts by combined cytopathology and cystic content analysis. World J Gastrointest Endosc. 2015;7:1157-1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Munigala S, Gelrud A, Agarwal B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastrointest Endosc. 2016;84:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152-161. [PubMed] [Cited in This Article: ] |
| 8. | Ge N, Sun S. Endoscopic ultrasound: An all in one technique vibrates virtually around the whole internal medical field. J Transl Intern Med. 2014;2:104-106. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Bhutani MS. Role of endoscopic ultrasound for pancreatic cystic lesions: Past, present, and future! Endosc Ultrasound. 2015;4:273-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Alkaade S, Chahla E, Levy M. Role of endoscopic ultrasound-guided fine-needle aspiration cytology, viscosity, and carcinoembryonic antigen in pancreatic cyst fluid. Endosc Ultrasound. 2015;4:299-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1238] [Cited by in F6Publishing: 1532] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 12. | Yoon WJ, Brugge WR. Endoscopic ultrasound and pancreatic cystic lesions-diagnostic and therapeutic applications. Endosc Ultrasound. 2012;1:75-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Lu X, Zhang S, Ma C, Peng C, Lv Y, Zou X. The diagnostic value of EUS in pancreatic cystic neoplasms compared with CT and MRI. Endosc Ultrasound. 2015;4:324-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Wright GP, Morrow JB, Shaheen M, Goslin BJ, Baatenburg L, Chung MH. Accuracy of endoscopic ultrasound in the evaluation of cystic pancreatic neoplasms: a community hospital experience. Pancreas. 2014;43:465-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Gilani SM, Tashjian R, Barawi M, Al-Khafaji B. Cytologic features of solid pseudopapillary neoplasms of the pancreas: a single institutional experience based on evaluation of diagnostic utility of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Pathologica. 2014;106:45-50. [PubMed] [Cited in This Article: ] |
| 17. | Lim LG, Lakhtakia S, Ang TL, Vu CK, Dy F, Chong VH, Khor CJ, Lim WC, Doshi BK, Varadarajulu S. Factors determining diagnostic yield of endoscopic ultrasound guided fine-needle aspiration for pancreatic cystic lesions: a multicentre Asian study. Dig Dis Sci. 2013;58:1751-1757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Buscail L, Faure P, Bournet B, Selves J, Escourrou J. Interventional endoscopic ultrasound in pancreatic diseases. Pancreatology. 2006;6:7-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 285] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Vege SS, Ziring B, Jain R, Moayyedi P; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-822; quize12-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 629] [Cited by in F6Publishing: 663] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 21. | Ardengh JC, Lopes CV, de Lima-Filho ER, Kemp R, Dos Santos JS. Impact of endoscopic ultrasound-guided fine-needle aspiration on incidental pancreatic cysts. A prospective study. Scand J Gastroenterol. 2014;49:114-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | de Jong K, Poley JW, van Hooft JE, Visser M, Bruno MJ, Fockens P. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: initial results from a prospective study. Endoscopy. 2011;43:585-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Oguz D, Öztaş E, Kalkan IH, Tayfur O, Cicek B, Aydog G, Kurt M, Beyazit Y, Etik D, Nadir I. Accuracy of endoscopic ultrasound-guided fine needle aspiration cytology on the differentiation of malignant and benign pancreatic cystic lesions: a single-center experience. J Dig Dis. 2013;14:132-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Frossard JL, Amouyal P, Amouyal G, Palazzo L, Amaris J, Soldan M, Giostra E, Spahr L, Hadengue A, Fabre M. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol. 2003;98:1516-1524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Okasha HH, Ashry M, Imam HM, Ezzat R, Naguib M, Farag AH, Gemeie EH, Khattab HM. Role of endoscopic ultrasound-guided fine needle aspiration and ultrasound-guided fine-needle aspiration in diagnosis of cystic pancreatic lesions. Endosc Ultrasound. 2015;4:132-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Frampton AE, Krell J, Prado MM, Gall TM, Abbassi-Ghadi N, Del Vecchio Blanco G, Funel N, Giovannetti E, Castellano L, Basyouny M. Prospective validation of microRNA signatures for detecting pancreatic malignant transformation in endoscopic-ultrasound guided fine-needle aspiration biopsies. Oncotarget. 2016;7:28556-28569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Lubezky N, Loewenstein S, Ben-Haim M, Brazowski E, Marmor S, Pasmanik-Chor M, Oron-Karni V, Rechavi G, Klausner JM, Lahat G. MicroRNA expression signatures in intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2013;153:663-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Caponi S, Funel N, Frampton AE, Mosca F, Santarpia L, Van der Velde AG, Jiao LR, De Lio N, Falcone A, Kazemier G. The good, the bad and the ugly: a tale of miR-101, miR-21 and miR-155 in pancreatic intraductal papillary mucinous neoplasms. Ann Oncol. 2013;24:734-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Mitra V, Nayar MK, Leeds JS, Wadehra V, Haugk B, Scott J, Charnley RM, Oppong KW. Diagnostic performance of endoscopic ultrasound (EUS)/endoscopic ultrasound--fine needle aspiration (EUS-FNA) cytology in solid and cystic pancreatic neuroendocrine tumours. J Gastrointestin Liver Dis. 2015;24:69-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | O’Toole D, Palazzo L, Hammel P, Ben Yaghlene L, Couvelard A, Felce-Dachez M, Fabre M, Dancour A, Aubert A, Sauvanet A. Macrocystic pancreatic cystadenoma: The role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointest Endosc. 2004;59:823-829. [PubMed] [Cited in This Article: ] |
| 31. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [PubMed] [Cited in This Article: ] |
| 32. | Bektas M, Krishna SG, Ross WA, Weston B, Katz MH, Fleming JB, Lee JH, Bhutani MS. Prevalence of extra-pancreatic cysts in patients with cystic pancreatic lesions detected by endoscopic ultrasound. Endosc Ultrasound. 2015;4:219-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Khashab MA, Kim K, Lennon AM, Shin EJ, Tignor AS, Amateau SK, Singh VK, Wolfgang CL, Hruban RH, Canto MI. Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas. 2013;42:717-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Gillis A, Cipollone I, Cousins G, Conlon K. Does EUS-FNA molecular analysis carry additional value when compared to cytology in the diagnosis of pancreatic cystic neoplasm? A systematic review. HPB (Oxford). 2015;17:377-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [PubMed] [Cited in This Article: ] |
| 36. | Barresi L, Tarantino I, Traina M, Granata A, Curcio G, Azzopardi N, Baccarini P, Liotta R, Fornelli A, Maimone A. Endoscopic ultrasound-guided fine needle aspiration and biopsy using a 22-gauge needle with side fenestration in pancreatic cystic lesions. Dig Liver Dis. 2014;46:45-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Carrara S, Arcidiacono PG, Mezzi G, Petrone MC, Boemo C, Testoni PA. Pancreatic endoscopic ultrasound-guided fine needle aspiration: complication rate and clinical course in a single centre. Dig Liver Dis. 2010;42:520-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | O’Toole D, Palazzo L, Arotçarena R, Dancour A, Aubert A, Hammel P, Amaris J, Ruszniewski P. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Rodríguez-D’Jesús A, Fernández-Esparrach G, Marra-Lopez C, Orive-Calzada A, Sendino O, Araujo IK, Rodríguez de Miguel C, Vázquez-Sequeiros E, Córdova H, Sánchez-Montes C. Adverse events of EUS-guided FNA of pancreatic cystic and solid lesions by using the lexicon proposed in an ASGE workshop: a prospective and comparative study. Gastrointest Endosc. 2016;83:780-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Tarantino I, Fabbri C, Di Mitri R, Pagano N, Barresi L, Mocciaro F, Maimone A, Curcio G, Repici A, Traina M. Complications of endoscopic ultrasound fine needle aspiration on pancreatic cystic lesions: final results from a large prospective multicenter study. Dig Liver Dis. 2014;46:41-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Al-Haddad M, Wallace MB, Woodward TA, Gross SA, Hodgens CM, Toton RD, Raimondo M. The safety of fine-needle aspiration guided by endoscopic ultrasound: a prospective study. Endoscopy. 2008;40:204-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Fujii LL, Levy MJ. Basic techniques in endoscopic ultrasound-guided fine needle aspiration for solid lesions: Adverse events and avoiding them. Endosc Ultrasound. 2014;3:35-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Lee LS, Saltzman JR, Bounds BC, Poneros JM, Brugge WR, Thompson CC. EUS-guided fine needle aspiration of pancreatic cysts: a retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3:231-236. [PubMed] [Cited in This Article: ] |
| 44. | Siddiqui AA, Shahid H, Shah A, Khurana T, Huntington W, Ghumman SS, Loren DE, Kowalski TE, Laique S, Hayat U. High risk of acute pancreatitis after endoscopic ultrasound-guided fine needle aspiration of side branch intraductal papillary mucinous neoplasms. Endosc Ultrasound. 2015;4:109-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Al-Haddad M, Gill KR, Raimondo M, Woodward TA, Krishna M, Crook JE, Skarvinko LN, Jamil LH, Hasan M, Wallace MB. Safety and efficacy of cytology brushings versus standard fine-needle aspiration in evaluating cystic pancreatic lesions: a controlled study. Endoscopy. 2010;42:127-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Hernandez LV, Mishra G, Forsmark C, Draganov PV, Petersen JM, Hochwald SN, Vogel SB, Bhutani MS. Role of endoscopic ultrasound (EUS) and EUS-guided fine needle aspiration in the diagnosis and treatment of cystic lesions of the pancreas. Pancreas. 2002;25:222-228. [PubMed] [Cited in This Article: ] |
| 47. | Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology. 2006;17:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: a retrospective, comparative analysis. Gastrointest Endosc. 2011;74:81-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 50. | Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc. 2006;63:622-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |