Published online Jan 7, 2019. doi: 10.3748/wjg.v25.i1.118
Peer-review started: October 17, 2018
First decision: November 22, 2018
Revised: December 16, 2018
Accepted: December 19, 2018
Article in press: December 19, 2018
Published online: January 7, 2019
In recent decades, neoadjuvant therapy (NT) has been the standardized treatment for locally advanced rectal cancer (LARC). Approximately 8%-35% of patients with LARC who received NT were reported to have achieved a complete pathological response (pCR). If the pathological response (PR) can be accurately predicted, these patients may not need surgery. In addition, no response after NT implies that the tumor is destructive, resistant to both chemotherapy and radiotherapy, and prone to having a high metastatic potential. Therefore, developing accurate models to predict PR has great clinical significance and can help achieve individualized treatment in LARC patients.
To establish nomograms for predicting PR to different NT regimens based on pretreatment parameters for patients with LARC.
Rectal cancer patients were identified from the database of The Sixth Affiliated Hospital, Sun Yat-sen University from January 2012 to December 2016. Logistic regression and nomograms were developed to predict the probability of pCR and good downstaging to ypT0-2N0M0 (ypTNM 0-I), respectively, based on pretreatment parameters for all LARC patients. Nomograms were also developed for three NT regimens (capecitabine/deGramont-RT, mFOLFOX6, and mFOLFOX6-RT) to predict pCR probability.
Four hundred and three patients were included in this study; 72 (17.9%) had pCR at the final pathology report, and 177 (43.9%) achieved good downstaging to ypT0-2N0M0 (ypTNM 0-I). The nomogram for predicting pCR probability showed that NT regimens, tumor differentiation, mesorectal fascia (MRF) status, and tumor length significantly influenced pCR probability. When predicting the probability of good downstaging, tumor differentiation, MRF status, and clinical T stage were the significant factors. Nomograms were developed based on NT regimens. For the capecitabine/de Gramont-RT group, the multivariate analysis showed that the neutrophil-lymphocyte ratio (NLR) was the only significant factor, thus we could not develop a nomogram for this regimen. For the mFOLFOX6-RT group, the analysis showed that the significant factors were tumor length and MRF status; and for the mFOLFOX6 group, the significant factors were tumor length and tumor differentiation.
We established accurate nomograms for predicting the PR to preoperative NT regimens based on pretreatment parameters for LARC patients.
Core tip: In this study, we established accurate nomograms for predicting the pathological response (PR) to preoperative neoadjuvant therapy (NT) regimens based on pretreatment parameters for locally advanced rectal cancer (LARC) patients. Logistic regression and nomograms were developed to predict the probability of complete pathological response and good downstaging, respectively, for all patients and for subgroups based on NT regimens. In conclusion, nomograms have been established for predicting the PR to different NT regimens for LARC patients; and these nomograms can be used to facilitate developing individualized treatments.
- Citation: Ren DL, Li J, Yu HC, Peng SY, Lin WD, Wang XL, Ghoorun RA, Luo YX. Nomograms for predicting pathological response to neoadjuvant treatments in patients with rectal cancer. World J Gastroenterol 2019; 25(1): 118-137
- URL: https://www.wjgnet.com/1007-9327/full/v25/i1/118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i1.118
In recent decades, neoadjuvant therapy (NT) has been the standardized treatment for locally advanced rectal cancer (LARC)[1]. NT was reported to decrease the risk of local recurrence and have reduced toxicity[2,3]. Pathological complete response (pCR) is characterized as complete elimination of malignant cells in a resected specimen[4,5]. Approximately 8%-35% of patients with LARC who received NT was reported to have achieved pCR[6-9]. Researchers have also found that good pathological response are associated with a longer disease-free survival (DFS) and lower local and distant recurrence rates[10-15].
Individualized treatment for LARC patients can be achieved by developing an accurate model to predict the probability of pCR or good downstaging. Some authors suggest that if pCR can be accurately predicted, these patients can be strictly followed without requiring surgery[16-18]. Radical surgery can drastically reduce the quality of life by impairing normal intestinal and genitourinary functions[19]. However, other authors argue that follow-up alone is unsafe and that the pathology cannot be accurately assessed without surgery after NT[20]. In addition, no tumor response or progression after NT implies that the tumor is destructive, resistant to both chemotherapy and radiotherapy, and prone to having a high metastatic potential. Thus, identifying potential responders and non-responders may aid in predicting treatment outcomes and choices.
Previous studies have reported that low carcinoembryonic antigen (CEA) levels[21,22], high pretreatment hemoglobin (HB) levels, early clinical T stage (cT)[23], early clinical N stage (cN), small tumor size, and long radiation surgery interval[6,23-25] are related to pCR probability. However, few models or nomograms have been established and even fewer are used clinically to predict a good pathological response after NT for LARC. Additionally, few models are available to predict neoadjuvant treatments. Therefore, developing accurate models to predict pathological responses has great clinical significance and remains a great challenge.
In this study, by analyzing pretreatment parameters in LARC patients before NT at our institution, we established accurate models and nomograms to predict the probability of pCR and good downstaging, respectively, with currently available pretreatment parameters that can be easily used in clinical decision-making.
Rectal cancer patients were identified from the database of The Sixth Affiliated Hospital, Sun Yat-sen University from January 2012 to December 2016. Four hundred and three patients who met the following criteria were included: histopathologically confirmed rectal adenocarcinoma, >18 years old, tumor located no more than 12 cm above the anal verge, clinical stage of cT3/4 or lymph node (+), and non-metastatic. All patients were assessed via abdominal-pelvic computed tomography (CT) and pelvic magnetic resonance imaging (MRI), and 44 (11%) patients received transrectal ultrasound testing. All received NT followed by total mesorectal excision (TME) radical surgery.
We collected all available clinical information before treatment: gender, age, body mass index (BMI), cT, cN, mesorectal fascia (MRF) status, tumor differentiation, tumor length (TL), distance of tumor from the anal verge (DTAV), tumor circumferential extent (TCE), serum tumor marker CEA, HB, neutrophil-lymphocyte ratio (NLR), platelets (PLT), apolipoprotein A-1 (ApoA1), apolipoprotein B (ApoB), and NT regimen type. All tumor-related parameters such as cT, cN, MRF status, DTAV, and TCE were assessed by MRI. Tumor length was also assessed by using MRI to measure the maximum diameter of tumor. CT, transrectal ultrasound, and endoscopy provided additional verification. Tumor differentiation was identified by enteroscopic pathology.
This retrospective study was approved by the Institutional Review Board of The Sixth Affiliated Hospital, Sun Yat-sen University.
During the period we identified patients for the current study, a clinical trial (FOWARC) was conducted at our institution comparing the effectiveness and safety of administering only chemotherapy with mFOLFOX6 or mFOLFOX6 plus radiotherapy to LARC patients with the effectiveness and safety in patients undergoing a standard NT regimen with fluorouracil plus radiotherapy. Consequently, 273 (67.7%) patients in our study were included in the FOWARC trial. The NT regimens included in our study were capecitabine /fluorouracil plus radiotherapy (standard group, capecitabine/deGramont-RT), mFOLFOX6 without radiotherapy (mFOLFOX6), and mFOLFOX6 plus radiotherapy (mFOLFOX6-RT). Details of all these treatments have been reported in previous studies[26,27]. The radiation dose for the radiotherapy was 46.0-50.4 Gy, delivered as 1.8-2.0 Gy/d, and the dose was the same in the capecitabine/deGramont-RT and mFOLFOX6-RT groups. Patients in the capecitabine/deGramont-RT and mFOLFOX6-RT groups underwent standard TME radical surgery after NT. The interval between radiation and surgery was 6-12 wk in mFOLFOX6-RT and de Gramont–RT groups. The interval between chemotherapy and TME radical surgery was about 2-4 wk in the mFOLFOX6 group.
All resected specimens were examined to determine the post-TN staging according to the American Joint Committee on Cancer-International Union Against Cancer (seventh edition), which is currently considered the most accurate and standard staging system in this period[28]. pCR was defined as no malignant cells found in the resected specimens, including the primary tumor and lymph nodes, and ypT0-2N0M0 (ypTNM 0-I) was classified as good downstaging.
Chi-square analysis was selected for the univariate logistic regression analysis of the countable data. Normal distribution tests were performed for the metrological data, nonparametric test was used for the indicators that were not normally distributed, and the expression form of the median (upper quartile to lower quartile) is used.
Parameters such as age (≤ 60 years vs > 60 years), BMI (< 25 kg/cm2vs ≥ 25 kg/cm2), CEA (> 5 ng/mL vs ≤ 5 ng/mL), HB (≤ 125 g/L vs > 125 g/L), NLR (> 3 vs ≤ 3), DTAV (< 5 cm vs ≥ 5 cm) and TL (> 3 cm vs ≤ 3 cm) were dichotomized according to previous studies[24,29,30]. PLT, ApoA1, ApoB, and the interval were used as continuous variables, however, all these variables were not normally distributed, so a nonparametric test was used.
Univariate logistic regression analysis was used to analyze variables related to the probability of pCR or good downstaging. Variables that achieved significance at P ≤ 0.05 in the univariate logistic regression analysis were further analyzed into the forward stepwise multivariable logistic regression. Multivariate logistic regression analysis was used to construct nomograms. Because the NT regimen was a statistically significant factor for predicting pCR probability, all patients were divided into three subgroups (the capecitabine/deGramont-RT, mFOLFOX6-RT, or mFOLFOX6 groups) based on the NT regimens. We then attempted to develop three nomograms based on the different NT regimens to predict pCR probability. The C-index was acquired for the nomogram, and internal validation using the bootstrap method was performed to determine the adjusted C-index. Calibration curves of the nomograms were generated to show the relationship between the predicted and observed outcomes. All statistical analyses were performed using SPSS 24.0 and R 3.5.1.
Of the 403 patients in our study, 281 (69%) were men. As assessed pathologically, 72 (17.86%) individuals achieved pCR; 177 (43.9%) patients achieved ypTNM 0-I and were classified as having good downstaging.
The interval between radiation and surgery was 52 (47-59) d in the mFOLFOX6-RT group and 54 (49-58.25) d in the deGramont–RT group, and there was no significance difference between the two groups. The interval between chemotherapy and surgery was 22 (18-25.75) d in the mFOLFOX6 group, which was much shorter than those in the other two groups.
All patients received TME surgery (28 underwent APR and 375 underwent sphincter-saving surgery).
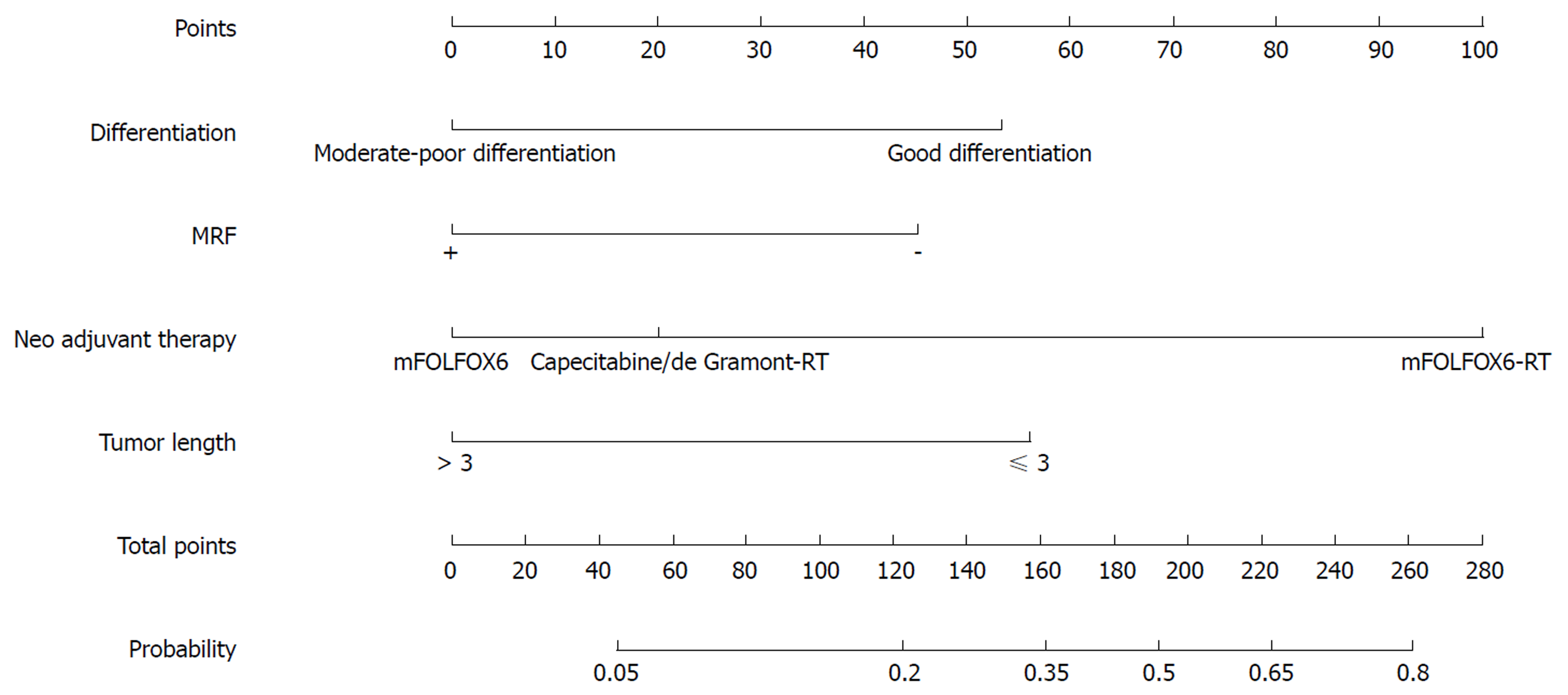
pCR patients and non-pCR patients did not differ significantly in terms of gender, BMI, CEA, NLR, HB, PLT, ApoA1, ApoB, cT, cN, or TCE in the univariate analysis (P > 0.05); however, significant differences were found for age, tumor differentiation, TL, DTAV, MRF status, interval, and NT regimen (Table 1). Statistically significant factors in the univariate logistic regression analysis (P ≤ 0.05) to predict pCR were entered into a multivariate analysis, in which NT regimen types (aP < 0.05), tumor differentiation (bP < 0.05), TL (cP < 0.05), and MRF status (dP < 0.05) were significantly associated with pCR probability (Table 2). For the NT regimens, the odds ratio (OR) was 5.339 [95% confidence interval (CI): 2.394-11.903] for the mFOLFOX6-RT regimen compared with the capecitabine/deGramont-RT regimen. The mFOLFOX6 regimen and capecitabine/deGramont-RT regimen did not differ significantly. For tumor differentiation, the OR was 2.966 (95%CI: 1.449-6.069) for well tumor differentiation compared with moderate-poor differentiation. For TL (> 3 cm) compared with TL (≤ 3 cm), the OR was 2.608 (95%CI: 1.347-5.052), and for MRF(-) compared with MRF(+), the OR was 2.729 (95%CI: 1.199-6.211).
| Variable | Non-pCR (n = 331) n P50 (P25-P75) | pCR (n = 72) n P50 (P25-P75) | pCR rate | P-value | |
| Gender | Male | 232 | 49 | 17.44% | 0.653 |
| Female | 96 | 23 | 19.33% | ||
| Age (yr) | ≤ 60 | 210 | 54 | 20.45% | 0.07 |
| > 60 | 119 | 18 | 13.14% | ||
| BMI (kg/cm2) | < 25 | 231 | 53 | 18.66% | 0.581 |
| ≥ 25 | 78 | 15 | 16.13% | ||
| Hemoglobin (g/L) | ≤ 125 | 109 | 16 | 12.80% | 0.126 |
| > 125 | 172 | 41 | 19.25% | ||
| NLR | > 3 | 37 | 11 | 22.92% | 0.227 |
| ≤ 3 | 244 | 46 | 15.86% | ||
| Platelet (× 109/L) | 237.5 (200.25-286.75) | 246 (200.5-268.5) | 0.981 | ||
| ApoA1 (g/L) | 1.29 (1.13-1.44) | 1.3 (1.15-1.52) | 0.454 | ||
| ApoB (g/L) | 0.98 (0.79-1.14) | 0.97 (0.82-1.19) | 0.382 | ||
| The interval (d) | 39 (22.25-54) | 50 (42-56.5) | 0 | ||
| CEA (ng/mL) | > 5 | 163 | 28 | 14.66% | 0.217 |
| ≤ 5 | 118 | 29 | 19.73% | ||
| Differentiation | Moderate-poor | 274 | 44 | 13.84% | 0.001 |
| Well | 44 | 20 | 31.25% | ||
| DTAV (cm) | < 5 | 140 | 41 | 22.65% | 0.024 |
| ≥ 5 | 191 | 31 | 13.96% | ||
| TL (cm) | > 3 | 237 | 41 | 14.75% | 0.001 |
| ≤ 3 | 73 | 30 | 29.13% | ||
| TCE | < 50% | 37 | 7 | 15.91% | 0.772 |
| ≥ 50% | 256 | 55 | 17.68% | ||
| cT | 2 | 16 | 5 | 23.81% | 0.405 |
| 3 | 234 | 56 | 19.31% | ||
| 4 | 54 | 8 | 12.90% | ||
| cN | + | 235 | 57 | 19.52% | 0.465 |
| - | 78 | 15 | 16.13% | ||
| MRF | - | 231 | 61 | 20.89% | 0.013 |
| + | 97 | 11 | 10.19% | ||
| NT regimen | Capecitabine/de Gramont-RT | 102 | 13 | 11.30% | 0 |
| mFOLFOX6 | 148 | 14 | 8.64% | ||
| mFOLFOX6-RT | 81 | 45 | 35.71% | ||
| Variable | P-value | OR | 95%CI | |
| Age (yr) | ≤ 60 | 0.703 | 0.873 | 0.434-1.756 |
| > 60 | 1 | |||
| Differentiation | Well | 0.003 | 2.966 | 1.449-6.069 |
| Moderate-poor | 1 | |||
| TL (cm) | ≤ 3 | 0.004 | 2.608 | 1.347-5.052 |
| > 3 | 1 | |||
| DTAV | ≥ 5 | 0.07 | 0.56 | 0.299-1.049 |
| < 5 | 1 | |||
| MRF | - | 0.017 | 2.729 | 1.199-6.211 |
| + | 1 | |||
| NT regimen | mFOLFOX6-RT | 0 | 5.339 | 2.394-11.903 |
| mFOLFOX6 | 0.402 | 1.821 | 0.449-7.387 | |
| Capecitabine/de Gramont-RT | 1 | |||
| The interval | 0.093 | 1.029 | 0.995-1.064 | |
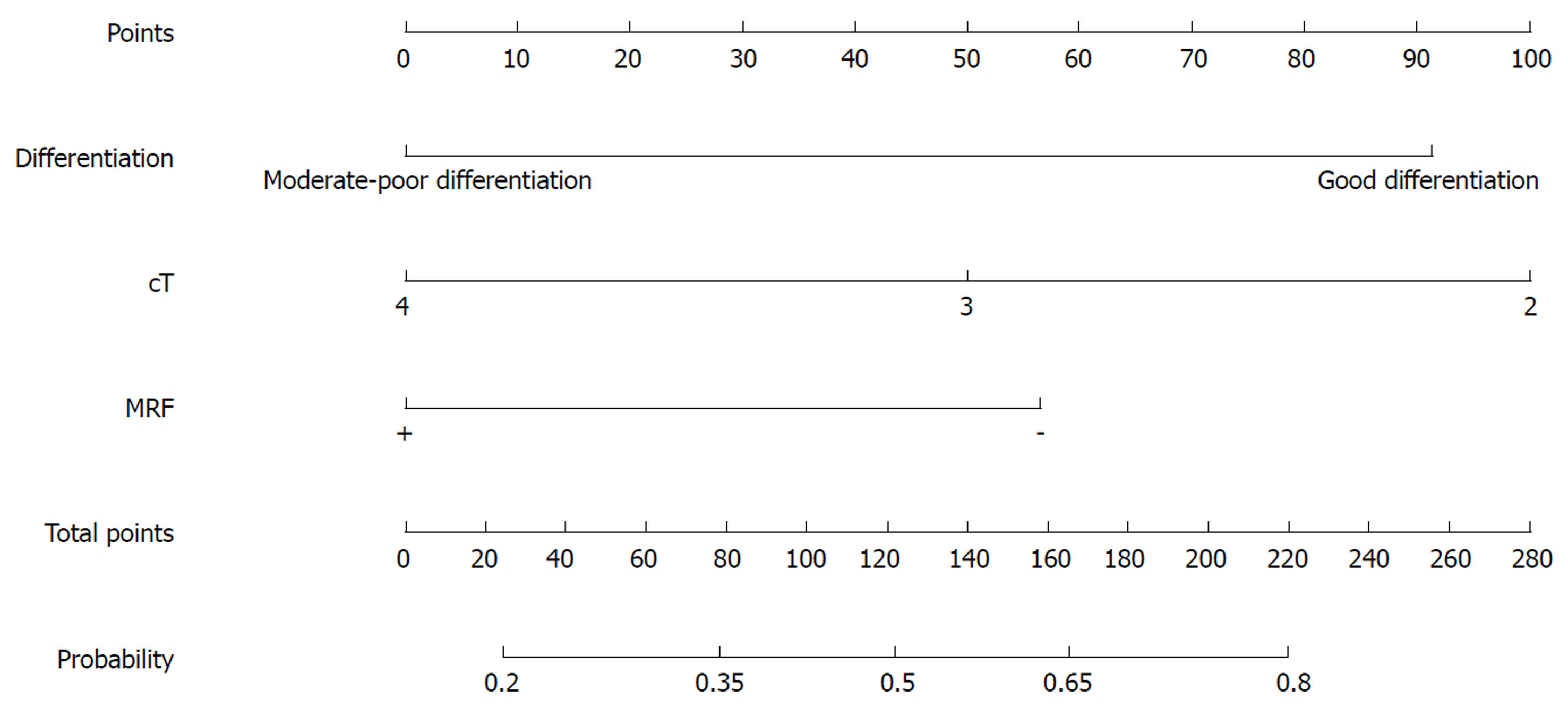
Patients with good downstaging and bad downstaging did not significantly differ in terms of age, gender, BMI, NLR, HB, PLT, ApoA1, ApoB, cN, TCE, the interval, or NT regimen in the univariate analysis (P > 0.05); however, significant differences were found for CEA, tumor differentiation, DTAV, TL, cT, and MRF status in the univariate logistic regression analysis for good downstaging (Table 3). In the multivariate analysis, tumor differentiation (eP < 0.05), MRF statuses (fP < 0.05), and cT (gP < 0.05) were significantly associated with the probability of good downstaging (Table 4). The OR was 4.814 (95%CI: 2.343-9.892) for well differentiation compared with moderate-poor differentiation, 4.226 (95%CI: 1.894-9.426) for MRF(-) compared with MRF(+), and 0.248 (95%CI: 0.063-0.974) for cT3 compared with cT2.
| Variable | Bad downstaging (n = 226) n P50 (P25-P75) | Good downstaging (n = 177) n P50 (P25-P75) | Good downstaging rate | P-value | |
| Gender | Male | 164 | 117 | 41.64% | 0.106 |
| Female | 59 | 60 | 50.42% | ||
| Age (yr) | ≤ 60 | 144 | 120 | 45.45% | 0.462 |
| > 60 | 80 | 57 | 41.61% | ||
| BMI (kg/cm2) | < 25 | 159 | 125 | 44.01% | 0.475 |
| ≥ 25 | 56 | 37 | 39.78% | ||
| Hemoglobin (g/L) | ≤ 125 | 69 | 56 | 44.80% | 0.426 |
| > 125 | 127 | 86 | 40.38% | ||
| NLR | > 3 | 27 | 21 | 43.75% | 0.792 |
| ≤ 3 | 169 | 121 | 41.72% | ||
| Platelet (×109/L) | 241 (207-294) | 236 (193-272.25) | 0.125 | ||
| ApoA1 (g/L) | 1.27 (1.13-1.44) | 1.31 (1.15-1.48) | 0.228 | ||
| ApoB (g/L) | 0.98 (0.79-1.13) | 0.97 (0.79-1.18) | 0.88 | ||
| The interval | 39 (23-54) | 48 (25.75-55) | 0.062 | ||
| CEA (ng/mL) | > 5 | 125 | 66 | 34.55% | 0.002 |
| ≤ 5 | 71 | 76 | 51.70% | ||
| Differentiation | Moderate-poor | 194 | 124 | 38.99% | 0 |
| Well | 20 | 44 | 68.75% | ||
| DTAV (cm) | < 5 | 85 | 96 | 53.04% | 0.001 |
| ≥ 5 | 141 | 81 | 36.49% | ||
| TL (cm) | > 3 | 167 | 111 | 39.93% | 0.002 |
| ≤ 3 | 44 | 59 | 57.28% | ||
| TCE | < 50% | 23 | 21 | 47.73% | 0.508 |
| ≥ 50% | 179 | 132 | 42.44% | ||
| cT | 2 | 4 | 17 | 80.95% | 0 |
| 3 | 160 | 130 | 44.83% | ||
| 4 | 46 | 16 | 25.81% | ||
| cN | + | 166 | 126 | 43.15% | 0.074 |
| - | 43 | 50 | 53.76% | ||
| MRF | - | 142 | 150 | 51.37% | 0 |
| + | 81 | 27 | 25.00% | ||
| NT regimen | Capecitabine/de Gramont-RT | 64 | 51 | 44.35% | 0.061 |
| mFOLFOX6-RT | 61 | 65 | 51.59% | ||
| mFOLFOX6 | 101 | 61 | 37.65% | ||
| Variable | P-value | OR | 95%CI | |
| CEA (ng/mL) | ≤ 5 | 0.095 | 1.565 | 0.925-2.647 |
| > 5 | 1 | |||
| Differentiation | Well | 0 | 4.814 | 2.343-9.892 |
| Moderate-poor | 1 | |||
| DTAV (cm) | ≥ 5 | 0.052 | 0.588 | 0.345-1.004 |
| < 5 | 1 | |||
| TL (cm) | ≤ 3 | 0.9 | 1.04 | 0.566-1.909 |
| > 3 | 1 | |||
| cT | 3 | 0.046 | 0.248 | 0.063-0.974 |
| 4 | 0.127 | 0.282 | 0.056-1.434 | |
| 2 | 1 | |||
| MRF | - | 0 | 4.226 | 1.894-9.426 |
| + | 1 | |||
Because the type of NT regimen was a statistically significant factor for predicting pCR probability, patients were divided into three subgroups (the capecitabine/deGramont-RT, mFOLFOX6, and mFOLFOX6-RT groups) based on the NT regimen. Table 5 shows the distribution of pretreatment clinical parameters in the NT regimen groups. No differences were found in any factors between the three groups except age (hP < 0.05) and DTAV (iP < 0.05). In the univariate analysis of the capecitabine/deGramont-RT group, NLR was the only significant factor for predicting pCR probability (Table 6). NLR (> 3) (jP < 0.05) was the only significant factor, with an OR of 4.278 (95%CI: 1.051-17.413) compared with NLR ≤ 3 in the further multivariate analysis (Table 7). We could not develop a nomogram to predict pCR probability in this case.
| Variable | Capecitabine/deGramont-RT n (%) P50 (P25-P75) | mFOLFOX6 n (%) P50 (P25-P75) | mFOLFOX6-RT n (%) P50 (P25-P75) | P-value | |
| Gender | Male | 76 (66.67) | 107 (66.88) | 98 (77.78) | 0.083 |
| Female | 38 (33.33) | 53 (33.13) | 28 (22.22) | ||
| Age (yr) | ≤ 60 | 66 (57.39) | 102 (63.75) | 96 (76.19) | 0.007 |
| > 60 | 49 (42.61) | 58 (36.25) | 30 (23.81) | ||
| BMI (kg/cm2) | < 25 | 83 (76.85) | 118 (76.62) | 83 (72.17) | 0.641 |
| ≥ 25 | 25 (23.15) | 36 (23.38) | 32 (27.83) | ||
| Hemoglobin (g/L) | ≤ 125 | 34 (34.34) | 55 (39.29) | 36 (36.36) | 0.729 |
| > 125 | 65 (65.66) | 85 (60.71) | 63 (63.64) | ||
| NLR | > 3 | 16 (16.16) | 17 (12.14) | 15 (15.15) | 0.646 |
| ≤ 3 | 83 (83.84) | 123 (87.86) | 84 (84.85) | ||
| CEA (ng/mL) | > 5 | 56 (56.57) | 79 (56.43) | 56 (56.57) | 1 |
| ≤ 5 | 43 (43.43) | 61 (43.57) | 43 (43.43) | ||
| Differentiation | Moderate-poor | 90 (81.08) | 135 (87.1) | 93 (80.17) | 0.246 |
| Well | 21 (18.92) | 20 (12.9) | 23 (19.83) | ||
| DTAV (cm) | < 5 | 61 (53.04) | 62 (38.27) | 58 (46.03) | 0.049 |
| ≥ 5 | 54 (46.96) | 100 (61.73) | 68 (53.97) | ||
| TL (cm) | > 3 | 82 (74.55) | 105 (70) | 91 (75.21) | 0.572 |
| ≤ 3 | 28 (25.45) | 45 (30) | 30 (24.79) | ||
| TCE | < 50% | 10 (10) | 22 (15.28) | 12 (10.81) | 0.389 |
| ≥ 50% | 90 (90) | 122 (84.72) | 99 (89.19) | ||
| cN | + | 84 (75) | 110 (73.33) | 98 (79.67) | 0.462 |
| - | 28 (25) | 40 (26.67) | 25 (20.33) | ||
| MRF | - | 85 (74.56) | 119 (74.38) | 88 (69.84) | 0.627 |
| + | 29 (25.44) | 41 (25.63) | 38 (30.16) | ||
| pCR | Non-pCR | 102 (88.7) | 148 (91.36) | 81 (64.29) | 0 |
| pCR | 13 (11.3) | 14 (8.64) | 45 (35.71) | ||
| Downstaging | Bad | 64 (55.65) | 101 (62.35) | 61 (48.41) | 0.061 |
| Good | 51 (44.35) | 61 (37.65) | 65 (51.59) | ||
| cT | 2 | 9 (8.26) | 10 (6.85) | 2 (1.69) | 0.19 |
| 3 | 85 (77.98) | 112 (76.71) | 93 (78.81) | ||
| 4 | 15 (13.76) | 24 (16.44) | 23 (19.49) | ||
| PLT (×109/L) | 230 (188.75-267.25) | 236.5 (200.25-290.75) | 244 (212-281) | 0.168 | |
| ApoA1 (g/L) | 1.31 (1.14-1.47) | 1.29 (1.12-1.44) | 1.28 (1.15-1.5) | 0.73 | |
| ApoB (g/L) | 0.97 (0.8-1.09) | 0.98 (0.78-1.14) | 0.98 (0.81-1.21) | 0.425 | |
| The interval | 54 (49-58.25) | 22 (18-25.75) | 52 (47-59) | 0 | |
| Variable | Non-pCR (n = 102) n P50 (P25-P75) | pCR (n = 13) n P50 (P25-P75) | pCR rate | P-value | |
| Gender | Male | 70 | 6 | 7.89% | 0.096 |
| Female | 31 | 7 | 18.42% | ||
| Age (yr) | ≤ 60 | 56 | 10 | 15.15% | 0.131 |
| > 60 | 46 | 3 | 6.12% | ||
| BMI (kg/cm2) | < 25 | 75 | 8 | 9.64% | 0.375 |
| ≥ 25 | 21 | 4 | 16.00% | ||
| Hemoglobin (g/L) | ≤ 125 | 33 | 1 | 2.94% | 0.087 |
| > 125 | 56 | 9 | 13.85% | ||
| NLR | > 3 | 12 | 4 | 25.00% | 0.031 |
| ≤ 3 | 77 | 6 | 7.23% | ||
| PLT (×109/L) | 228 (188.25-266.75) | 252 (188.75-319) | 0.338 | ||
| ApoA1 (g/L) | 1.3 (1.14-1.48) | 1.34 (1.11-1.46) | 0.912 | ||
| ApoB (g/L) | 0.97 (0.8-1.09) | 0.92 (0.78-1.02) | 0.667 | ||
| The interval | 53.97 ± 8.94 | 52.38 ± 10.79 | 0.588 | ||
| CEA (ng/mL) | > 5 | 51 | 5 | 8.93% | 0.659 |
| ≤ 5 | 38 | 5 | 11.63% | ||
| Differentiation | Moderate-poor | 82 | 8 | 8.89% | 0.177 |
| Well | 17 | 4 | 19.05% | ||
| DTAV (cm) | < 5 | 52 | 9 | 14.75% | 0.214 |
| ≥ 5 | 50 | 4 | 7.41% | ||
| TL (cm) | > 3 | 74 | 8 | 9.76% | 0.252 |
| ≤ 3 | 23 | 5 | 17.86% | ||
| TCE | ≤ 50% | 10 | 0 | 0.00% | 0.241 |
| > 50% | 79 | 11 | 12.22% | ||
| cT | 2 | 7 | 2 | 22.22% | 0.521 |
| 3 | 75 | 10 | 11.76% | ||
| 4 | 14 | 1 | 6.67% | ||
| cN | + | 73 | 11 | 13.10% | 0.394 |
| - | 26 | 2 | 7.14% | ||
| MRF | - | 74 | 11 | 12.94% | 0.377 |
| + | 27 | 2 | 6.90% | ||
| Variable | P value | OR | 95%CI | |
| NLR | > 3 | 0.042 | 4.278 | 1.051-17.413 |
| ≤ 3 | 1 | |||
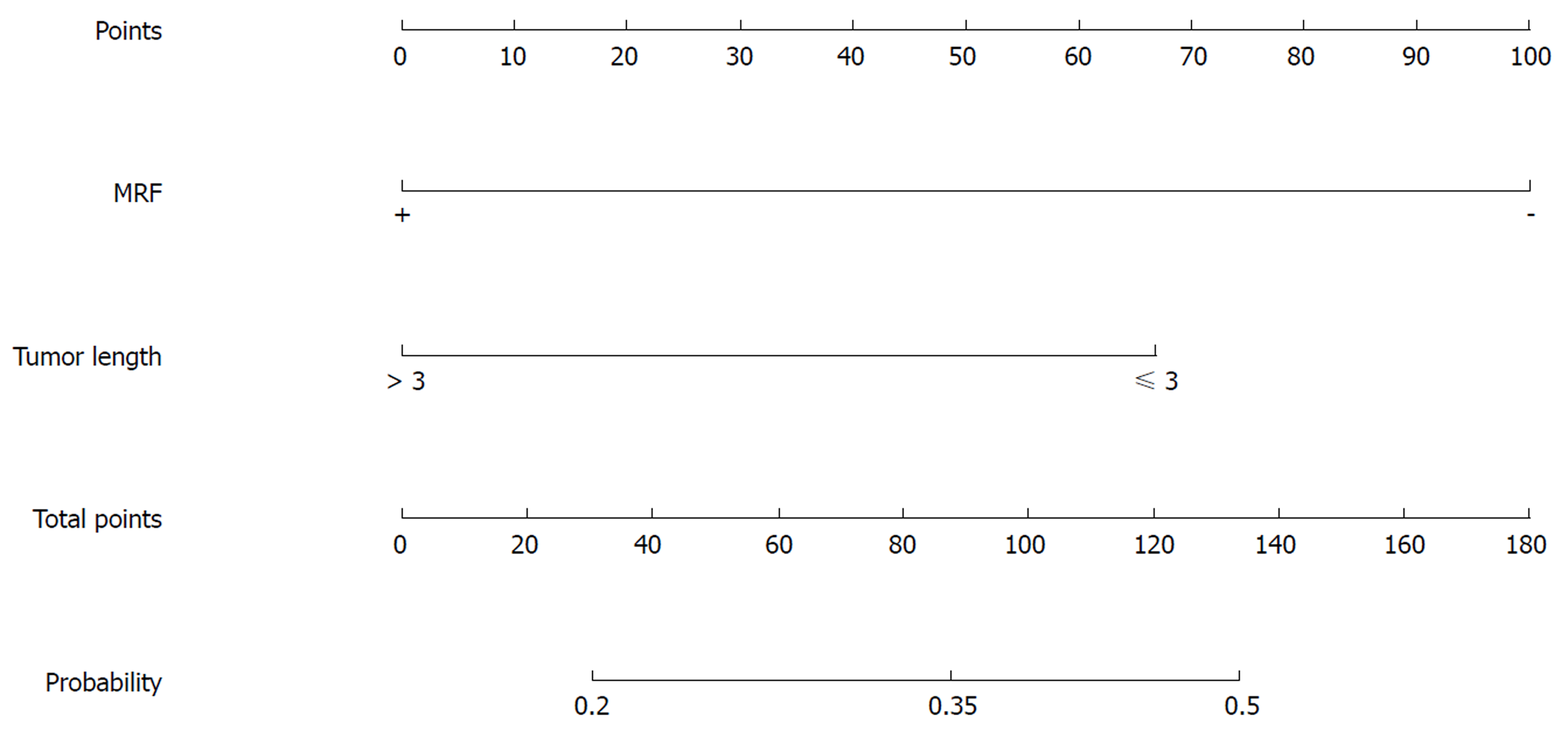
Table 8 shows that TL and MRF status were significant factors predicting pCR probability in the univariate analysis of the mFOLFOX6-RT regimen. TL (kP < 0.05) and MRF(+) (lP < 0.05) were significant factors, with an OR of 2.452 (95%CI: 1.015-5.926) for TL(≤ 3 cm) compared with TL(> 3 cm) and an OR of 3.829 (95%CI: 1.42-10.325) for MRF(-) compared with MRF(+) in the further multivariate analysis (Table 9).
| Variable | Non-pCR (n = 81) n P50 (P25-P75) | pCR (n = 45) n P50 (P25-P75) | pCR rate | P-value | |
| Gender | Male | 63 | 35 | 35.71% | 1 |
| Female | 18 | 10 | 35.71% | ||
| Age (yr) | ≤ 60 | 62 | 34 | 35.42% | 0.901 |
| > 60 | 19 | 11 | 36.67% | ||
| BMI (kg/cm2) | < 25 | 49 | 34 | 40.96% | 0.202 |
| ≥ 25 | 23 | 9 | 28.13% | ||
| Hemoglobin (g/L) | ≤ 125 | 23 | 13 | 36.11% | 0.905 |
| > 125 | 41 | 22 | 34.92% | ||
| NLR | > 3 | 9 | 6 | 40.00% | 0.683 |
| ≤ 3 | 55 | 29 | 34.52% | ||
| PLT (×109/L) | 246.5 (214.25-289.75) | 239 (197-269) | 0.22 | ||
| ApoA1 (g/L) | 1.26 (1.15-1.44) | 1.3 (1.15-1.55) | 0.453 | ||
| ApoB (g/L) | 1 (0.81-1.24) | 0.97 (0.81-1.17) | 0.725 | ||
| The interval | 51.5 (43-58.75) | 54 (50-62) | 0.116 | ||
| CEA (ng/mL) | > 5 | 40 | 16 | 28.57% | 0.107 |
| ≤ 5 | 24 | 19 | 44.19% | ||
| Differentiation | Moderate-poor | 63 | 30 | 32.26% | 0.311 |
| Well | 13 | 10 | 43.48% | ||
| DTAV (cm) | < 5 | 33 | 25 | 43.10% | 0.11 |
| ≥ 5 | 48 | 20 | 29.41% | ||
| TL (cm) | > 3 | 64 | 27 | 29.67% | 0.008 |
| ≤ 3 | 13 | 17 | 56.67% | ||
| TCE | < 50% | 8 | 4 | 33.33% | 0.944 |
| ≥ 50% | 65 | 34 | 34.34% | ||
| cT | 2 | 0 | 2 | 100.00% | 0.061 |
| 3 | 58 | 35 | 37.63% | ||
| 4 | 18 | 5 | 21.74% | ||
| cN | + | 62 | 36 | 36.73% | 0.946 |
| - | 16 | 9 | 36.00% | ||
| MRF | - | 49 | 39 | 44.32% | 0.002 |
| + | 32 | 6 | 15.79% | ||
| Variable | P value | OR | 95%CI | |
| TL (cm) | ≤ 3 | 0.046 | 2.452 | 1.015-5.926 |
| > 3 | 1 | |||
| MRF | - | 0.008 | 3.829 | 1.42-10.325 |
| + | 1 | |||
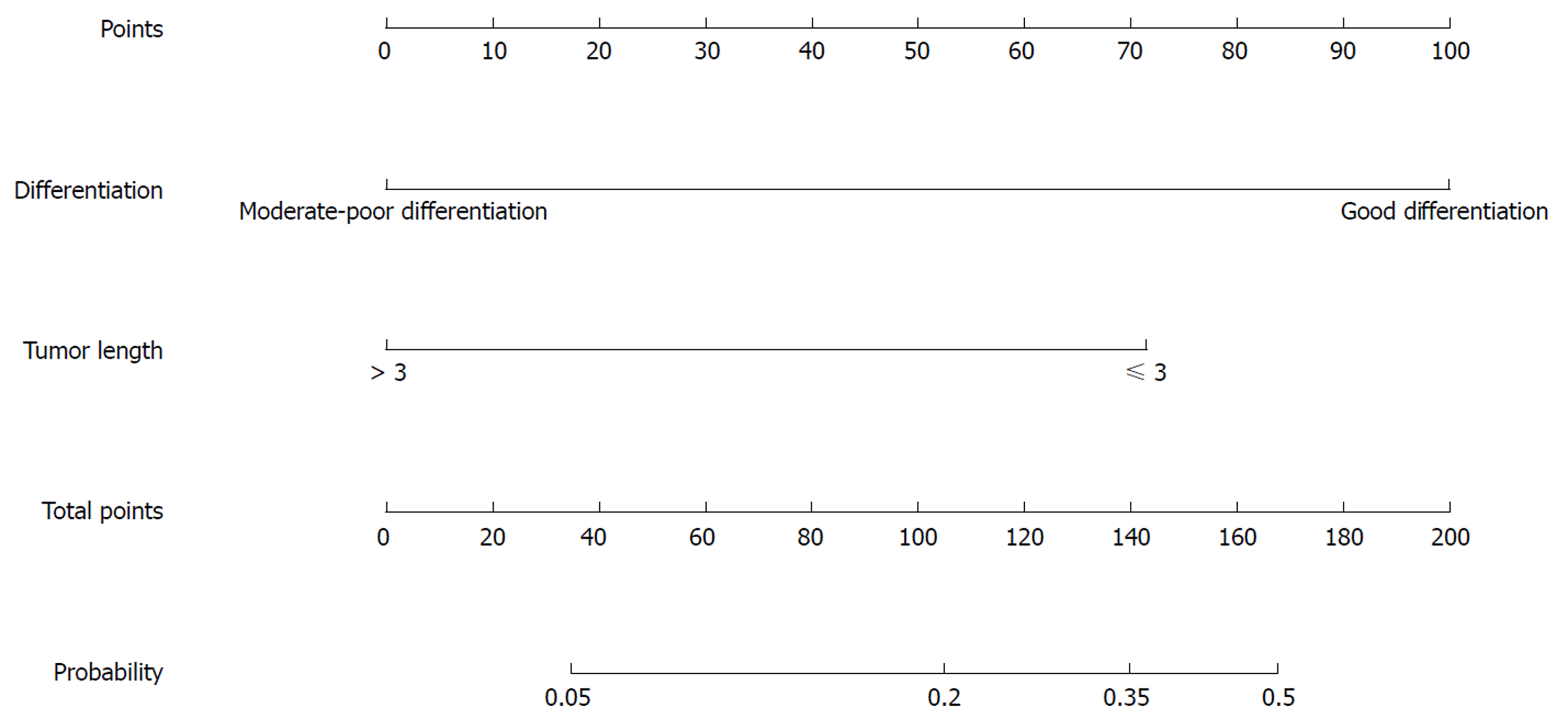
In the univariate analysis of the mFOLFOX6 regimen, tumor differentiation and TL were significant factors for predicting pCR probability (Table 10). Further multivariate analysis showed that differentiation (mP < 0.05) and TL (nP < 0.05) were significant factors, with an OR of 8.881 (95%CI: 2.263-34.85) for well tumor differentiation compared with moderate-poor differentiation and an OR of 4.805 (95%CI: 1.25-18.466) for TL (≤ 3 cm) compared with TL (> 3 cm) (Table 11).
| Variable | Non-pCR (n = 148) n P50 (P25-P75) | pCR (n = 14) n P50 (P25-P75) | pCR rate | P-value | |
| Gender | Male | 99 | 8 | 7.48% | 0.418 |
| Female | 47 | 6 | 11.32% | ||
| Age (yr) | ≤ 60 | 92 | 10 | 9.80% | 0.532 |
| > 60 | 54 | 4 | 6.90% | ||
| BMI (kg/cm2) | < 25 | 107 | 11 | 9.32% | 0.477 |
| ≥ 25 | 34 | 2 | 5.56% | ||
| Hemoglobin (g/L) | ≤ 125 | 53 | 2 | 3.64% | 0.093 |
| > 125 | 75 | 10 | 11.76% | ||
| NLR | > 3 | 16 | 1 | 5.88% | 0.673 |
| ≤ 3 | 112 | 11 | 8.94% | ||
| PLT (×109/L) | 127.5 (117.5-139) | 137.5 (127-142.25) | 0.82 | ||
| ApoA1 (g/L) | 1.28 (1.11-1.42) | 1.3 (1.15-1.46) | 0.542 | ||
| ApoB (g/L) | 0.96 (0.77-1.13) | 1.13 (0.86-1.3) | 0.051 | ||
| The interval | 21.5 (18-25) | 25 (19.25-26.75) | 0.09 | ||
| CEA (ng/mL) | > 5 | 72 | 7 | 8.86% | 0.889 |
| ≤ 5 | 56 | 5 | 8.20% | ||
| Differentiation | Moderate-poor | 129 | 6 | 4.44% | 0 |
| Well | 14 | 6 | 30.00% | ||
| DTAV (cm) | < 5 | 55 | 7 | 11.29% | 0.345 |
| ≥ 5 | 93 | 7 | 7.00% | ||
| TL (cm) | > 3 | 99 | 6 | 5.71% | 0.02 |
| ≤ 3 | 37 | 8 | 17.78% | ||
| TCE | < 50% | 19 | 3 | 13.64% | 0.413 |
| ≥ 50% | 112 | 10 | 8.20% | ||
| cT | 2 | 9 | 1 | 10.00% | 0.974 |
| 3 | 101 | 11 | 9.82% | ||
| 4 | 22 | 2 | 8.33% | ||
| cN | + | 100 | 10 | 9.09% | 0.866 |
| - | 36 | 4 | 10.00% | ||
| MRF | - | 108 | 11 | 9.24% | 0.707 |
| + | 38 | 3 | 7.32% | ||
| Variable | P-value | OR | 95%CI | |
| Differentiation | Well | 0.002 | 8.881 | 2.263-34.85 |
| Moderate-poor | 1 | |||
| TL | ≤ 3 | 0.022 | 4.805 | 1.25-18.466 |
| > 3 | 1 | |||
Nomograms were developed based on the significant factors in the multivariate logistic regression analysis. The nomogram for predicting pCR probability showed that NT regimen and tumor differentiation influenced the probability of pCR, followed by TL and MRF status (Figure 1). When developing the nomogram to predict the probability of good downstaging, tumor differentiation and MRF status were the most important, followed by cT (Figure 2). We attempted to develop three nomograms to predict pCR probability based on NT regimens, because only one significant factor was found for the capecitabine/deGramont-RT regimen, we could not develop a nomogram. MRF status and TL were the significant factors for the mFOLFOX6-RT group (Figure 3). For the mFOLFOX6 group, tumor differentiation and TL were the significant factors in the nomogram for predicting pCR probability (Figure 4). Using the nomograms, we could easily calculate the probability of pCR and ypTNM (0-I), and we calculated pCR probabilities based on the NT regimens.
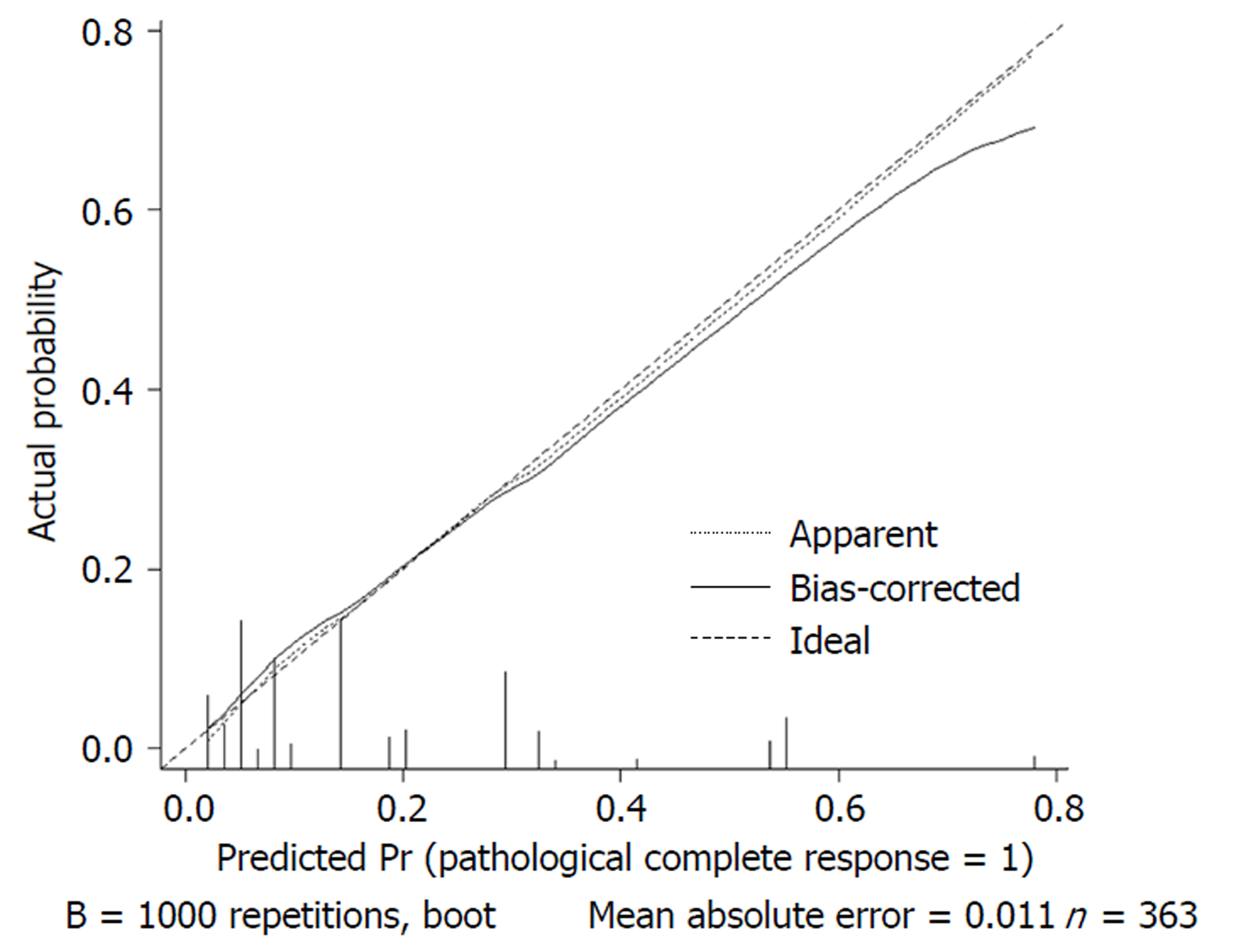
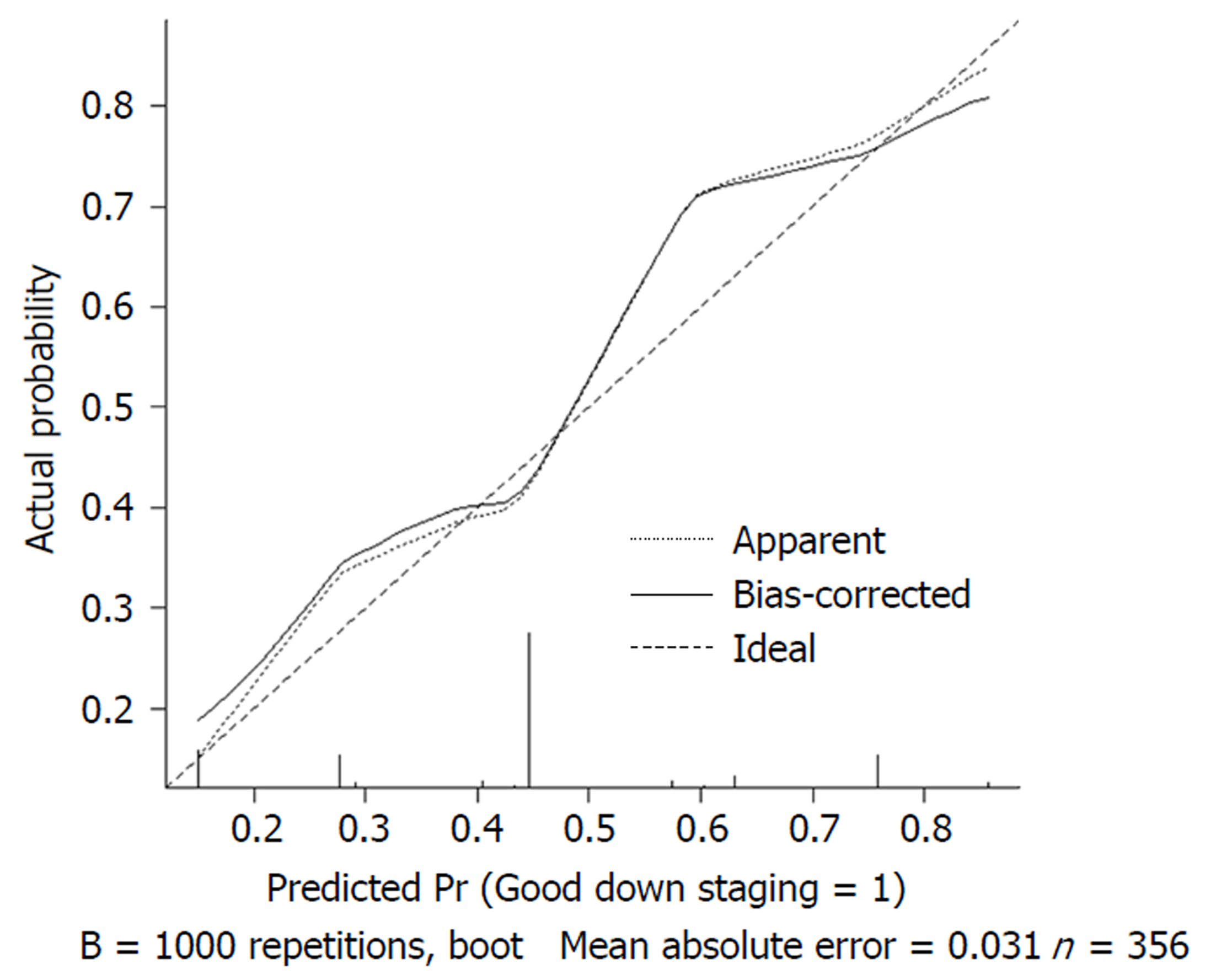
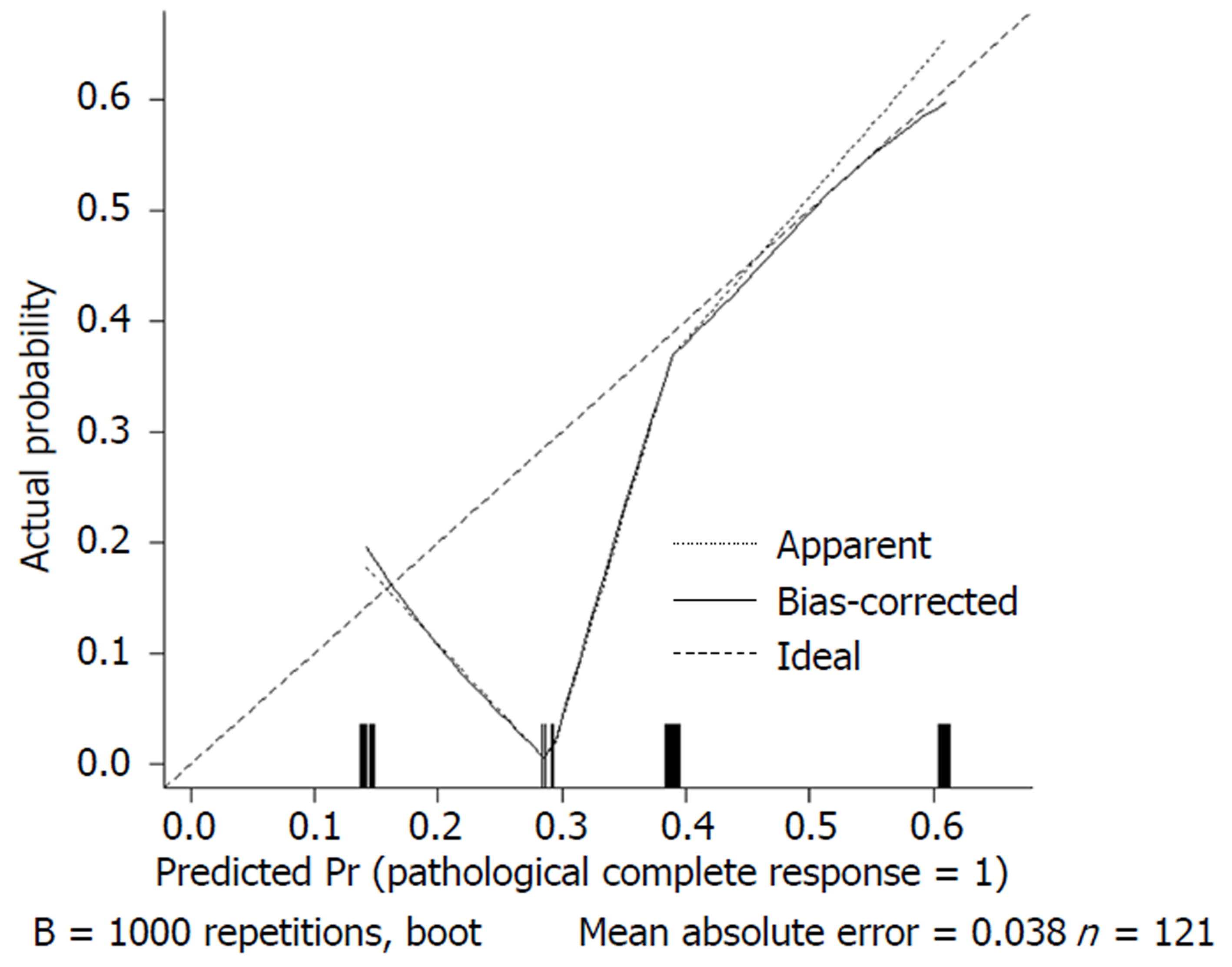
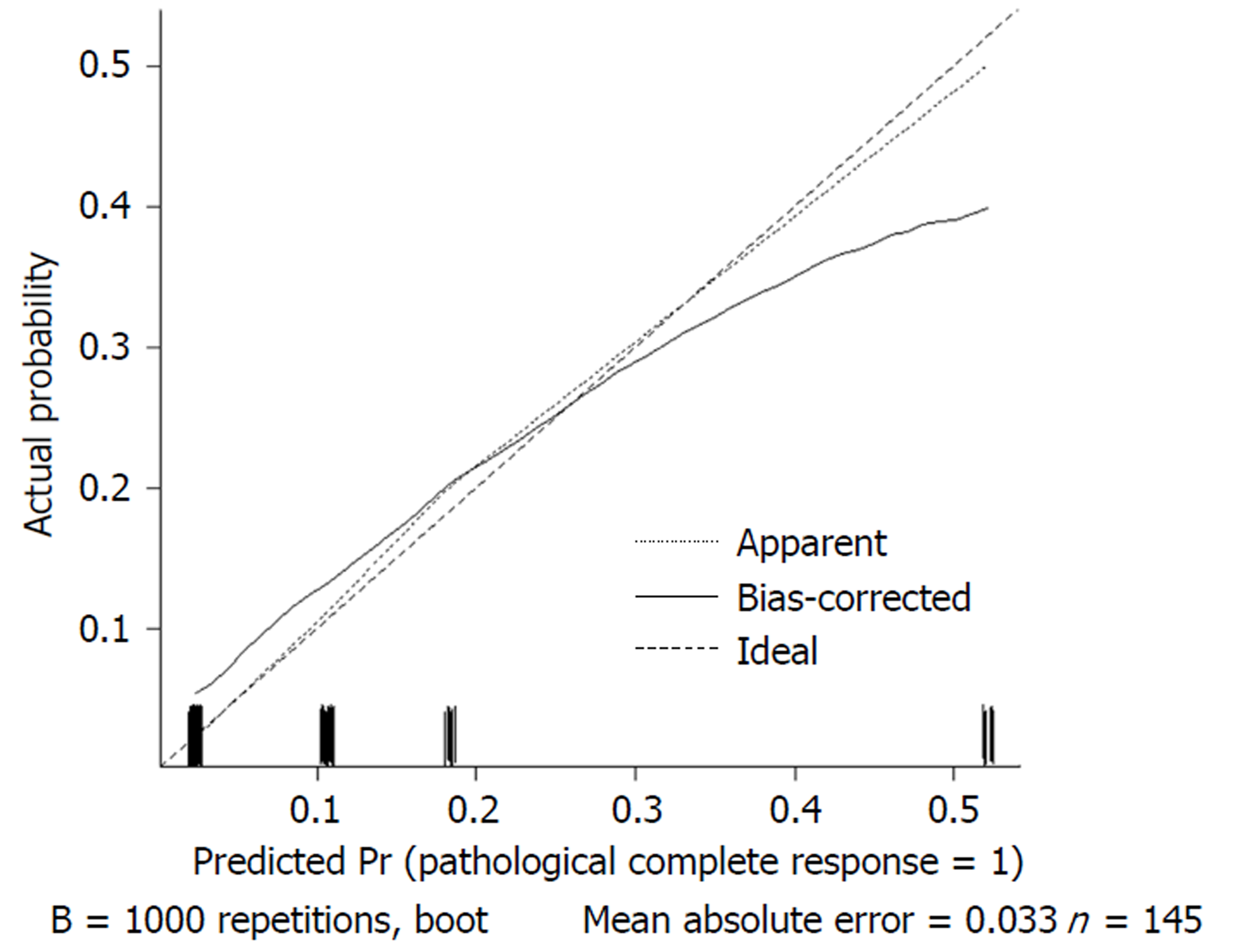
We used 1000 bootstrap resamples to compute an adjusted C-index, which was 79.34% for predicting pCR (95%CI: 73.48%-85.21%) for all patients, with a C-index of 69.85% (95%CI: 60.94%-78.76%) for the mFOLFOX6-RT group and 83.39% (95%CI: 67.26%-93.52%) for the mFOLFOX6 group. For predicting good downstaging, the adjusted C-index was 68.08% (95%CI: 63.08%-73.07%) for all patients. Calibration curves between predicted and actual observations by internal validation demonstrated that these nomograms showed good statistical performance for predicting the probability of pCR and good downstaging. Figures 5-8 show the calibration curve between the predicted and actual observations by internal validation and demonstrates that these nomograms showed good statistical performance for predicting the probability of pCR and good downstaging.
At present, preoperative NT is the standard treatment for patients with LARC. Patients who respond well to preoperative treatment have shown to have an excellent long-term prognosis. Knowledge of these factors ultimately leads to individualized treatment strategies; for example, patients who do not respond to the usual management can choose an aggressive preoperative regimen before NT. Conversely, to accurately determine an excellent pathological response after NT, surgeons may choose to perform local excision or a “watch and wait” strategy. In some cases, radical surgical resection may not benefit for some patients who achieve a good response because radical surgical resection may be associated with high rates of temporary or permanent stomas, defecatory disorders, urinary and sexual dysfunction, and unnecessary mortality[31,32]. pCR after NT is reported to have an excellent long-term prognosis irrespective of the treatment strategy, so noninvasive treatment strategies, such as a “watch and wait” strategy, have become more popular for patients who achieve a good response[33,34]. Thus, understanding the factors that predict the pathological response to NT is becoming crucial.
Our study identified clinical variables related to the pCR and good downstaging of LARC patients after NT. In the nomogram, we demonstrated that type of NT regimen, tumor differentiation, MRF status, and TL predicted pCR, whereas tumor differentiation, MRF status, and cT predicted good downstaging.
In our model, the mFOLFOX6-RT group had a higher probability of pCR compared with the capecitabine/de Gramont-RT group. We acknowledge that a potential selection bias may contribute to this high pCR rate. The data missing were more frequently in patients not reaching pCR than those with pCR, possibly resulting from more attentions pCR-patients got in clinical practice, follow-up, or research work. The pCR rate was 35.7% for mFOLFOX6-RT, which is higher than that of FOWARC[26,27]. It is expected since this is a single-center statistic result, while the FORWARC trial is a muli-center study. Although the benefits of oxaliplatin have not been demonstrated and it is not part of standard NT regimens, oxaliplatin is a standard component of chemotherapy for treating colon cancer[35]. Importantly, it has been reported in more and more studies[36,37] that the regimen combining mFOLFOX 6 with RT is getting a higher pCR rate of 38% in a clinical trial published in Lancet Oncology[38]. However, the role of oxaliplatin adding to fluorouracil-based neoadjuvant chemoradiotherapy (CRT) is unclear for LARC patients, and more studies are needed in the future.
pCR probabilities did not significantly differ between the capecitabine/deGramont-RT and themFOLFOX6 groups. Additionally, NT regimen was not a significant factor for predicting the probability of good downstaging. To avoid radiotherapeutic harm to LARC patients, the use of neoadjuvant chemotherapy alone has been proposed. Our model showed that patients treated with the mFOLFOX6 regimen alone had an acceptable probability of pCR and good downstaging. Thus, some chemosensitive patients can avoid radiation therapy.
Tumor differentiation was associated with both pCR and good downstaging. Well differentiation was associated with a higher pCR probability, which is consistent with previous studies[23,39], and was related to good downstaging compared with moderate-poor differentiation. Patients with a well differentiated tumor have a higher pCR probability indicating that a mild NT regimen, local resection, or “watch and wait” strategy can be considered after NT. Patients with moderate-poor differentiation may have a poor likelihood of pCR and good downstaging indicating that a “watch and wait” strategy requires careful selection.
Factors associated with pCR and good downstaging both included MRF status. MRF(+) implies that the tumor is aggressive, and even after NT, patients with MRF(+) may have a poor likelihood of pCR and good downstaging, indicating that an enhanced NT regimen and radical surgery are needed and a “watch and wait” strategy requires careful selection. While patients with MRF(-) may have a higher pCR probability indicating that a mild NT regimen, local resection, or “watch and wait” strategy can be considered after NT.
TL was also a significant factor in the multivariate logistic regression analysis for predicting pCR probability in all patients. Van Stiphout et al[40] reported that TL was related to the probability of pCR after NT, although this study was based on data from positron emission tomography (PET)-CT results. TL (> 3 cm) implies an aggressive tumor, and even after NT, patients with TL (> 3 cm) may have a lower pCR probability indicating that an enhanced NT regimen and radical surgery are needed. While for patients with TL (≤ 3 cm) may have a higher pCR probability indicating that a mild NT regimen, local resection, or “watch and wait” strategy can be considered after NT.
For predicting the probability of good downstaging, cT was also a significant factor in the multivariate logistic regression analysis. In a study performed by Joye et al[41], a low cT stage was linked with ypT0-1N0, and it, together with other factors, could be used as a selection tool for organ-preserving strategies. Our study also showed that low cT stage was more likely to achieve good downstaging with NT, and indicated that less invasive surgery can be selected.
For the capecitabine/de Gramont-RT regimen, the only significant factor was the NLR. Kim et al[42] showed that an elevated NLR before CRT can be used to predict poor tumor response and adverse prognostic factors. As lymphocytes decrease and neutrophils increase, NLR affects the adverse tumor reaction and adverse prognosis. Our study showed that the NLR before NT was related to better pathological responses to the capecitabine/deGramont-RT regimen; thus, further studies are needed to validate the relationship between NLR and pathological response to NT.
For the mFOLFOX6-RT regimen, the significant factors for predicting pCR probability were MRF status and TL. MRF(+) and long TL indicated that the tumor was aggressive and patients have heavy tumor load, and were related to poor neoadjuvant pathological responses. Patients with moderate-poor differentiation and TL (> 3 cm) have a lower pCR probability indicating that the efficacy of CRT is poor for these patients, and radical surgery can be directly selected without NT to avoid complications caused by CRT.
For the mFOLFOX6 regimen, the nomogram for predicting pCR probability showed that differentiation and TL were significant factors. Poor differentiation and long TL indicated an aggressive tumor, and they were related to a poor neoadjuvant pathological response. Patients with moderate-poor differentiation and TL (> 3 cm) will have a lower probability of pCR indicating that radical surgery after NT is needed, or mFOLFOX6-RT regimen is chosen to increase pCR probability. However, good differentiation and short TL were related to a good neoadjuvant pathological response and high probability of pCR indicating that local resection or a “watch and wait” strategy can be chosen.
To the best of our knowledge, our study is the first to use different NT regimen types to predict a pathological response. We established an accurate model with easily obtained variables to predict the probability of pCR and good downstaging. Our analysis was also strengthened through cross-validation. These models can be used to assist with individualized therapy as follows. For LARC patients expected to have a poor pathological response, NT and NT-related harm can be avoided. For patients expected to have good pathological responses to chemotherapy alone, radiotherapy can be avoided. For patients who are not expected to have good pathological response from a standard NT regimen, an enhanced mFOFOLX6-RT regimen can be considered. For patients who are not expected to have good pathological response from an enhanced regimen, radical surgery can be directly chosen without NT to avoid complications caused by CRT. For patients with a high probability of pCR after NT, local resection or a “watch and wait” strategy can be used to avoid complications.
Our analysis had several limitations. First, this was a retrospective study, in which some factors associated with pCR were unavailable, such as smoking status, molecular subtype and so on. Second, mFOLFOX6 and mFOLFOX6-RT are not the standard regimens for LARC, and both regimens remain in the clinical trial phase. Finally, our nomograms are based on the experience of our single institution. These results must be validated in a group of independent external institutions.
The nomograms established in our study can be used to evaluate the probability of a pathological responses before NT and after NT. However, additional studies are required to answer clinical questions, regarding which patients can be treated only with neoadjuvant chemotherapy, which patients need oxaliplatin added to the neoadjuvant CRT, which patients need radical surgery, which patients can undergo local excision, and which patients can be managed with a “watch and wait” strategy after achieving a good response.
We established accurate nomograms to predicting the pathological responses to different preoperative NT regimens based on pretreatment parameters for LARC patients. These nomograms can be used to distinguish patient types and facilitate developing individualized treatments.
In recent decades, neoadjuvant therapy (NT) has been the standardized treatment for locally advanced rectal cancer (LARC). Approximately 8-35% of patients with LARC who received NT were reported to have achieved a complete pathological response (pCR). If the pathological response can be accurately predicted, these patients may not need surgery. In addition, no response after NT implies that the tumor is destructive, resistant to both chemotherapy and radiotherapy, and prone to having a high metastatic potential.
Few models or nomograms have been established and even fewer are used clinically to predict a good pathological response after NT for LARC. Therefore, developing accurate models to predict pathological response (PR) has great clinical significance and can help achieve individualized treatment in LARC patients.
Our goal was to establish nomograms that can be used to assist with individualized therapy as follows: for which patients NT and NT-related harm can be avoided; which patients will have good pathological responses to chemotherapy alone and radiotherapy can be avoided; which patients will have a good pathological response from a standard NT regimen, which patients need an enhanced mFOFOLX6-RT regimen; and which patients can use local resection or a “watch and wait” strategy to avoid complications. Solving these problems may aid in clinical treatment choices.
Our main objective was to establish nomograms for predicting a pathological response to different NT regimens based on pretreatment parameters for patients with LARC. We established accurate nomograms for predicting the pathological response to preoperative NT regimens based on pretreatment parameters for LARC patients. These nomograms can be used to distinguish patient types and facilitate developing individualized treatments.
Rectal cancer patients were identified from the database of The Sixth Affiliated Hospital, Sun Yat-sen University from January 2012 to December 2016. Four hundred and three patients who met the criteria were included. We collected all available clinical information before treatment.
The NT regimens included in our study were capecitabine/fluorouracil plus radiotherapy (standard group, capecitabine/deGramont-RT), mFOLFOX6 without radiotherapy (mFOLFOX6), and mFOLFOX6 plus radiotherapy (mFOLFOX6-RT). The radiation dose for the radiotherapy was 46.0-50.4 Gy, delivered as 1.8-2.0 Gy/d.
pCR was defined as no malignant cells found in the resected specimens, including the primary tumor and lymph nodes, and ypT0-2N0M0 (ypTNM 0-I) was classified as good downstaging.
Univariate logistic regression analysis was used to analyze variables related to the probability of pCR or good downstaging. Variables that achieved significance at P ≤ 0.05 in the univariate logistic regression analysis were further analyzed into the forward stepwise multivariable logistic regression. Multivariate logistic regression analysis was used to construct the nomograms. Because the NT regimen was a statistically significant factor for predicting pCR probability, we then attempted to develop three nomograms based on the different NT regimens to predict pCR probability. The C-index was acquired for the nomogram, and internally validated using the bootstrap method to determine the adjusted C-index. Calibration curves of the nomograms were generated to show the relationship between the predicted and observed outcomes.
All statistical analyses were performed using SPSS 24.0 and R 3.5.1.
Of the 403 patients in our study, 281 (69%) were men. As assessed pathologically, 72 (17.86%) individuals achieved a pCR; 177 (43.9%) patients achieved ypTNM 0-I and were classified as having good downstaging.
Significant differences were found for age, tumor differentiation, TL, DTAV, mesorectal fascia (MRF) status, interval, and NT regimen in the univariate analysis. In the multivariate analysis, NT regimen types, tumor differentiation, TL, and MRF status were significantly associated with pCR probability.
Significant differences were found for carcinoembryonic antigen (CEA), tumor differentiation, distance of tumor from the anal verge (DTAV), tumor length (TL), cT, and MRF status in the univariate logistic regression analysis for good downstaging. In the multivariate analysis, tumor differentiation, MRF statuses, and cT were significantly associated with the probability of good downstaging.
Table 5 shows the distribution of pretreatment clinical parameters in the NT regimen groups. No differences were found in any factors between the three groups except age and DTAV.
In the univariate analysis of the capecitabine/deGramont-RT group, NLR was the only significant factor for predicting pCR probability. NLR (> 3) was the only significant factor compared with NLR ≤ 3 in the further multivariate analysis. We could not develop a nomogram to predict pCR probability in this case.
In the univariate analysis of the mFOLFOX6-RT regimen, TL and MRF status were significant factors predicting pCR probability. TL and MRF(+) were significant factors in multivariate analysis.
In the univariate analysis of the mFOLFOX6 regimen, tumor differentiation and TL were significant factors for predicting pCR probability. Further multivariate analysis showed that differentiation and TL were significant factors.
Nomograms were developed based on the significant factors in the multivariate logistic regression analysis. We used 1000 bootstrap resamples to compute an adjusted C-index. Calibration curves between predicted and actual observations by internal validation demonstrated that these nomograms showed good statistical performance for predicting the probability of pCR and good downstaging.
We established accurate nomograms to predicting the pathological responses to different preoperative NT regimens based on pretreatment parameters for LARC patients. These nomograms can be used to distinguish patient types and facilitate developing individualized treatments.
To the best of our knowledge, our study is the first to use different NT regimen types to predict a pathological response. We established an accurate model with easily obtained variables to predict the probability of pCR and good downstaging. Our analysis was also strengthened through cross-validation. These models can be used to assist with individualized therapy as follows. For LARC patients expected to have a poor pathological response, NT and NT-related harm can be avoided. For patients expected to have good pathological responses to chemotherapy alone, radiotherapy can be avoided. For patients who are not expected to have good pathological response from a standard NT regimen, an enhanced mFOFOLX6-RT regimen can be considered. For patients with a high probability of pCR after NT, local resection or a “watch and wait” strategy can be used to avoid complications.
Our analysis had several limitations. First, this was a retrospective study, in which some factors associated with pCR were unavailable, such as smoking status, molecular subtypes and so on. Second, mFOLFOX6 and mFOLFOX6-RT are not the standard regimens for LARC, and both regimens remain in the clinical trial phase. Finally, our nomograms are based on the experience of our single institution. These results must be validated in a group of independent external institutions.
The nomograms established in our study can be used to evaluate the probability of a pathological responses before NT and after NT. However, additional studies are required to answer clinical questions regarding which patients can be treated only with neoadjuvant chemotherapy, which patients need oxaliplatin added to the neoadjuvant chemoradiotherapy, which patients need radical surgery, which patients can undergo local excision, and which patients can be managed with a “watch and wait” strategy after achieving a good response.
In the future, we plan to include a larger number of patients to enhance the accuracy of the prediction. On the other hand, we plan to add a second external cohort for validation to strengthen the reliability of the nomogram.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gerard JP, Young CJ S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Kalyan A, Rozelle S, Benson A 3rd. Neoadjuvant treatment of rectal cancer: where are we now? Gastroenterol Rep (Oxf). 2016;4:206-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4342] [Cited by in F6Publishing: 4228] [Article Influence: 211.4] [Reference Citation Analysis (1)] |
| 3. | Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926-1933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1251] [Cited by in F6Publishing: 1352] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 4. | de Campos-Lobato LF, Stocchi L, da Luz Moreira A, Geisler D, Dietz DW, Lavery IC, Fazio VW, Kalady MF. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011;18:1590-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 424] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 6. | Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661-2667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1189] [Cited by in F6Publishing: 1313] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 8. | Duldulao MP, Lee W, Streja L, Chu P, Li W, Chen Z, Kim J, Garcia-Aguilar J. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum. 2013;56:142-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM; Timing of Rectal Cancer Response to Chemoradiation Consortium. Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg. 2011;254:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Jalilian M, Davis S, Mohebbi M, Sugamaran B, Porter IW, Bell S, Warrier SK, Wale R. Pathologic response to neoadjuvant treatment in locally advanced rectal cancer and impact on outcome. J Gastrointest Oncol. 2016;7:603-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688-8696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 918] [Cited by in F6Publishing: 923] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 12. | Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, Ghadimi M, Mrak K, Merkel S, Raab HR, Sauer R, Wittekind C, Rödel C. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 13. | Valentini V, Coco C, Picciocchi A, Morganti AG, Trodella L, Ciabattoni A, Cellini F, Barbaro B, Cogliandolo S, Nuzzo G, Doglietto GB, Ambesi-Impiombato F, Cosimelli M. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 271] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, Miccichè F, Ricci R, Morganti AG, Gambacorta MA, Maurizi F, Coco C. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Reggiani Bonetti L, Lionti S, Domati F, Barresi V. Do pathological variables have prognostic significance in rectal adenocarcinoma treated with neoadjuvant chemoradiotherapy and surgery? World J Gastroenterol. 2017;23:1412-1423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Appelt AL, Pløen J, Harling H, Jensen FS, Jensen LH, Jørgensen JC, Lindebjerg J, Rafaelsen SR, Jakobsen A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16:919-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 17. | Habr-Gama A, Sabbaga J, Gama-Rodrigues J, São Julião GP, Proscurshim I, Bailão Aguilar P, Nadalin W, Perez RO. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56:1109-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 299] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 18. | Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, Hulsewé KW, Buijsen J, Beets GL. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633-4640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 724] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 19. | Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-7; discussion 717-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1120] [Cited by in F6Publishing: 1208] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 20. | Glynne-Jones R, Hughes R. Critical appraisal of the ‘wait and see’ approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg. 2012;99:897-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 21. | Park YA, Sohn SK, Seong J, Baik SH, Lee KY, Kim NK, Cho CW. Serum CEA as a predictor for the response to preoperative chemoradiation in rectal cancer. J Surg Oncol. 2006;93:145-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Wallin U, Rothenberger D, Lowry A, Luepker R, Mellgren A. CEA - a predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum. 2013;56:859-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 23. | Al-Sukhni E, Attwood K, Mattson DM, Gabriel E, Nurkin SJ. Predictors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer. Ann Surg Oncol. 2016;23:1177-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, Fazio VW. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250:582-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 25. | Lorimer PD, Motz BM, Kirks RC, Boselli DM, Walsh KK, Prabhu RS, Hill JS, Salo JC. Pathologic Complete Response Rates After Neoadjuvant Treatment in Rectal Cancer: An Analysis of the National Cancer Database. Ann Surg Oncol. 2017;24:2095-2103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34:3300-3307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 27. | Zhang J, Cai Y, Hu H, Lan P, Wang L, Huang M, Kang L, Wu X, Wang H, Ling J, Xiao J, Wang J, Deng Y. Nomogram basing pre-treatment parameters predicting early response for locally advanced rectal cancer with neoadjuvant chemotherapy alone: a subgroup efficacy analysis of FOWARC study. Oncotarget. 2016;7:5053-5062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, O’Donoghue DP, Moriarty M, Fennelly D, Sheahan K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 434] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 29. | Restivo A, Zorcolo L, Cocco IM, Manunza R, Margiani C, Marongiu L, Casula G. Elevated CEA levels and low distance of the tumor from the anal verge are predictors of incomplete response to chemoradiation in patients with rectal cancer. Ann Surg Oncol. 2013;20:864-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Berkel AE, Woutersen DP, van der Palen J, Klaase JM. Prognostic factors for postoperative morbidity and tumour response after neoadjuvant chemoradiation followed by resection for rectal cancer. J Gastrointest Surg. 2014;18:1648-1657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Guren MG, Eriksen MT, Wiig JN, Carlsen E, Nesbakken A, Sigurdsson HK, Wibe A, Tveit KM; Norwegian Rectal Cancer Group. Quality of life and functional outcome following anterior or abdominoperineal resection for rectal cancer. Eur J Surg Oncol. 2005;31:735-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2013;15:1130-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, Temple LK, Nash GM, Paty PB. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256:965-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 34. | Stijns RCH, Tromp MR, Hugen N, de Wilt JHW. Advances in organ preserving strategies in rectal cancer patients. Eur J Surg Oncol. 2018;44:209-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1502] [Cited by in F6Publishing: 1544] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 36. | Huerta S, Hrom J. Oxaliplatin as a radiosensitizing agent in rectal cancer. Anticancer Drugs. 2011;22:317-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Martin LK, Bekaii-Saab T. Optimizing neoadjuvant therapy for rectal cancer with oxaliplatin. J Natl Compr Canc Netw. 2013;11:298-307; quiz 307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 424] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 39. | García-Flórez LJ, Gómez-Álvarez G, Frunza AM, Barneo-Serra L, Martínez-Alonso C, Fresno-Forcelledo MF. Predictive markers of response to neoadjuvant therapy in rectal cancer. J Surg Res. 2015;194:120-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Joye I, Debucquoy A, Fieuws S, Wolthuis A, Sagaert X, D’Hoore A, Haustermans K. Can clinical factors be used as a selection tool for an organ-preserving strategy in rectal cancer? Acta Oncol. 2016;55:1047-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Rödel C, Hofheinz R, Fokas E. Rectal cancer: Neoadjuvant chemoradiotherapy. Best Pract Res Clin Gastroenterol. 2016;30:629-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Kim IY, You SH, Kim YW. Neutrophil-lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancer. BMC Surg. 2014;14:94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |