Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.842
Peer-review started: August 28, 2016
First decision: October 20, 2016
Revised: October 22, 2016
Accepted: November 16, 2016
Article in press: November 16, 2016
Published online: February 7, 2017
To identify a set of contributors, and weight and rank them on a pathophysiological basis.
Patients who have undergone a lactulose or glucose hydrogen breath test to rule out small intestinal bacterial overgrowth (SIBO) for various clinical symptoms, including diarrhoea, weight loss, abdominal pain, cramping or bloating, were seen as eligible for inclusion in a retrospective single-centre study. Clinical data such as co-morbidities, medication, laboratory parameters and other possible risk factors have been identified from the electronic data system. Cases lacking or with substantially incomplete clinical data were excluded from the analysis. Suspected contributors were summarised under four different pathophysiological pathways (impaired gastric acid barrier, impaired intestinal clearance, immunosuppression and miscellaneous factors including thyroid gland variables) and investigated using the χ2 test, Student’s t-test and logistic regression models.
A total of 1809 patients who had undergone hydrogen breath testing were analysed. Impairment of the gastric acid barrier (gastrectomy, odds ratio: OR = 3.5, PPI therapy OR = 1.4), impairment of intestinal clearance (any resecting gastric surgery OR = 2.6, any colonic resection OR = 1.9, stenosis OR = 3.4, gastroparesis OR = 3.4, neuropathy 2.2), immunological factors (any drug-induced immunosuppression OR = 1.8), altered thyroid gland metabolism (hypothyroidism OR = 2.6, levothyroxine therapy OR = 3.0) and diabetes mellitus (OR = 1.9) were associated significantly to SIBO. Any abdominal surgery, ileocecal resection, vagotomy or IgA-deficiency did not have any influence, and a history of appendectomy decreased the risk of SIBO. Multivariate analysis revealed gastric surgery, stenoses, medical immunosuppression and levothyroxine to be the strongest predictors. Levothyroxine therapy was the strongest contributor in a simplified model (OR = 3.0).
The most important contributors for the development of SIBO in ascending order are immunosuppression, impairment of intestinal clearance and levothyroxine use, but they do not sufficiently explain its emergence.
Core tip: Several contributors to small intestinal overgrowth have been described, but the impact of particular risk factors is poorly understood. We aimed to determine the influence of several pathomechanisms, such as impaired gastric acid barrier function, impaired intestinal clearance, impairment of defence mechanisms and miscellaneous factors, as well as to weight and rank a large set of potential contributors by means of a retrospective cohort study of 1809 consecutive patients who had undergone a hydrogen breath test to rule out small intestinal bacterial overgrowth. Overall, levothyroxine therapy, impaired intestinal clearance and immunosuppression are the strongest contributors, while an impaired gastric acid barrier only plays a minor role.
- Citation: Brechmann T, Sperlbaum A, Schmiegel W. Levothyroxine therapy and impaired clearance are the strongest contributors to small intestinal bacterial overgrowth: Results of a retrospective cohort study. World J Gastroenterol 2017; 23(5): 842-852
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/842.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.842
Small intestinal bacterial overgrowth (SIBO) is defined as an increase in the number of bacteria in the upper gastrointestinal tract. The aetiology and pathogenesis of SIBO are incompletely understood. It is believed that endogenous defence mechanisms prevent abundant microbial growth in the small intestine[1] under physiological conditions, so that the development of SIBO is usually seen to be associated with disorders of protective antimicrobial mechanisms, anatomical abnormalities or motility disorders. High recurrence rates after successful antibiotic treatment emphasise the need to identify aetiological factors in order to potentially remedy the situation[2].
Gastric acidity constitutes an effective barrier against the invasion of ingested microorganisms. Although the data is weak and contradictory, reduction of the acid barrier function, as suspected for atrophic gastritis[3,4], use of proton pump inhibitors[5] and gastrectomy[6,7], is thought to lead to higher microbial loads in the small intestine.
Although several reviews highlight anatomic pathologies associated with small intestinal obstruction and stagnation, for example, strictures, adhesions, tumours of the small bowel, duodenal and jejunal diverticula, previous abdominal surgery such as blind loop syndrome after Billroth-II or Roux-en-Y procedure, or bariatric bypass surgery, to be associated with SIBO, only very few or no data at all support these hypotheses. Overall abdominal surgery was not associated with SIBO in a small retrospective study[8]. However, gastrectomy and bariatric surgery in morbid obese patients often leads to the development of SIBO[6,7,9]; the existence of blind loops might be the common mechanism. Additionally, conditions that predispose stool reflux, such as ileocecal resection or low ileocecal valve pressure, are discussed as SIBO predisposing factors[10].
More evidence exists concerning impaired motility, such as small intestinal pseudo-obstruction and several neurological diseases or diabetes[1,11]. It has been demonstrated for a long time that a subset of patients with SIBO show reduced motility[12] with fewer phase III contractions of the migrating motor complexes and a mutual influence since eradication of bacterial overgrowth improved motility[13]. Gastroparesis, which has been shown to be associated with SIBO[14,15], might indicate gastrointestinal autonomous neuropathy. Otherwise, little is known about the effect of drugs used to deteriorate intestinal motility, even though “narcotics” have been identified as contributing towards SIBO[16].
Data about the role of the immune system are also scarce and contradictory. A higher bacterial load of jejunal aspirates have been shown in ten paediatric patients with IgA deficiency and seven with other immune syndromes[17], while data concerning adult patients are lacking. In fact, medical therapy is the most common reason for immunosuppression in adults, though SIBO was not shown to be associated with immunosuppressive medication in patients with Crohn’s disease (CD)[18,19], while steroids predisposed to SIBO in a more unselected cohort[20].
Various other diseases and disorders have been described as being associated with or complicated by SIBO, such as alcohol consumption[21], liver cirrhosis[22-25], non-alcoholic steatohepatitis[26], hypothyroidism[27] or chronic pancreatitis[28].
In summary, several contributors to the development of SIBO have been proposed, but only a few have been proven in clinical studies, which often refer to small and selected cohorts. Additionally, it is uncertain which pathomechanisms are more and which are less important contributors. We aimed, therefore, to (1) evaluate a larger set of potential risk factors; (2) arrange them in a pathogenetic model; and (3) rank the contributors referring to their particular weight in a largely unselected cohort of SIBO and non-SIBO patients in an extensive retrospective cohort study.
We conducted a retrospective single-centre study of patients undergoing lactulose or glucose hydrogen breath testing between 1995 and 2010, who were referred to the Department of Gastroenterology at the University Hospital Bergmannsheil Bochum, Germany. Patients underwent breath tests to rule out SIBO for various clinical symptoms, including diarrhoea, weight loss, abdominal pain, cramping or bloating. All cases with an original examination report available were considered eligible for the study. Patients with both missing or incomplete clinical data, or incomplete or aborted examination were excluded. Patients with multiple hydrogen breath tests within the study period were considered only once.
All patients underwent a hydrogen breath test with lactulose, glucose or both in combination. Additionally, patients underwent a routine diagnostic work-up following a clinical algorithm by a symptom-based diagnostic approach including clinical evaluation (history, symptomatology, clinical examination, complaints, clinical course), laboratory including stool testing, and endoscopy (ileocolonoscopy, esophagogastroduodenoscopy) including histopathology and transabdominal ultrasound. Further diagnostic tools may have been used, for example, small bowel magnetic resonance imaging, endoscopic ultrasound, small bowel endoscopy, manometry or extended function testing.
Breath tests were performed according to a standardised protocol with either 25 g lactulose or 75 g glucose in 300 mL water, respectively. Only one test was performed per day. Breath samples were collected using an AlveoSamplerTM (Campro Scientific GmbH, Germany) every 10 min over a period of 2 h. In cases of failed hydrogen exhalation during a lactulose breath test, collecting periods were extended up to 180 min.
An increase in hydrogen of at least 20 ppm was considered positive; for a lactulose breath test, this increase had to have occurred at least 15 min before a sustained rise in hydrogen exhalation indicating colonic lactulose metabolism.
Additional clinical data was collected from the electronic database. Cases lacking or with substantially incomplete clinical data were excluded from the analysis.
Statistical analysis was performed with SPSS 23 (IBM, Armonk, United states). The χ2 test was used to determine statistical significance with categorial variables, and the Student’s t-test for metric variables. Regarding the multivariate analysis, binary logistic regression was performed in each pathophysiological pathway suspected separately (hypo-/achlorhydria, impaired clearance, immunosuppression, thyroid gland variables and miscellaneous factors) and with statistically significant variables in a summarising model. Finally, the highest ranked parameter of each particular section was chosen to calculate a simplified ranking model. The odds ratios (OR) and 95%CI were estimated for specific clinical factors using logistic regression models. Analysis was considered significant with a P value ≤ 0.05.
The study was approved by the institutional review board (registration number 4864-13). Informed consent was obtained from the patients before particular examinations.
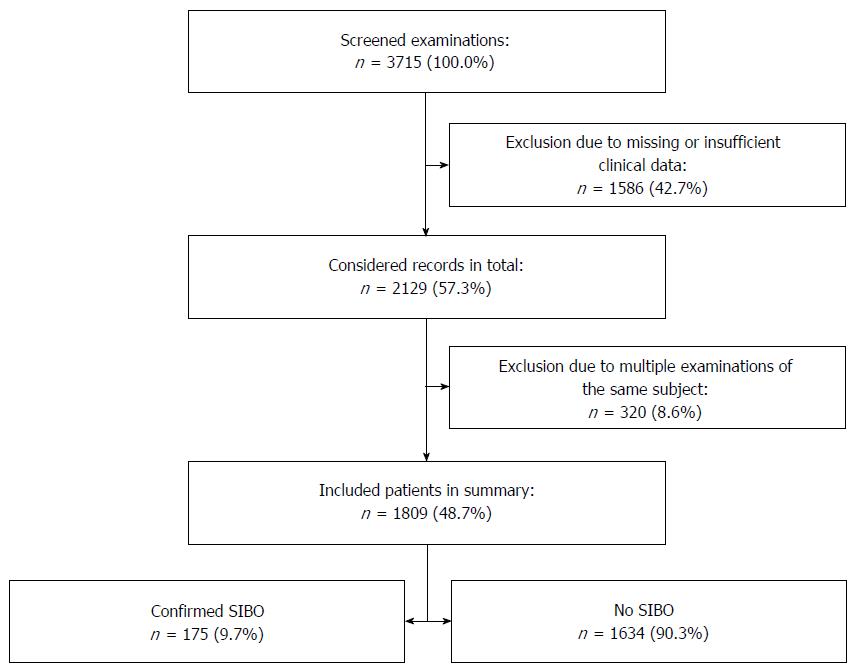
A total of 3715 hydrogen breath test examinations was considered eligible, 1586 of these were excluded due to missing or insufficient clinical information, and 320 records were excluded due to repetitive examinations of the same subject (Figure 1), therefore, our study population in summary contained a total of 1809 patients, with a slight, but not significant excess of women (Table 1). The age did not differ significantly between the gender groups. The overall basic characteristics of the patients were equally distributed as displayed, but patients with SIBO took slightly more drugs (1.32% vs 1.61%), especially spasmolytics and antiemetics (4.1% vs 14.9% and 8.8% vs 11.6%, respectively). Supplementation of iron and cobalamin was more common in SIBO patients.
| SIBO | Non-SIBO | χ2 or Student’s t-test | |||
| n = 175 | % | n = 1634 | % | P value | |
| Age (yr) | 48.7 ± 17.9 | - | 49.3 ± 18.0 | - | 0.70 |
| Sex (female) | 107 | 61.1 | 924 | 56.5 | 0.139 |
| Pathological LHBT | 102 | 58.3 | 212 | 13.0 | < 0.0011 |
| Pathological GHBT | 146 | 83.4 | 9 | 0.6 | < 0.0011 |
| Diarrhoea | 72 | 41.1 | 648 | 39.7 | 0.380 |
| Constipation | 4 | 2.3 | 38 | 2.3 | 0.616 |
| Weight Loss | 39 | 22.3 | 313 | 19.2 | 0.185 |
| Malabsorption | 9 | 5.1 | 53 | 3.2 | 0.138 |
| Iron Deficiency Anaemia | 2 | 1.1 | 38 | 2.3 | 0.240 |
| Vitamin B12 Anaemia | 1 | 0.6 | 4 | 0.2 | 0.399 |
| Number of drugs used | 1.61 | - | 1.32 | - | 0.0051 |
| Supplementation of folic acid | 3 | 1.7 | 22 | 1.3 | 0.442 |
| Supplementation of iron | 17 | 9.7 | 18 | 1.1 | 0.0221 |
| Supplementation of vitamin B12 | 6 | 3.4 | 18 | 1.1 | 0.0231 |
| Abdominal ultrasound | 152 | 86.9 | 1508 | 92.3 | 0.0221 |
| EGD | 120 | 68.6 | 1187 | 72.6 | 0.146 |
| Colonoscopy | 100 | 57.1 | 1069 | 65.4 | 0.0191 |
| Enteroclysis | 45 | 25.7 | 357 | 21.8 | 0.143 |
| Video capsule endoscopy | 7 | 4.0 | 19 | 1.2 | 0.0091 |
| Enteroscopy | 1 | 0.6 | 4 | 0.2 | 0.399 |
The SIBO patients were more likely to undergo small bowel visualisation (video capsule endoscopy 4.0% vs 1.2%) while colonoscopy was performed more often in the non-SIBO group (57.1% vs 65.4%).
Hypo-/achlorhydria: Patients with a history of total gastrectomy were more likely to develop SIBO (2.3% vs 0.7%). The OR was 3.45 (Table 2). Current PPI therapy led to a higher SIBO rate (40.0% vs 31.8%), although the effect was small (OR = 1.43).
| SIBO | Non-SIBO | χ2 test | OR | RR | |||
| n = 175 | % | n = 1634 | % | P value | |||
| Gastrectomy | 4 | 2.3 | 11 | 0.7 | 0.0491 | 3.451 | 0.295 |
| Atrophic gastritis | 2 | 1.1 | 5 | 0.3 | 0.141 | - | - |
| PPI therapy | 70 | 40.0 | 519 | 31.8 | 0.0341 | 1.432 | 0.794 |
| Ulcer indicated PPI therapy | 5 | 2.9 | 47 | 2.9 | 0.611 | - | - |
| Gastritis indicated PPI therapy | 20 | 11.4 | 195 | 11.9 | 0.481 | - | - |
| GERD indicated PPI therapy | 20 | 11.4 | 179 | 11.0 | 0.464 | - | - |
| Indicated PPI therapy | 42 | 24.0 | 395 | 24.2 | 0.522 | - | - |
| Not indicated PPI therapy | 58 | 33.1 | 370 | 22.6 | 0.0021 | 1.728 | 0.693 |
Impaired bowel clearance - anatomical alterations and surgery: Overall abdominal surgery was not associated with a higher risk of SIBO (Table 3). Patients after gastrectomy and patients with a history of any resecting gastric surgery had a higher prevalence of SIBO (6.9% vs 2.8%, OR = 2.60). By contrast, neither Bilroth-II resection nor the existence of blind intestinal loops exhibited a higher prevalence of SIBO, although the latter group showed a tendency (4.6% vs 2.5%). Loss of the ileocecal valve did not increase risk, while a history of appendectomy occurred more often in non-SIBO patients (8.2% vs 4.6%), indicating a protective factor with an OR of 0.46. On the other hand, functional appendectomy (including those patients with ileocecal resection) did not show such an effect. Obstetric surgery, sigmoid and small intestinal resection did not affect development of SIBO, while any colonic resection was associated with an OR of 1.93 (10.9% vs 5.9%). Cholecystectomy tended to be protective (4.6% vs 7.8%). Stenoses of the intestinal tract were associated with SIBO (5.7% vs 1.8%) with an OR of 3.36.
| SIBO | Non-SIBO | χ2 test | OR | RR | |||
| n = 175 | % | n = 1634 | % | P value | |||
| Any abdominal surgery | 51 | 29.1 | 487 | 29.8 | 0.466 | - | - |
| Obstetric surgery | 2 | 1.1 | 45 | 2.8 | 0.135 | - | - |
| Abdominal w/o obstetric surgery | 49 | 28.0 | 444 | 27.2 | 0.438 | - | - |
| Gastrectomy | 4 | 2.3 | 11 | 0.7 | 0.0491 | 3.451 | 0.295 |
| BII-resection | 5 | 2.9 | 31 | 1.9 | 0.265 | - | - |
| Existence of blind loops | 8 | 4.6 | 41 | 2.5 | 0.0942 | 1.861 | 0.549 |
| Any resecting gastric surgery | 12 | 6.9 | 45 | 2.8 | 0.0071 | 2.600 | 0.402 |
| Resection of Ileocecum | 7 | 4.0 | 41 | 2.5 | 0.176 | - | - |
| Appendectomy | 7 | 4.0 | 134 | 8.2 | 0.0271 | 0.466 | 2.050 |
| Functional appendectomy | 14 | 8.0 | 175 | 10.7 | 0.163 | - | - |
| Cholecystectomy | 8 | 4.6 | 127 | 7.8 | 0.0782 | 0.568 | 1.700 |
| Resection of small intestine | 11 | 6.3 | 80 | 4.9 | 0.260 | - | - |
| Any colonic resection | 19 | 10.9 | 97 | 5.9 | 0.0131 | 1.930 | 0.547 |
| Sigmoid resection | 3 | 1.7 | 13 | 0.8 | 0.196 | - | - |
| Vagotomy | 2 | 1.1 | 6 | 0.4 | 0.177 | - | - |
| Fistula | 2 | 1.1 | 16 | 1.0 | 0.532 | - | - |
| Stenosis | 10 | 5.7 | 29 | 1.8 | 0.0031 | 3.354 | 0.311 |
| Impaired motility | 17 | 9.7 | 9 | 0.6 | < 0.0011 | 5.157 | 0.202 |
| Gastroparesis | 5 | 2.9 | 24 | 1.5 | 0.0301 | 3.403 | 0.300 |
| Neuropathy | 9 | 5.1 | 39 | 2.4 | 0.0371 | 2.217 | 0.464 |
| Opioid use | 10 | 5.7 | 55 | 3.4 | 0.0902 | 1.740 | 0.589 |
Impaired bowel clearance - functionally impaired clearance: Impaired motility (9.7% vs 0.6%), gastroparesis (2.9% vs 1.5%) and neuropathy (5.1% vs 2.4%), but not vagotomy were associated with higher risks of SIBO; referring to Table 3, the ORs were 5.16, 3.40 and 2.22, respectively. Prevalence of SIBO tended to be higher under opioid medication, but did not achieve statistical significance (5.7% vs 3.4%).
Immunological factors: Immunoglobulin deficiency did not change the risk of developing SIBO (Table 4), while the use of steroids (20.6% vs 13.6%) or classical immunosuppressants (4.6% vs 1.9%), any immunosuppressive therapy (22.3% vs 14.1%) and the combination of steroids and immunosuppressants led to a higher risk of SIBO (4.0% vs 1.4%). The OR was highest in the combination group (steroid plus immunosuppressant: OR = 2.92).
| SIBO | Non-SIBO | χ2 test | OR | RR | |||
| n = 175 | % | n = 1634 | % | P value | |||
| IgA-deficiency | 0 of 6 | 0.0 | 13 of 75 | 17.3 | 0.337 | - | - |
| IgG-deficiency | 3 of 5 | 60.0 | 15 of 52 | 28.8 | 0.175 | - | - |
| IgM-deficiency | 0 of 5 | 0.0 | 15 of 54 | 21.7 | 0.311 | - | - |
| 5-Aminosalicylates | 24 | 13.7 | 183 | 11.2 | 0.191 | - | - |
| Steroid use | 36 | 20.6 | 222 | 13.6 | 0.0101 | 1.647 | 0.660 |
| Immunosuppressant use | 8 | 4.6 | 31 | 1.9 | 0.0291 | 2.477 | 0.415 |
| Azathioprin use | 7 | 4.0 | 31 | 1.9 | 0.0672 | 2.155 | 0.474 |
| Metotrexate use | 3 | 1.7 | 0 | 0.0 | 0.0011 | n/a | n/a |
| Any drug-induced immunosuppression | 39 | 22.3 | 230 | 14.1 | 0.0041 | 1.751 | 0.632 |
| Steroid plus immunosuppressant | 7 | 4.0 | 23 | 1.4 | 0.0211 | 2.918 | 0.352 |
Thyroid gland metabolism: As referred to in Table 5, patients with hypothyroidism and patients with levothyroxine therapy showed a higher prevalence of SIBO (9.7% vs 4.0% and 17.1% vs 6.5%, respectively) while a history of thyroidectomy slightly failed to.
| SIBO | Non-SIBO | χ2 test | OR | RR | |||
| n = 175 | % | n = 1634 | % | P value | |||
| Thyroid gland surgery | 7 | 4.0 | 33 | 2.0 | 0.0852 | 2.021 | 0.505 |
| Hypothyroidism | 17 | 9.7 | 66 | 4.0 | 0.0021 | 2.556 | 0.416 |
| Hyperthyroidism | 1 | 0.6 | 24 | 1.5 | 0.287 | - | - |
| Levothyroxine use | 30 | 17.1 | 106 | 6.5 | < 0.0011 | 2.982 | 0.378 |
| Adipositas | 31 | 17.7 | 307 | 18.8 | 0.410 | - | - |
| Diabetes mellitus | 25 | 14.3 | 132 | 8.1 | 0.0061 | 1.896 | 0.565 |
| Steatosis hepatis | 22 | 12.6 | 158 | 9.7 | 0.139 | - | - |
| Hepatitis | 7 | 4.0 | 61 | 3.7 | 0.492 | - | - |
| Liver cirrhosis | 2 | 1.1 | 35 | 2.1 | 0.289 | - | - |
| Renal insufficiency | 2 | 1.1 | 16 | 1.0 | 0.532 | - | - |
| Colonic diverticulosis | 6 | 3.4 | 37 | 2.3 | 0.229 | - | - |
| Sigmoid diverticulosis | 8 | 4.6 | 151 | 9.2 | 0.0201 | 0.470 | 2.021 |
| Crohn’s disease | 19 | 10.9 | 134 | 8.2 | 0.146 | 1.363 | 0.755 |
| Ulcerative colitis | 2 | 1.1 | 52 | 3.2 | 0.0922 | 0.352 | 2.785 |
| Alcoholism | 3 | 1.7 | 36 | 2.2 | 0.468 | - | - |
| Smokers | 10 | 5.7 | 52 | 3.2 | 0.0702 | 1.844 | 0.557 |
| NSAID use | 29 | 16.6 | 284 | 17.4 | 0.442 | - | - |
| Laxative use | 3 | 1.7 | 58 | 3.5 | 0.142 | - | - |
| Antidiarrhoics use | 9 | 5.1 | 118 | 7.2 | 0.195 | - | - |
| Spasmolytics use | 9 | 5.1 | 257 | 15.7 | < 0.0011 | 0.290 | 3.058 |
| Antiemetics use | 22 | 12.6 | 144 | 8.8 | 0.0712 | 1.488 | 0.701 |
| Irritable bowel syndrome | 8 | 4.6 | 322 | 19.7 | < 0.0011 | 0.195 | 4.311 |
Miscellaneous: Diabetes mellitus was associated with a 1.90-fold increased risk of developing SIBO (14.3% vs 8.1%; Table 5). Sigmoid, but not colonic diverticulosis was associated with a lower prevalence of SIBO (4.6% vs 9.2%).
Multivariate analysis: The different pathomechanistic pathways were tested in a binary logistic regression analysis. The strongest particular independent variables were PPI therapy and gastrectomy for hypo- or achlorhydria. Both showed statistical significance with an OR of 1.45 and 3.64, respectively.
All variables which potentially impair intestinal clearance were studied in a further model. Any resecting gastric surgery, stenoses, gastroparesis and any colonic resection were significantly associated with the presence of SIBO (P < 0.05): the ORs were 6.49, 3.19, 3.25 and 1.85, respectively, while gastrectomy, neuropathy, existence of blind loops, appendectomy and cholecystectomy were not.
The model for immunosuppression did not show any significant parameters. Binary logistic regression for thyroid gland variables proved statistically significant for levothyroxine use with an OR of 2.8, while thyroidectomy and hypothyroidism did not. The only significant parameter in the model of miscellaneous variables was sigmoid diverticulosis with an OR of 0.453; the other factors were opioid use, smoking, diabetes and ulcerative colitis.
Finally, the variables PPI therapy, history of gastrectomy, history of any resecting gastric surgery, presence of stenoses, use of levothyroxine, presence of diabetes, neuropathy or gastroparesis, medical immunosuppression and therapy with opioids have been included in a summarising model (Table 6). The Omnibus test results are highly significant (P < 0.001). The Hosmer-Lemeshow test (P = 0.500) indicates that the independent variables form a good model to predict SIBO (54 cases observed, 56 cases expected); Nagelkerke’s R2 was 0.070. Variables with significant influence were any resecting gastric surgery (P = 0.037, OR = 2.40), stenoses (P = 0.008, OR = 2.81), any medical immunosuppression (P = 0.036, OR = 1.53), levothyroxine therapy (P < 0.001, OR = 2.92) and presence of sigmoid diverticulosis (P = 0.028, OR = 2.30).
| Equation variables | Regression coefficient B | Wald | Significance | Exp (B) | 95%CI for Exp (B) | |
| Lower limit | Upper limit | |||||
| PPI therapy | 0.241 | 2.015 | 0.156 | 1.273 | 0.912 | 1.776 |
| Gastrectomy | 0.600 | 0.676 | 0.411 | 1.821 | 0.436 | 7.604 |
| Any resecting gastric surgery | 0.875 | 4.369 | 0.037 | 2.399 | 1.056 | 5.450 |
| Stenoses | 1.033 | 7.011 | 0.008 | 2.809 | 1.308 | 6.033 |
| Gastroparesis | 1.016 | 3.244 | 0.072 | 2.762 | 0.914 | 8.345 |
| Any colon resection | 0.479 | 3.012 | 0.083 | 1.614 | 0.940 | 2.772 |
| Any medical immunosuppression | 0.428 | 4.380 | 0.036 | 1.534 | 1.028 | 2.291 |
| Levothyroxine therapy | 1.070 | 20.980 | 0.000 | 2.916 | 1.845 | 4.609 |
| Diabetes mellitus | -0.453 | 3.223 | 0.073 | 0.636 | 0.388 | 1.042 |
| Sigmoid diverticulosis | 0.832 | 4.835 | 0.028 | 2.298 | 1.095 | 4.823 |
Of the variables “impairment of acid barrier” (PPI therapy and gastrectomy), “impairment of intestinal clearance” (history of any resecting gastric surgery, presence of stenoses, gastroparesis), “impairment of immune response” (any medical suppression) or hypothyroidism (levothyroxine supplementation), the latter three were significantly associated with SIBO with odds ratios of 2.2, 1.6 and 3.0, respectively, in a simplified model in which every factor that was significant in the particular logistic regression was summarised (Table 7).
| Equation variables | Regression coefficient B | Wald | Significance | Exp (B) | 95%CI for Exp (B) | |
| Lower limit | Upper limit | |||||
| Impaired acid barrier | 0.277 | 2.780 | 0.095 | 1.319 | 0.953 | 1.827 |
| Impaired clearance | 0.772 | 14.463 | 0.0001 | 2.164 | 1.454 | 3.221 |
| Impaired immune response | 0.453 | 5.161 | 0.0231 | 1.572 | 1.064 | 2.324 |
| Hypothyroidism | 1.083 | 22.514 | 0.0001 | 2.953 | 1.888 | 4.620 |
The pathogenesis of SIBO and underlying predisposing conditions are insufficiently understood. Several risk factors have been proposed, but most studies refer to one or very few variables in selected populations. Furthermore, no study investigated and ranked the underlying main pathomechanistic pathways. In this retrospective study, we sought to identify, categorise and, finally, rank the influence of potential contributors to SIBO in a large and widely unselected population. Three main pathogenetic pathways have been hypothesised: hypo- or achlorhydria, impaired intestinal clearance and immunosuppression. Further factors of unknown action, such as hypothyroidism, inflammatory bowel disease or sigmoid diverticulosis, were also considered.
Both PPI therapy and, even more predominantly, gastrectomy were significantly associated with SIBO (Table 2). These findings coincide with results in the literature, although glucose hydrogen breath tests usually failed to find an association between SIBO and PPI therapy[5-7]. Consequently, gastrectomy was a stronger predictor of SIBO in binary regression analysis than PPI therapy (OR 3.6 vs 1.5). Moreover, the altered anatomical situation with the establishment of blind loops might play a role in the development of SIBO after gastrectomy, although our data does not confirm blind loops as a pathophysiological factor (Table 3). However, both variables are of minor relevance and lost their significance in multivariate models.
Anatomic modifications which might lead to impairment of intestinal clearance derive mostly from surgery, but, similar to a smaller retrospective study by Petrone et al[8], overall abdominal surgery was not associated with an increased risk of SIBO, neither were sigmoid nor ileocecal resection nor obstetric surgery (Table 3). Our data, therefore, does not confirm the previously assumed protective function of the ileocecal valve[10]. By contrast, total gastrectomy, any resecting gastric surgery and stenoses which lead to stasis of the chymus contributed to the development of SIBO with odds ratios between 1.9 and 3.6. Since blind loops of any reason were astonishingly not associated to SIBO, the combination of intestinal diversion and loss of acid barrier is of greater relevance.
Since small intestinal motility is difficult to quantify as a promoter of clearance, this effect might be estimated by measuring gastroparesis as a surrogate parameter. This association has already been described in other studies[14,15]. Our data shows that conditions believed to be associated with intestinal paralysis, such as polyneuropathy, are indeed associated with gastroparesis (P < 0.001, χ2 test). Both are linked to SIBO, but multivariate analysis reveals that gastroparesis is the better parameter (Tables 3 and 6), by which it is easier to quantify than intestinal motility itself.
Any resecting gastric surgery, stenoses and gastroparesis were associated with SIBO in binary regression analysis, and the particular OR varied between 1.8 and 6.5, with the highest value for overall gastric surgery.
Data about the role of the immune system is scarce, biased and contradictory. Higher bacterial loads of jejunal aspirates have been shown in ten paediatric patients with IgA deficiency and seven with other immune syndromes[17]. Therapy with immunosuppressives in CD did not increase the occurrence of SIBO[18,19], while steroids did so in a more unselected cohort by lowered levels of IgA, as hypothesised by the authors[20]. Since neither IgA nor IgG deficiency were associated to SIBO in our analysis, we conclude that IgA deficiency does not - either directly or indirectly triggered by steroids - contribute to SIBO in adult patients. On the other hand, therapy with classical immunosuppressants, such as azathioprine or methotrexate, with steroids alone or in combination with immunosuppressants leads to a higher risk of SIBO (Table 4), while other anti-inflammatory drugs, such as 5-aminosalicylates, that usually serve as reference did not change the risk, suggesting that immunosuppression is the underlying mechanism. The association was quite loose, with odds ratios between 1.6 and 2.5, but was still found to be a minor contributor to SIBO in the multivariate analysis therapy with any immunosuppressant drug, i.e., azathioprine, methotrexate or steroids.
Hypothyroidism and levothyroxine therapy are the most strongly associated to SIBO in our cohort. A case control study by Lauritano et al[27] has already revealed a high prevalence of SIBO in patients with autoimmune thyroiditis and hypothyroidism, but the influence of the autoimmune process was a questionable biasing factor. Multivariate analysis confirmed that levothyroxine therapy is a stronger predictor of SIBO than hypothyroidism. The underlying mechanism is unclear. One might speculate that hypothyroidism leads to hypomotility, but, surprisingly, levothyroxine therapy was even more associated to SIBO and not able to reverse the effect of hypothyroidism.
Although SIBO was more frequent in patients with cholecystectomy in one study[29], our data propose that cholecystectomy is a protective factor. This finding is supported by another retrospective study[21]. We assume that the prolonged contact time and the optimised environment due to constant excretion of the bile allow its antimicrobial effects to better develop as a potential underlying mechanism.
Though reported as a slight risk factor for the development[20] and recurrence[2] of SIBO, in our cohort, appendectomy was prevalent more often in non-SIBO patients (8.2% vs 4.6%, P = 0.027, χ2 test), indicating that it is a protective factor with an OR of 0.455. The potential underlying mechanism is unclear, but an effect on other bowel diseases, especially ulcerative colitis, has already been shown[30].
As in previous studies[11], diabetes was associated with SIBO, but lost its effect in multivariate analysis (Tables 5 and 6), proposing that diabetes does not contribute directly, but as a consequence of complications such as neuropathy, which is supported by a strong association between diabetes and gastroparesis (P < 0.001, χ2 test).
Binary logistic regression reveals the history of any resecting gastric surgery, stenoses, medical immunosuppression, levothyroxine supplementation or presence of sigmoid diverticulosis to be associated to a higher risk of SIBO (Table 6). In this model, a reduced gastric acid barrier lost its significance as a pathogenetic factor. The highest odds ratios were seen for factors that, similar to stenoses, lead to impaired clearance, on the one hand, and supplementation of levothyroxine, on the other hand (Table 6). In this model, several independent factors of impaired clearance, but only one of immunosuppression and thyroid gland metabolism were confirmed.
In a simplified model in which any impairment of the acid barrier, of intestinal clearance, or of immune response and hypothyroidism were analysed, the impaired acid barrier again lost its significance (Table 7). Hypothyroidism or, respectively, levothyroxine therapy was most the important single factor with an odds ratio of 3.0; the second most important was impaired intestinal clearance (OR = 2.2) and the third most important was impaired immune response (OR = 1.6).
Due to selective information on outpatients, our study population consists mostly of inpatients, which might have led to a certain bias. Furthermore, the retrospective study design itself is associated with certain limitations, such as primary lack or secondary loss of information, uncertainty of adherence to protocols or unequal power between groups. We, therefore, included only a subset of possible contributors that were available for retrospective analysis. Diagnosis of SIBO referred to clinical work-up and is mainly based on the hydrogen breath test. That approach covers advantages such as independence from jejunal cultures, which is neither well standardised nor able to culture all respective flora, but also disadvantages such as unclear reliability. Finally, not every potential contributor who underwent clinical work-up was carefully ruled out in every subject. Therefore, some suspected contributors, such as gastroparesis or polyneuropathy, might be underreported.
To the best of our knowledge, this is the largest study that investigated contributors to SIBO in a widely spread population. The results confirm that impairment of acid barrier, impaired intestinal clearance, immunosuppression and especially levothyroxine therapy/hypothyroidism to be important pathomechanistic pathways for the development of SIBO. The strongest effects derive from levothyroxine supplementation/hypothyroidism, although the relevant mechanism of action remains unclear, and intestinal stasis.
We would like to thank the staff of the Department of Gastroenterology and Hepatology for their support throughout the study, and Professor Burkhard May who pioneered functional gastrointestinal testing at the University-Hospital Bergmannsheil and deceased in 2015. Special thanks go to the members of the laboratory of gastroenterological function testing, i.e., Beckmann A, Kuehl A, Nolte C and Dretaki-Schnackenberg E.
Small intestinal bacterial overgrowth (SIBO) is characterised by an excessive colonisation of the small bowel. Pathogenesis is incompletely understood, but recurrence rates after successful antibiotic treatment are high. Several risk factors that contribute to the development have been proposed, and some potential contributors have been shown in mostly small studies with selected populations.
SIBO is defined as an increase in the number of bacteria in the upper gastrointestinal tract. Related symptoms are nonspecific and include bloating, abdominal pain or diarrhea, and thus overlap widely with symptoms of irritable bowel syndrome. Diagnosis of SIBO still is a challenging clinical problem. Several methods are available, but up to now, a standard of choice is not yet defined. Furthermore, the pathogenesis of SIBO is incompletely understood and studies are needed to identify etiology and pathogenesis. Therapy of SIBO is unsatisfactory; empiric and often broad-spectrum antibiotic treatment fails frequently and suffers from high recurrence rates.
The aim of the study was to identify a set of risk factors, weight and rank them on a pathophysiological basis in a large and widely unselected population. Potential contributors were summarised under four different pathophysiological pathways. Impairment of the gastric acid barrier (gastrectomy, odds ratio: OR = 3.5, PPI therapy OR = 1.4), impairment of intestinal clearance (any resecting gastric surgery OR = 2.6, any colonic resection OR = 1.9, stenosis OR = 3.4, gastroparesis OR = 3.4, neuropathy 2.2), immunological factors (any drug-induced immunosuppression OR = 1.8), altered thyroid gland metabolism (hypothyroidism OR = 2.6, levothyroxine therapy OR = 3.0) and diabetes mellitus (OR = 1.9) were associated significantly to SIBO. Any abdominal surgery, ileocecal resection, vagotomy or IgA-deficiency did not have any influence, and a history of appendectomy decreased the risk of SIBO. Multivariate analysis revealed gastric surgery, stenoses, medical immunosuppression and levothyroxine to be the strongest predictors. Levothyroxine therapy was the strongest contributor in a simplified model (OR = 3.0).
The most important contributors for the development of SIBO in ascending order are immunosuppression, impairment of intestinal clearance and levothyroxine use, but they do not sufficiently explain its emergence. The knowledge of respective contributors potentially enables to intervene in the pathogenesis and therefore minimise the risk of recurrence.
SIBO is characterised by an increase of bacterial colonisation of the small bowel.
It is an interesting and well performed study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sinha RA S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 383] [Cited by in F6Publishing: 331] [Article Influence: 23.6] [Reference Citation Analysis (3)] |
| 2. | Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Novi M, Sottili S, Vitale G, Cesario V, Serricchio M, Cammarota G. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am J Gastroenterol. 2008;103:2031-2035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Urita Y, Watanabe T, Maeda T, Arita T, Sasaki Y, Ishii T, Yamamoto T, Kugahara A, Nakayama A, Nanami M. Extensive atrophic gastritis increases intraduodenal hydrogen gas. Gastroenterol Res Pract. 2008;2008:584929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Ardatskaia MD, Loginov VA, Minushkin ON. [Syndrome of bacterial overgrowth in patients with the reduced stomach acid secretion: some aspects of the diagnosis]. Eksp Klin Gastroenterol. 2014;30-36. [PubMed] [Cited in This Article: ] |
| 5. | Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 216] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 6. | Iivonen MK, Ahola TO, Matikainen MJ. Bacterial overgrowth, intestinal transit, and nutrition after total gastrectomy. Comparison of a jejunal pouch with Roux-en-Y reconstruction in a prospective random study. Scand J Gastroenterol. 1998;33:63-70. [PubMed] [Cited in This Article: ] |
| 7. | Paik CN, Choi MG, Lim CH, Park JM, Chung WC, Lee KM, Jun KH, Song KY, Jeon HM, Chin HM. The role of small intestinal bacterial overgrowth in postgastrectomy patients. Neurogastroenterol Motil. 2011;23:e191-e196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Petrone P, Sarkisyan G, Fernández M, Coloma E, Akopian G, Ortega A, Kaufman HS. Small intestinal bacterial overgrowth in patients with lower gastrointestinal symptoms and a history of previous abdominal surgery. Arch Surg. 2011;146:444-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Lakhani SV, Shah HN, Alexander K, Finelli FC, Kirkpatrick JR, Koch TR. Small intestinal bacterial overgrowth and thiamine deficiency after Roux-en-Y gastric bypass surgery in obese patients. Nutr Res. 2008;28:293-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Roland BC, Ciarleglio MM, Clarke JO, Semler JR, Tomakin E, Mullin GE, Pasricha PJ. Low ileocecal valve pressure is significantly associated with small intestinal bacterial overgrowth (SIBO). Dig Dis Sci. 2014;59:1269-1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Faria M, Pavin EJ, Parisi MC, Lorena SL, Brunetto SQ, Ramos CD, Pavan CR, Mesquita MA. Delayed small intestinal transit in patients with long-standing type 1 diabetes mellitus: investigation of the relationships with clinical features, gastric emptying, psychological distress, and nutritional parameters. Diabetes Technol Ther. 2013;15:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158-1166. [PubMed] [Cited in This Article: ] |
| 13. | Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002;47:2639-2643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | George NS, Sankineni A, Parkman HP. Small intestinal bacterial overgrowth in gastroparesis. Dig Dis Sci. 2014;59:645-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Reddymasu SC, McCallum RW. Small intestinal bacterial overgrowth in gastroparesis: are there any predictors? J Clin Gastroenterol. 2010;44:e8-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Choung RS, Ruff KC, Malhotra A, Herrick L, Locke GR, Harmsen WS, Zinsmeister AR, Talley NJ, Saito YA. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther. 2011;33:1059-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Pignata C, Budillon G, Monaco G, Nani E, Cuomo R, Parrilli G, Ciccimarra F. Jejunal bacterial overgrowth and intestinal permeability in children with immunodeficiency syndromes. Gut. 1990;31:879-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Klaus J, Spaniol U, Adler G, Mason RA, Reinshagen M, von Tirpitz C C. Small intestinal bacterial overgrowth mimicking acute flare as a pitfall in patients with Crohn’s Disease. BMC Gastroenterol. 2009;9:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Sánchez-Montes C, Ortiz V, Bastida G, Rodríguez E, Yago M, Beltrán B, Aguas M, Iborra M, Garrigues V, Ponce J. Small intestinal bacterial overgrowth in inactive Crohn’s disease: influence of thiopurine and biological treatment. World J Gastroenterol. 2014;20:13999-14003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Schatz RA, Zhang Q, Lodhia N, Shuster J, Toskes PP, Moshiree B. Predisposing factors for positive D-Xylose breath test for evaluation of small intestinal bacterial overgrowth: a retrospective study of 932 patients. World J Gastroenterol. 2015;21:4574-4582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Gabbard SL, Lacy BE, Levine GM, Crowell MD. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Dig Dis Sci. 2014;59:638-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 22. | Madrid AM, Cumsille F, Defilippi C. Altered small bowel motility in patients with liver cirrhosis depends on severity of liver disease. Dig Dis Sci. 1997;42:738-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29:1273-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Bauer TM, Steinbrückner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, Pelz K, Kist M, Blum HE. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol. 2001;96:2962-2967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 598] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 27. | Lauritano EC, Bilotta AL, Gabrielli M, Scarpellini E, Lupascu A, Laginestra A, Novi M, Sottili S, Serricchio M, Cammarota G. Association between hypothyroidism and small intestinal bacterial overgrowth. J Clin Endocrinol Metab. 2007;92:4180-4184. [PubMed] [Cited in This Article: ] |
| 28. | Kumar K, Ghoshal UC, Srivastava D, Misra A, Mohindra S. Small intestinal bacterial overgrowth is common both among patients with alcoholic and idiopathic chronic pancreatitis. Pancreatology. 2014;14:280-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Sung HJ, Paik CN, Chung WC, Lee KM, Yang JM, Choi MG. Small Intestinal Bacterial Overgrowth Diagnosed by Glucose Hydrogen Breath Test in Post-cholecystectomy Patients. J Neurogastroenterol Motil. 2015;21:545-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Gardenbroek TJ, Eshuis EJ, Ponsioen CI, Ubbink DT, D’Haens GR, Bemelman WA. The effect of appendectomy on the course of ulcerative colitis: a systematic review. Colorectal Dis. 2012;14:545-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |