Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7139
Peer-review started: April 1, 2017
First decision: June 5, 2017
Revised: July 6, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: October 21, 2017
To report adalimumab (Ada) efficacy on articular-gastrointestinal disease and health-related quality of life (HRQoL) in patients with enteropathic spondyloarthritis (ES).
A cohort of 52 patients with ES was evaluated in the departments of gastroenterology and internal medicine. At baseline, all patients underwent assessment by an integrated gastro-rheumatologic evaluation of articular and gastrointestinal activity, as well patient reported outcomes (PROs) of the HRQoL questionnaires. After this integrated evaluation and following a specific working flowchart, the Ada anti-tumor necrosis factor (TNF)-inhibitor was assigned to a cohort of 30 patients and its clinical efficacy was evaluated at baseline and after 6-mo and 12-mo treatment by the following tests: (1) Ankylosing Spondylitis Disease Activity Score-C-Reactive Protein (ASDAS-CRP); Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI) and Bath Ankylosing Spondylitis Metrology Index (BASMI) for articular activity; (2) Inflammatory Bowel Disease Questionnaire (IBDQ), Crohn’s Disease Activity Index (CDAI) and partial Mayo (pMayo) score for gastrointestinal symptoms and activity; and (3) Health Assessment Questionnaire (HAQ), Patient Global Assessment (PGA) and Short Form-36 health survey (SF-36) questionnaires for PROs of the HRQoL.
Integrated evaluation and management of the patients affected by ES, carried out simultaneously by a gastroenterologist and a rheumatologist, allowed clinicians to choose the optimal therapeutic strategy. In a cohort of 30 ES patients affected by active articular and gastrointestinal disease, or axial active articular inflammation, Ada led to fast and sustained improvement of both articular and gastrointestinal disease activities. In fact, all the clinimetric evaluation tests exploring articular or gastrointestinal activity, as well as all the HRQoL scores, showed a significant improvement having been achieved at the earliest (6-mo) assessment. This important clinical improvement was maintained at the 12-mo follow-up. Importantly, global and gastrointestinal quality of life significantly correlated with articular disease activity, providing evidence to support that the integrated evaluation is the best option to manage patients with ES.
Ada treatment, upon multidisciplinary (gastro-rheumatologic) evaluation, significantly improves both articular and gastrointestinal inflammation, thereby improving the HRQoL in patients affected by ES.
Core tip: Enteropathic spondyloarthritis (ES) is characterized by articular inflammation in patients with inflammatory bowel diseases, such as Crohn’s disease or ulcerative colitis. Correct management, especially covering both of the two clinical manifestations (gastro-rheumatologic), remains a challenge. In this study, we demonstrated that the integrated gastroenterological and rheumatologic evaluation of ES patients achieved the best therapeutic approach. In particular, we demonstrated that in a real-life cohort of ES patients, the tumor necrosis factor-inhibitor, adalimumab, led to fast and sustained improvement of articular and gastrointestinal inflammation, with a consequent improvement in the global and gastrointestinal quality of life.
- Citation: Luchetti MM, Benfaremo D, Ciccia F, Bolognini L, Ciferri M, Farinelli A, Rossini M, Mosca P, Triolo G, Gabrielli A. Adalimumab efficacy in enteropathic spondyloarthritis: A 12-mo observational multidisciplinary study. World J Gastroenterol 2017; 23(39): 7139-7149
- URL: https://www.wjgnet.com/1007-9327/full/v23/i39/7139.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i39.7139
Enteropathic spondyloarthritis (ES) is a seronegative spondyloarthropathy (SpA) characterized by the presence of articular inflammation in patients affected by inflammatory bowel diseases (IBDs), such as Crohn’s disease (CD) or ulcerative colitis (UC)[1,2]. Arthritis is the most frequent extra-intestinal manifestation in patients with IBDs[1] and it may primarily involve the axial joints, presenting with definite ankylosing spondylitis (AS) and/or isolated sacroiliitis, or peripheral joints and/or peri-articular structures, such as tendons and entheses[3]. The articular manifestations significantly affect health-related quality of life (HRQoL) of ES patients[4].
Although a link between inflammation of the joints and gut has been demonstrated[5,6], only half of ES patients are actually evaluated by a rheumatologist for proper diagnosis and, thus, for an integrated therapeutic approach through a coordinated action with the treating gastroenterologist[7]. Thus, an integrated clinical evaluation and therapeutic approach encompassing both the intestinal and articular features in ES patients, will likely be beneficial, particularly for the clinical outcomes. It has been demonstrated by recent studies that tumor necrosis factor (TNF)-alpha inhibitors could be effective therapeutic agents against ES[8], but few to date have reported on their real-life efficacy in this disease.
Herein, we have investigated the role of a gastro-rheumatologic multidisciplinary management and therapeutic approach in ES patients through evaluation of the efficacy of the TNF-alpha inhibitor adalimumab (Ada), assessing the efficacy on both gastrointestinal and rheumatologic disease activities and on the patient-reported HRQoL.
This study was carried out in a cohort of 52 patients with ES, including 31 affected by CD (59.6%) and 21 by UC (40.3%), collectively representing 23.6% of the 220 overall patient population with IBD in the Spondyloarthritis in IBD Project (commonly referred to as SPIB), described elsewhere[9]. The patients’ clinical and laboratory data are shown in Table 1, and the patient group is henceforth defined as the “ES-AN” cohort (Enteropathic spondyloarthritis from Ancona, Italy).
| ES-AN, n = 52 | ES-AN/Ada, n = 30 | |
| Crohn’s disease:Ulcerative colitis | 31 (60):21 (40) | 19 (63):11 (37) |
| Males:Females | 22 (42):30 (58) | 17 (57):13 (43) |
| Age in years | 47.2 ± 14.2 | 46.2 ± 14.4 |
| Disease duration of IBD in years | 11.3 ± 10.1 | 8.8 ± 7.9 |
| Smokers:Ex-smokers | 9 (17):20 (38) | |
| HLA-B27 positivity | 5 (10) | 4 (13) |
| Prior surgical intervention for IBD | 13 (25) | 5 (17) |
| Previous extra-intestinal disease | 6 (11) | 5 (17) |
| Eritema nodosum | 2 | 1 |
| Uveitis | 3 | 3 |
| Pioderma gangrenosum | 1 | 1 |
| Crohn’s disease activity by CDAI | ||
| Inactive | 14 (45) | 7 (37) |
| Moderate | 10 (32) | 8 (42) |
| Moderate-to-Severe | 7 (23) | 4 (21) |
| Ulcerative colitis activity by partial Mayo | ||
| Mild | 18 (86) | 8 (73) |
| Moderate | 3 (14) | 3 (27) |
| Severe | 0 | 0 |
| Current medication at baseline | ||
| Non-steroids anti-inflammatory drugs | 3 | 0 |
| Sulfasalazine | 3 | 2 |
| Mesalazine | 25 | 12 |
| Cyclosporine | 1 | 1 |
| Azathioprine | 9 | 5 |
| Oral steroids | 12 | 7 |
| Topical steroids | 3 | 2 |
| Metotrexate | 2 | 1 |
| Infliximab | 6 | 4 |
| Adalimumab | 2 | 0 |
| Spondyloarthritis features | ||
| Ankylosing spondylitis according to Modified New York Criteria | 16 (31) | 10 (33) |
| Non-radiographic Axial-Spondyloarthritis by ASAS Criteria | 13 (25) | 10 (33) |
| Peripheral- Spondyloarthritis | 23 (44) | 10 (33) |
| Type of axial involvement | n = 29 | n = 20 |
| Syndesmophytosis | 8 (28) | 6 (30) |
| Bamboo spine | 2 (7) | 2 (10) |
| Sacroiliitis by MRI and/or X-ray | 29 (100) | 20 (100) |
| Type of articular involvement in Crohn’s disease | n = 31 | n = 19 |
| Axial | 16 (52) | 11 (58) |
| Axial and peripheral | 4 | 3 |
| Peripheral only | 15 (48) | 8 (42) |
| Enthesitis | 9 (29) | 5 (26) |
| Type of articular involvement in ulcerative colitis | n = 21 | n = 11 |
| Axial | 13 (62) | 9 (82) |
| Axial and peripheral | 9 | 5 |
| Peripheral only | 8 (38) | 2 (18) |
| Enthesitis | 4 (19) | 2 (18) |
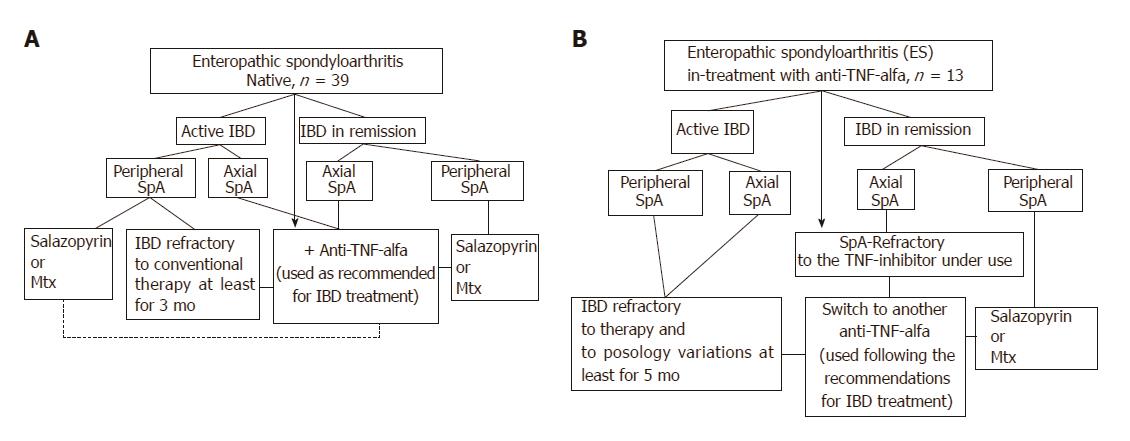
At each clinical observation, both the rheumatologist and the gastroenterologist collaborated in a shared session to develop the therapeutic strategy by applying a specifically designed algorithm (Figure 1) that was mainly based upon gastrointestinal-articular disease activity and the site of articular involvement at diagnosis (axial or peripheral arthritis). Briefly, the ES-AN patients were primarily separated into the following two groups for comparative analysis:
Group I (biological drugs-naïve group): This group encompassed three treatment subgroups. In the axial-ES-AN (Ax-ES-AN) subgroup, patients were administered Ada as first-line therapy, due to the absolute contraindication for a long-course treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) in case of IBD. In the peripheral-ES-AN (Per-ES-AN) subgroup, patients with active IBD or who were non-responders to a short course of corticosteroids (not more than 3 mo) or NSAIDs (not more than 2 wk) were administered either a disease-modifying anti-rheumatic drug (DMARD), such as either methotrexate (MTX) or sulfasalazine (SSZ), or Ada if erythrocyte sedimentation rate (ESR) was > 30 mm/h and/or C-reactive protein (CRP) concentration was > 0.5 mg/dL and/or in the presence of polyarticular inflammatory involvement. In the Per-ES-AN in inactive IBDs subgroup, patients were administered steroids or DMARDs, depending on count number of inflamed joints and systemic inflammation (evaluated by ESR and/or CRP).
Group II (TNF inhibitor-treated group): This group also encompassed three treatment subgroups. For the first, the Per-ES-AN consisting of patients with still active IBD were switched to another TNF-inhibitor (Ada). For the second, the Per-ES-AN consisting of patients with IBD in remission were administered a DMARD in addition to the TNF-inhibitor already in use. For the third, the Ax-ES-AN patients were switched to Ada, regardless of IBD activity.
In all patients, the TNF-inhibitor Ada was used as recommended for IBD treatment: 160 mg in the first week, 80 mg for the next 2 wk, and thereafter 40 mg once every 2 wk.
All patients of the ES-AN cohort were assessed for clinical disease activity and HRQoL (Table 2). Briefly, articular (SpA) disease activity was assessed by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)[10] and the Ankylosing Spondylitis Disease Activity Score-C-Reactive Protein (ASDAS-CRP) calculation[11]; gastrointestinal (IBD) disease activity was assessed with the Crohn’s Disease Activity Index (CDAI) for CD[12] and the partial Mayo (pMAYO) score for UC[13].
| Test | Items and interpretation |
| Bath Ankylosing Spondylitis Disease Index[10] | 6 items: (1) fatigue, (2) back pain, (3) peripheral pain/swelling, (4) discomfort at pressure, (5) morning discomfort, and (6) duration of morning stiffness; |
| Range from 0 to 10, with lower number representing less severe disease activity; | |
| Score > 4 = active disease. | |
| Ankylosing Spondylitis Disease Activity Score-C-Reactive Protein[11] | 5 items: (1) back pain, (2) morning stiffness, (3) patient global, (4) peripheral pain/swelling, and (5) C-reactive protein; |
| Score < 1.3 = inactive disease; Score 1.3 to < 2.1 = moderate activity; Score 2.1 to ≤ 3.5 = high activity; Score > 3.5 = very high activit; Change ≥ 1.1 = clinically important improvement; Change ≥ 2.0 = major improvement. | |
| Crohn’s Disease Activity Index[12] | 8 items: (1) liquid stools, (2) abdominal pain, (3) general well-being, (4) extra-intestinal manifestations (including arthralgia), (5) use of anti-diarrheals, (6) abdominal masses, and (7) hematocrit, 8) weight; |
| Final score is the sum of items, weighted by different factors; | |
| Score < 150 = non-active disease; Score > 150 = active disease; Score > 450 = extremely severe disease. | |
| Partial MAYO score[13] | 3 items: (1) stool frequency, (2) rectal bleeding, and (3) physician global assessment; |
| Range from 0 to 9; | |
| Score < 2 = disease remission; Score 2-4 = mild disease activity; Score 5-7 = moderate disease activity; Score > 7 = severe disease activity. | |
| Bath Ankylosing Spondylitis Functional Index[14] | 10 questions designed to determine the degree of functional limitation; |
| Final score ranges from 0 to 10, with lower score indicating less functional limitation. | |
| Inflammatory Bowel Disease Questionnaire[15] | 32 questions divided into 4 subscales: (1) bowel symptoms (10 questions); (2) systemic symptoms, including sleep disorders and fatigue (5 questions); (3) emotional function, such as depression, aggression and irritation (12 questions); and (4) social function, meaning the ability to participate in social activities and to work (5 questions); |
| The patient is invited to choose from 1 to 7 for every question; | |
| Total score ranges from 32 to 224 points, with lower scores reflecting worse quality of life. | |
| Patient Global Assessment (PtGA) | Collected on a numeric rating scale ranging from 0 to 10 for the question asking the patient: “Considering all the ways your disease affects you, how much do you think is active today?” |
| Short Form-36 health survey[16] | Generic health status instrument with 8 domains: (1) physical function, (2) body pain, (3) role limitations–physical, (4) general health, (5) vitality, (6) social function, (7) role limitations–emotional, and (8) mental health; |
| Greater scores reflect better health status; | |
| Summarized in two summary scores defined as the (1) physical component score (Sf-36/PCS) and (2) mental component score (Sf-36/MCS). |
Patient-reported outcomes (PROs) were assessed with tests specific for articular-related symptoms [i.e. the Bath Ankylosing Spondylitis Functional Index (BASFI)][14] and gastrointestinal-related symptoms [i.e. the Inflammatory Bowel Disease Questionnaire (IBDQ)][15]. Global wellness was assessed by use of the Health Assessment Questionnaire (HAQ), Patient Global Assessment (PtGA), and the Short Form-36 health survey (SF-36)[16].
Endpoints of this study were the disease activity indexes at baseline and at 6-mo and 12-mo follow-ups. Variables are presented as mean ± standard deviation. Comparisons between groups at baseline and between baseline and subsequent assessments were performed, respectively, with unpaired and paired Student’s t-tests. Correlations between variables were assessed using Pearson’s correlation coefficient. Data were analyzed using the SPSS software (v22.0; IBM SPSS Statistics for Windows, Armonk, NY, United States).
In the ES-AN cohort, 29 (57.6%) patients were affected by predominant axial SpA (Ax-ES-AN) and 23 (42.3%) by peripheral SpA (Per-ES-AN) (Table 1). Only 5 patients showed positivity for human leukocyte antigen-B27 (9.6%), including 4 affected by Ax-ES-AN and 1 by Per-ES-AN. Sacroiliitis was found in all the Ax-ES-AN patients, but 17 (55%) patients fulfilled the Modified New York Criteria for AS, whereas 13 (45%) were affected by non-radiographic axial-SpA[2]. Syndesmophytosis was found in 8 (27.6%) of the Ax-ES-AN patients at different levels, but only 2 (6.9%) presented a bamboo spine radiologic feature at baseline. Concurrent peripheral arthritis and enthesitis were present in about half of the Ax-ES-AN patients (44.8%).
According to the established criteria, IBD was active in 63.2% of the CD patients and in 98% of the UC patients, although the degrees of activity varied. At baseline, no differences were observed between the axial and peripheral spondyloarthritis patients and between the CD and UC patients for the articular disease activity (BASDAI, ASDAS-CRP) and the PROs [IBDQ, BASFI, PtGA, HAQ, SF-36 Physical Component Score (PCS) and Mental Component Score (MCS)] (data not shown). Following findings from the integrated evaluation and according to the algorithm presented in Figure 1, the criteria employed in our study to guide the therapeutic choice were (1) the presence and/or absence of active IBD (evaluated by both the gastroenterologist and by the results of the gastrointestinal tests and PROs); and (2) the site of articular involvement (axial or peripheral joints). Thus, Ada was assigned to a cohort of 30 patients, henceforth defined as the ES-AN/Ada cohort. Among this cohort, 4 were switched from infliximab for inefficacy, and the total was comprised of 20 patients with Ax-ES-AN and 10 patients with Per-ES-AN (Table 1).
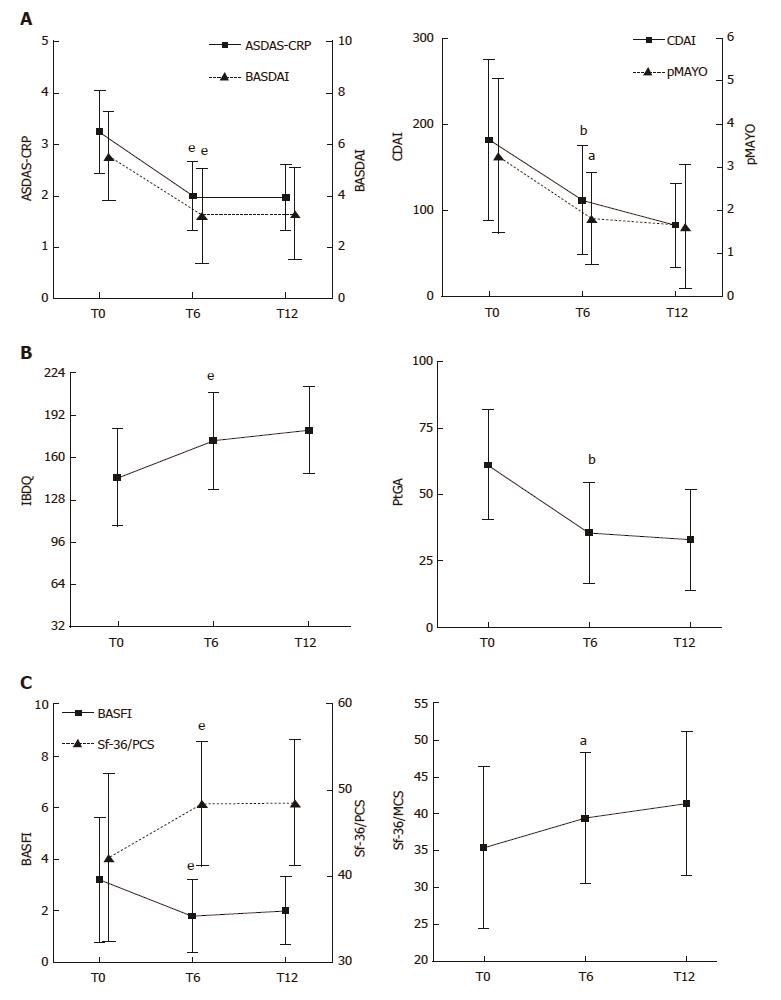
In most of the ES-AN/Ada patients, articular disease activity, as assessed by ASDAS-CRP and BASDAI, was significantly improved at 6-mo compared to baseline (ASDAS-CRP: 3.2 ± 0.8 vs 1.9 ± 0.6, P < 0.001; BASDAI: 5.5 ± 1.7 vs 3.2 ± 1.8, P < 0.001). A major clinical improvement (ASDAS change of ≥ 2) occurred in 21% and an important clinical improvement (ASDAS change of ≥ 1.1) occurred in 52% (Figure 2A). The improvement of articular disease was significant in both the axial and the peripheral subgroups of patients (P < 0.001 for both comparisons). The clinical improvement at the articular level was maintained at the 12-mo examination (Figure 2A).
Regarding the gastrointestinal disease activity, in the CD patients the treatment led to a fast, consistent and significant improvement of the gastrointestinal symptoms at 6 mo, as assessed by the CDAI score (181.3 ± 93.2 vs 112.3 ± 63.0, P < 0.01; Figure 2A), and maintained at 12 mo, when the clinical remission was observed in almost all patients (Figure 2A). In parallel, a significant clinical improvement was observed also in the UC patients, as shown by the decrease of the pMAYO score at the 6-mo and 12-mo examinations (Figure 2A). All the values and comparisons are detailed in Table 3.
| Baseline | T6 | T12 | |
| CDAI, n = 19 | 181.3 ± 93.2 | 112.3 ± 63.0b | 82.7 ± 48.7b |
| pMAYO, n = 11 | 3.27 ± 1.79 | 1.81 ± 1.07a | 1.63 ± 1.43a |
| IBDQ | 145.3 ± 36.8 | 172.8 ± 36.7e | 180.7 ± 33.0e |
| BASDAI | 5.5 ± 1.7 | 3.2 ± 1.8e | 3.3 ± 1.7e |
| BASFI | 3.2 ± 2.4 | 1.8 ± 1.4e | 2.0 ± 1.3a |
| ASDAS-CRP | 3.2 ± 0.8 | 1.9 ± 0.6e | 1.9 ± 0.6e |
| PtGA | 61.1 ± 20.6 | 35.6 ± 19.1b | 33.0 ± 19.1b |
| HAQ | 4.7 ± 8.5 | 2.0 ± 4.2a | 1.6 ± 3.5a |
| Sf-36/PCS | 42.2 ± 9.7 | 48.4 ± 7.2e | 48.5 ± 7.3b |
| Sf-36/MCS | 35.4 ± 10.9 | 39.4 ± 8.8a | 41.4 ± 9.7a |
| CRP in mg/dL | 2.7 ± 3.7 | 0.8 ± 1.2a | 0.5 ± 0.6a |
ES-AN/Ada patients reported significant improvement, from baseline to 6 mo, in the IBDQ (145.3 ± 36.8 vs 172.8 ± 36.7, P < 0.01), PtGA (61.1 ± 20.6 vs 35.6 ± 19.1, P < 0.01; Figure 2B) and HAQ (4.8 ± 7.9 vs 2.0 ± 4.2, P < 0.05) scores, and this improvement was maintained at 12 mo. Moreover, regarding the PROs impacting articular function and global health wellness, significant improvements were observed from baseline to 6 mo in the BASFI (3.2 ± 2.4 vs 1.8 ± 1.4, P < 0.01), Sf-36/PCS (42.2 ± 9.7 vs 48.4 ± 7.3, P < 0.01) and Sf-36/MCS scores (35.4 ± 10.9 vs 39.4± 8.8, P < 0.05; Figure 2C); again, the improvements were maintained at 12 mo. The improvement of all the scores for PROs was similar in both the Ax-ES-AN/Ada and Per-ES-AN/Ada subgroups at each follow-up observation. All the values and comparisons are detailed in Table 3.
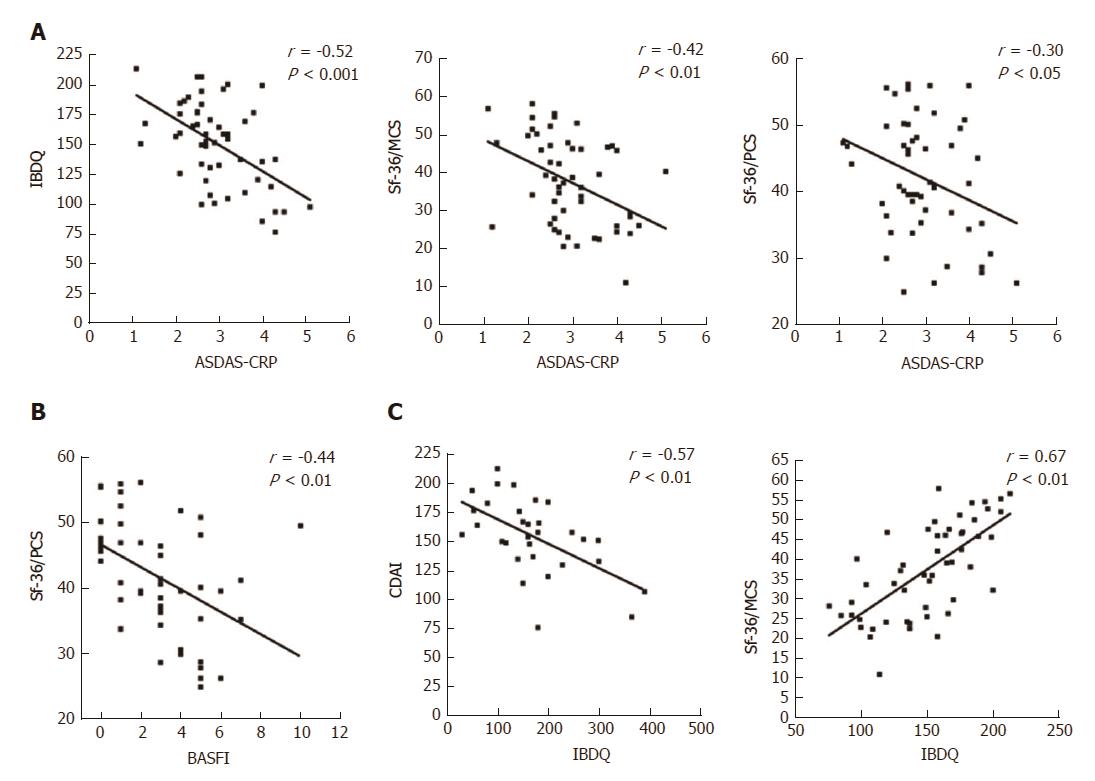
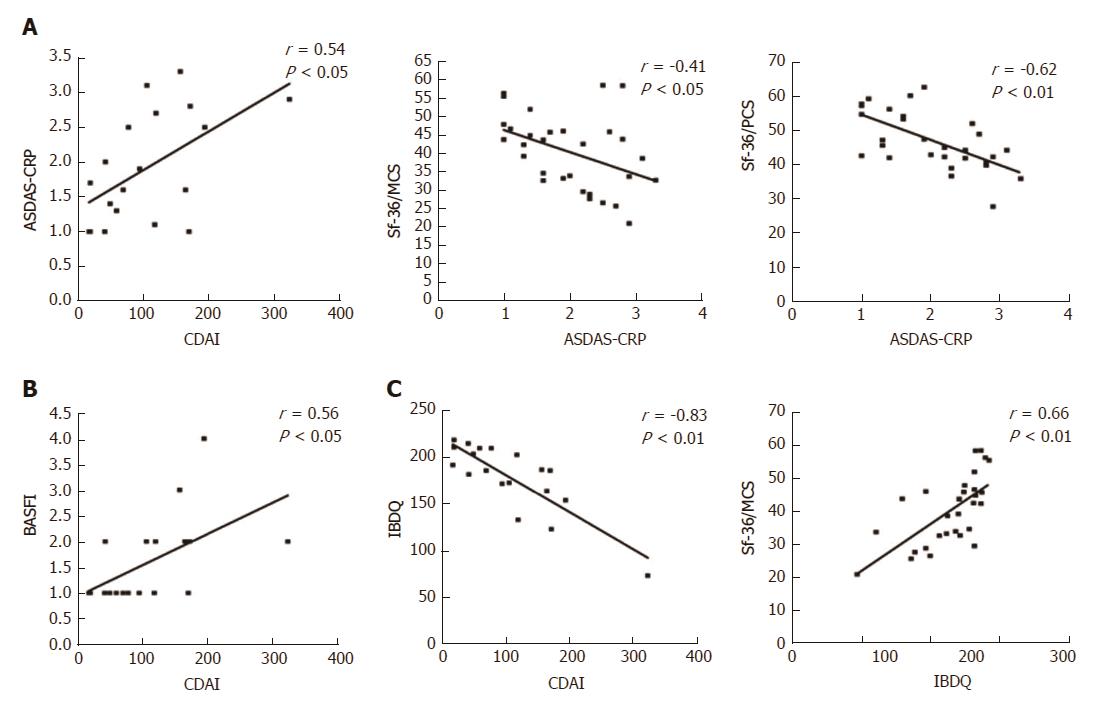
At baseline, it is noteworthy that a consistent significant correlation was observed between the IBDQ test (specific for the evaluation of the PROs related to the gastrointestinal disease activity) and the clinimetric scores of articular activity, as assessed by ASDAS-CRP (Figure 3A), BASDAI, and with most of the PROs of the HRQoL, as assessed by Sf-36/MCS and CDAI (Table 4). Moreover, articular disease activity at baseline was significantly correlated with worse results in the HRQoL tests, as shown by the Sf-36/PCS and Sf-36/MCS scores (Table 4). After 12 mo of Ada treatment, improvement of the articular activity (ASDAS-CRP) correlated significantly with decrease in the gastrointestinal disease activity scores, as assessed by CDAI in the CD patients (Figure 4A) and IBDQ in all of the patients, and with most of the PROs of the HRQoL, as shown by the Sf-36/PCS (r = -0.30, P < 0.05) and Sf-36/MCS (r = -0.41, P < 0.01) scores (Figure 2C), as well as the BASFI (r = 0.57, P < 0.001), PtGA (r = 0.37, P < 0.01) and HAQ (r = 0.28, P < 0.05) scores. All the correlations for the values at 12-mo examination are detailed in Table 4.
| CDAI | pMAYO | IBDQ | BASDAI | BASFI | ASDAS-CRP | PtGA | HAQ | Sf-36/ PCS | Sf-36/ MCS | CRP | |
| At baseline | |||||||||||
| CDAI | 1 | 1 | -0.57b | 0.19 | 0.29 | 0.21 | 0.14 | 0.12 | -0.24 | -0.33 | 0.35 |
| pMAYO | 1 | 1 | -0.48a | 0.34 | 0.38 | 0.37 | 0.15 | 0.18 | -0.14 | -0.11 | 0.28 |
| IBDQ | -0.57b | -0.48a | 1 | -0.38b | -0.34a | -0.52b | -0.26 | -0.19 | 0.27 | 0.67b | -0.26 |
| BASDAI | 0.19 | 0.34 | -0.38b | 1 | 0.64b | 0.69b | 0.24 | 0.26 | -0.22 | -0.31a | 0.00 |
| BASFI | 0.29 | 0.38 | -0.34a | 0.64b | 1 | 0.57b | 0.37b | 0.36b | -0.44b | -0.15 | 0.37b |
| ASDAS-CRP | 0.21 | 0.37 | -0.52b | 0.69b | 0.57b | 1 | 0.37b | 0.36b | -0.30a | -0.41b | 0.32a |
| PtGA | 0.14 | 0.15 | -0.26 | 0.24 | 0.37b | 0.37b | 1 | 0.22 | -0.14 | -0.22 | 0.14 |
| HAQ | 0.12 | 0.18 | -0.19 | 0.26 | 0.36b | 0.36b | 0.22 | 1 | -0.46b | -0.03 | 0.07 |
| Sf-36/PCS | -0.24 | -0.14 | 0.27 | -0.22 | -0.44e | -0.30a | -0.14 | -0.46b | 1 | 0.05 | -0.13 |
| Sf-36/MCS | -0.33 | -0.11 | 0.67b | -0.31a | -0.15 | -0.41b | -0.22 | -0.03 | 0.05 | 1 | -0.08 |
| After 12 mo of therapy with adalimumab (ES-AN/Ada cohort) | |||||||||||
| CDAI | 1 | 1 | -0.83b | 0.16 | 0.56a | 0.54a | 0.53a | 0.53a | -0.71b | -0.67b | 0.75b |
| pMAYO | 1 | 1 | -0.51 | 0.18 | 0.49 | 0.17 | 0.56 | -0.29 | -0.14 | -0.25 | 0.6 |
| IBDQ | -0.83b | -0.51 | 1 | -0.23 | -0.56b | -0.55b | -0.61b | -0.36a | 0.53b | 0.66b | -0.52b |
| BASDAI | 0.16 | 0.18 | -0.23 | 1 | 0.58b | 0.68b | 0.51b | 0.39a | -0.52b | -0.11 | -0.14 |
| BASFI | 0.56a | 0.49 | -0.56b | 0.58b | 1 | 0.56b | 0.64b | 0.54b | -0.52b | -0.45a | 0.16 |
| ASDAS-CRP | 0.54a | 0.17 | -0.55b | 0.68b | 0.56b | 1 | 0.67b | 0.38a | -0.62b | -0.41a | 0.31 |
| PtGA | 0.53a | 0.56 | -0.61b | 0.51b | 0.64b | 0.67b | 1 | 0.51b | -0.37a | -0.59b | 0.26 |
| HAQ | 0.53a | -0.29 | -0.36a | 0.39a | 0.54b | 0.38a | 0.51b | 1 | -0.47b | -0.40a | 0.10 |
| Sf-36/PCS | 1 | 1 | -0.83b | 0.16 | 0.56a | 0.54a | 0.53a | 0.53a | -0.71b | -0.67b | 0.75b |
| Sf-36/MCS | 1 | 1 | -0.51 | 0.18 | 0.49 | 0.17 | 0.56 | -0.29 | -0.14 | -0.25 | 0.60 |
| CRP | -0.83b | -0.51 | 1 | -0.23 | -0.56b | -0.55b | -0.61b | -0.36a | 0.53b | 0.66b | -0.52b |
Ada was well tolerated throughout the follow-up period. Side effects reported included recurrent upper respiratory tract infections (n = 2), bothersome hair loss (n = 1), and widespread itch (n = 2); but none necessitated suspension of the treatment. One patient suffered from a serious infection (axillary suppurative hidradenitis) that required prolonged antibiotic treatment. The other minor side effects reported were headache and fatigue.
The association between SpAs and IBDs has been known since the beginning of the last century[17]. However, gastroenterologists and rheumatologists continue to carry out their clinical evaluations and the therapeutic management of these diseases, now known collectively as ES, independently. In this regard, the different clinical guidelines employed by the two medical specialists may lead to different therapeutic decisions for the same clinical scenario, ultimately harboring potential for different clinical outcomes of the disease. A critical issue in the clinical management of ES is the correct therapeutic choice, and an important role has emerged recently for the TNF-alpha inhibitors in this regard. Infliximab was the first TNF-alpha inhibitor used in ES[18], but Ada has recently been applied to ES patients affected by CD and has achieved rates of clinical remission up to 50%[19].
To our knowledge, this is the first study demonstrating the efficacy of the integrated gastrorheumatologic approach for evaluation and therapeutic management of ES patients, with efficacy evidenced through assessments of disease activity and HRQoL over a 12-mo period. As dictated by our operative algorithm, both the gastroenterologist and the rheumatologist evaluated each of the patients in the study cohort, with both of the specialists collaborating to choose the optimal therapy. This integrated multidisciplinary approach, therefore, considered the gastrointestinal and articular disease activities simultaneously and, most importantly in the latter case, the site of articular inflammation (axial or peripheral). Thus, for example, in the case of axial spondyloarthritis, the therapy with anti-TNF-alpha inhibitors was mandatory, following the latest rheumatologic indications[20].
Since only few studies in the literature have so far evaluated the efficacy and safety of anti-TNF-alpha in ES, we employed the up and coming Ada treatment in our study. The results indicated that in ES patients, regardless of IBD type and/or site of articular involvement, Ada significantly improves the inflammation states of both the joints (assessed by ASDAS-CRP and BASDAI) and gut (assessed by CDAI or pMAYO score), as well as the HRQoL. Moreover, the benefits were already observable at 6 mo and the improvements were maintained at 12 mo. Ada also consistently improved not only the physical function in almost all ES patients (assessed by PtGA, BASFI, HAQ and Sf-36/PCS) but also their psychological function (assessed by Sf-36/MCS and IBDQ), from baseline to 6 mo.
It is noteworthy that in our study the strong linear relationship between articular-gastrointestinal disease activity and the PROs confirms the strong link between gut and joint inflammations. In fact, the IBDQ (specific for the gastrointestinal-related quality of life) strongly correlated with results of the clinimetric tests of articular activity (ASDAS-CRP, BASDAI and BASFI); additionally, the articular disease activity (assessed by ASDAS-CRP) strongly correlated with all of the PROs studied, even those specific for IBDs. To the contrary, however, at baseline the gastrointestinal disease activity scores (except for those of the IBDQ) did not correlate well with the other variables, but at 12 mo of therapy the CDAI correlated with most of the articular and PROs of HRQoL. This latter finding may be consequent to the different degrees of disease activities reported by the patients at baseline, which, after the treatment, tend to be globally ameliorated both at the gastrointestinal and articular levels.
Thus, since only the ASDAS-CRP test proved to be the most reliable in all patients in our study, we think that it should be necessary to develop composite scores and HRQoL questionnaires specific and suitable for ES patients at diagnosis, similar to those developed for other multidisciplinary diseases, such as psoriatic arthritis[21]. In conclusion, in our work that was carried out in a real-life cohort of a significant number of ES patients, we demonstrated that Ada produced a fast and significant improvement of both the gastrointestinal and articular scores of disease activity and, moreover, of the HRQoL. This clinical result was achieved by employing an integrated outpatient clinic specific for ES patients, as recently endorsed, particularly with regard to early diagnosis[22,23]. The integrated approach provided the optimal management of both the multidisciplinary clinical evaluation and the therapy of these patients. Further studies are certainly warranted to assess the long-term outcomes, and tolerability, of Ada and other TNF-alpha inhibitors in patients affected by ES.
Enteropathic spondyloarthritis (ES) is characterized by articular inflammation in patients with inflammatory bowel diseases (IBDs), such as Crohn’s disease or ulcerative colitis. Arthritis is the most frequent extra-intestinal manifestation found in IBD patients, yet only half of the ES patients are actually evaluated by a rheumatologist for a proper diagnosis and, thereafter, for receipt of an integrated therapeutic approach through a coordinated action between the two specialists.
Considering the multidisciplinary intrinsic “face” of IBDs and the novel therapeutic opportunities that comprise the biological drugs, as in the case of anti-tumor necrosis factor (TNF)-alpha inhibitors, an integrated approach and evaluation of all ES patients should be routinely employed and strongly encouraged to obtain the best therapeutic efficacy on all the clinical manifestations of this disease.
In this work, the authors have demonstrated that the integrated (simultaneous) evaluation of patients affected by ES, through the coordinated efforts of a gastroenterologist and rheumatologist, led to the best therapeutic approach, thereby allowing the patients to achieve a consistent clinical remission of both the articular and gastrointestinal inflammations.
Through this study authors have been able to generate a simple working flowchart of the multidisciplinary clinical care process applied during the patients’ integrated assessment. They suggest that in patients with ES and in consideration of the therapeutic choice, particular attention should be paid to the presence of active intestinal disease, presence of active articular (arthritis) and/or periarticular (enthesitis) disease and localization of the joints’ inflammation (peripheral or axial, as in the case of sacroiliitis).
ES belongs to the group of seronegative spondyloarthritis (SpA) and, as such, is characterized by the presence of arthritis in patients affected by IBDs; it is also known as SpA-IBD.
This is an interesting and well-conducted work.
We would like to acknowledge all the patients who enthusiastically participated in this study. We are also grateful with Miss Lucrezia Lombardi for her assistance in the writing and revision of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Pellicano R S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Peluso R, Di Minno MN, Iervolino S, Manguso F, Tramontano G, Ambrosino P, Esposito C, Scalera A, Castiglione F, Scarpa R. Enteropathic spondyloarthritis: from diagnosis to treatment. Clin Dev Immunol. 2013;2013:631408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2101] [Cited by in F6Publishing: 2164] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 3. | Rodríguez-Reyna TS, Martínez-Reyes C, Yamamoto-Furusho JK. Rheumatic manifestations of inflammatory bowel disease. World J Gastroenterol. 2009;15:5517-5524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 67] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Brakenhoff LK, de Wijs L, van den Berg R, van der Heijde DM, Huizinga TW, Fidder HH, Hommes DW. Impact of arthropathies on health-related quality of life in inflammatory bowel disease patients. J Crohns Colitis. 2012;6:S56-S57. [DOI] [Cited in This Article: ] |
| 5. | Colombo E, Latiano A, Palmieri O, Bossa F, Andriulli A, Annese V. Enteropathic spondyloarthropathy: a common genetic background with inflammatory bowel disease? World J Gastroenterol. 2009;15:2456-2462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Actis GC, Pellicano R. The pathologic galaxy modulating the genotype and phenotype of inflammatory bowel disease: comorbidity, contiguity, and genetic and epigenetic factors. Minerva Med. 2016;107:401-412. [PubMed] [Cited in This Article: ] |
| 7. | Stolwijk C, Pierik M, Landewé R, Masclee A, van Tubergen A. Prevalence of self-reported spondyloarthritis features in a cohort of patients with inflammatory bowel disease. Can J Gastroenterol. 2013;27:199-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Van den Bosch F, Kruithof E, De Vos M, De Keyser F, Mielants H. Crohn’s disease associated with spondyloarthropathy: effect of TNF-alpha blockade with infliximab on articular symptoms. Lancet. 2000;356:1821-1822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Avellini C, Bolognini L, Farinelli A, Ciferri M, Gambacorta G, Benfaremo D, Cedraro S, Rossini M, Capeci W, Manfredi L. Patient Reported Outcomes and Assessment of the Quality of Life in a Cohort of Patients Affected By Enteropathic Spondyloarthritis: Definitive Results of a Monocentric Prospective Observational Study at One Year. Arthritis Rheumatol. 2015;67:10 Available from: http://acrabstracts.org/abstract/patient-reported-outcomes-and-assessment-of-the-quality-of-life-in-a-cohort-of-patients-affected-by-enteropathic-spondyloarthritis-definitive-results-of-a-monocentric-prospective-observational-study. [Cited in This Article: ] |
| 10. | Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286-2291. [PubMed] [Cited in This Article: ] |
| 11. | Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, van der Linden S, van der Heijde D; Assessment of SpondyloArthritis international Society. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68:18-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 566] [Cited by in F6Publishing: 597] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 12. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] [Cited in This Article: ] |
| 13. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 589] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 14. | Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, Jenkinson T. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21:2281-2285. [PubMed] [Cited in This Article: ] |
| 15. | Ciccocioppo R, Klersy C, Russo ML, Valli M, Boccaccio V, Imbesi V, Ardizzone S, Porro GB, Corazza GR. Validation of the Italian translation of the Inflammatory Bowel Disease Questionnaire. Dig Liver Dis. 2011;43:535-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51:1025-1036. [PubMed] [Cited in This Article: ] |
| 17. | Wright V, Moll JH, Seronegative Polyarthritis. North Holland Publishing Company, Amsterdam, The Netherlands, 1976. . [Cited in This Article: ] |
| 18. | Generini S, Giacomelli R, Fedi R, Fulminis A, Pignone A, Frieri G, Del Rosso A, Viscido A, Galletti B, Fazzi M. Infliximab in spondyloarthropathy associated with Crohn’s disease: an open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann Rheum Dis. 2004;63:1664-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Löfberg R, Louis EV, Reinisch W, Robinson AM, Kron M, Camez A, Pollack PF. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn’s disease: results from CARE. Inflamm Bowel Dis. 2012;18:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, Sepriano A, Regel A, Ciurea A, Dagfinrud H, Dougados M. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1070] [Cited by in F6Publishing: 958] [Article Influence: 136.9] [Reference Citation Analysis (0)] |
| 21. | Chandran V, Maharaj AB. Assessing disease activity in psoriasis and psoriatic arthritis: impact on management and therapy. Expert Rev Clin Immunol. 2016;12:573-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Olivieri I, Cantini F, Castiglione F, Felice C, Gionchetti P, Orlando A, Salvarani C, Scarpa R, Vecchi M, Armuzzi A. Italian Expert Panel on the management of patients with coexisting spondyloarthritis and inflammatory bowel disease. Autoimmun Rev. 2014;13:822-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Conigliaro P, Chimenti MS, Ascolani M, Triggianese P, Novelli L, Onali S, Lolli E, Calabrese E, Petruzziello C, Pallone F. Impact of a multidisciplinary approach in enteropathic spondyloarthritis patients. Autoimmun Rev. 2016;15:184-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |