Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.111
Revised: January 11, 1999
Accepted: January 20, 1999
Published online: April 15, 1999
AIM To certify the relationship between AFP-mRNA and some pathological parameters of he patocellular carcinoma (HCC).
METHOD We detected the expression of AFP in mRNA level in tissue samples from 52 patients suffering from HCC by RT-PCR method.
RESULTS The positive rate of AFP mRNA was 76.9% in the HCC tumor tissues, and 69.4% in the paratumor tissues from the HCC patients with severe cirrhosis. However, in HCC patients without cirrhosis, the positive rate reached 50% in tumor tissues, but no AFP mRNA expression was found in the related paratumor tissues.
CONCLUSION The AFP protein was specially expressed by HCC cells and mutated hepatocytes. The AFP mRNA was positively related with cirrhosis, but no significant relationship was found between AFP mRNA and tumor size, capsule status and tumor metastasis.
- Citation: He P, Tang ZY, Ye SL, Liu BB. Relationship between expression of α-fetoprotein messenger RNA and some clinical parameters of human hepatocellular carcinoma. World J Gastroenterol 1999; 5(2): 111-115
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.111
The value of α-fetoprotein (AFP) as a useful marker for hepatocellular carcinoma (HCC) has been widely accepted since its discovery in 1956 and detection in serum of patients with HCC (reviewed by Abelev 1971)[1]. Further evaluation of AFP, particularly for much earlier detection of HCC and early detection of subclinical recurrence or metastasis, remains an important approach to improving the overall 5-year survival rate of HCC before breakthroughs are made in basic researches. Recent progress in the development of PCR amplification of AFP from HCC tissues, will assist the clinical evaluation of AFP.
Serum AFP concentrations are increased in the majority of patients with HCC. In China, the serum AFP level of 60%-70% of the HCC patients was higher than normal values. Histochemical studies have shown the presence of AFP in malignant hepatocytes[2], and serum AFP concentrations rapidly return to normal after radical resection of HCC, indicating that malignant hepatocytes are responsible for production of AFP. Possible explanations for the reinitiating of AFP synthesis by neoplastic hepatocytes include either increased transcription of the AFP gene or post-translational modifications affecting AFP production.
In the present study, we have developed the competitive reverse transcription followed by polymerase chain reaction (RT-PCR) assays to evaluate messenger RNA(mRNA) level of AFP in tissue specimens obtained by biopsy. In addition, AFP mRNA levels in human HCC were quantitated in comparison with those of non-neoplastic portion to elucidate the relationship of AFP gene expression in human HCC and some pathological parameters.
Surgical specimens of 52 patients with HCC were obtained in Liver Cancer Institu te of Shanghai Medical University from February 1996 to March 1998. Thirty-two cases (61.5%) had abnormal serum AFP ( > 20 μg/L). The controls were operative specimens of patients with liver cyst (3 cases) and hepatic hemangioma (2 cases). Surgical specimens were immediately frozen in liquid nitrogen and stored at -80 °C until use.
The primers for amplifying AFP and β-actin gene were synthesized by Shanghai Biochemistry Institute, Chinese Academy of Sciences. The sequences of primers for AFP were 5’-ATTCAGACTGCTGCAGCCAA-3’(sense, 272 bp-291 bp) and 5’-GTGCTCATGTACATGGGCCA-3’ (antisense, 728 bp-747 bp). The sequences of the sense and antisense primers for the β-actin gene were 5’-CTATTGGCAACGAGCGGTTC-3’ and 5’-CTTAGGAGTGGGGGTGGCTT-3’, respectively.
Total cellular RNA was isolated by the guanidinium isothiocyanate extraction method of Chomezynski and Sacchi[3]. Approximately 0.5 g-1.0 g of each tumor and corresponding non-tumorous liver tissues were minced and homogenized with 1mL denaturing solution (4 mol/L guanidinium isothiocy anate, 25 mmol/L- sodium citrate at pH 7.0, 0.5% sarkosyl, 0.1 mol/L mercaptoethanol). Sodium acetate 0.1 mL-2 mol/L (pH 4.0 ), 1 mL- phenol (water sa turated) and 0.2 mL- chloroform/isoamyl alcohol mixture (49:1) were sequentially added to the homogenate. The final suspension was shaken vigorously for 10s and cooled in ice-water for 15 min. After centrifugation at 10000 × g for 20 min at 4 °C, the aqueous phase, in whi ch RNA was present, was taken and mixed with 1 mL isopropanol, and then placed at -20 °C for 1 h to precipitate RNA. Sedimentation at 10000 × g for 20 min was performed again and the resulting RNA pellet was dissolved in 0.3 mL denaturing solution, transferred into a 1.5 mL Eppendorf tube, and precipitated with 0.3 mL- isopropanol at -20 °C for 1 h. After centrifugation for 10 min at 4 °C, the RNA pellet was dissolved in diethyl-pyrocarbonate (DEPC)-treated water.
A 5 μL sample of total RNA, 20IU RNasin, 5 μL- oligo (dT) (100 mg/L), 5 μL- dNTP (20 mM), 30IU avian myeloblastosis virus reverse transcriptase (Promega), 10 μL-5 × reaction buffer and 13 μL- DEPC-treated water were added to a 0.5 mL-tube and incubated at 42 °C for 1 h. The PCR system for amplifying AFP and β-actin gene each contained equal amounts of RT products (10 μL), sense and antisense primers (50 pmol each), Taq DNA polymerase (3 IU), PCR reaction buffer and double - distilled H2O. Samples were amplified through 35 consecutive cycles, each amplification cycle consisting of a denaturation at 94 °C for 1 min, primer anneal- ing at 55 °C for 1 min, and extension at 72 °C for 2 min. Cycles were preceded by incubation at 95 °C for 5 min to ensure full denaturation of the target DNA, and were followed by an extra 7 min of incubation at 72 °C after the final cycle to ensure full extension of the product. The PCR reactions were performed on a DNA thermal cycler (Perkin Elmer/Cetus Instruments, Norwalk, Conn.). The amplified fragments of AFP and β-actin gene were analyzed by 2% agarose gel.
The PCR products from HCC and paratumor tissues were separated by 2% agarose, the electrophoresis results were printed. The absorbance (A) and the areas of fragment were read by ImageMaster VDS (Pharmacia Biotech). The ex pression level of the AFP gene was calculated as follows:AFP gene expression (%) = [AFP fragment area × (Afragment-Abackground)] / [ β-actin fragment area × (Afragment-Abackground)].
The clinical parameters of patients with HCC were cirrhosis, HBsAg, differentiation, tumor size, metastasis and tumor capsule. The differences among the groups were analyzed by Chi-square test.
The total RNAs from samples were analyzed by electrophoresis (Figure 1). The RNAs containing 28 s, 18 s and 5 s were not contaminated by DNA and degraded.
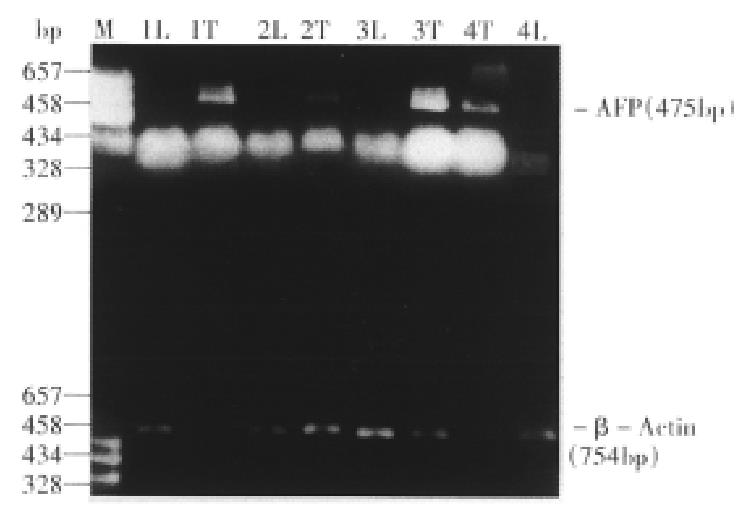
The expected sizes of the amplified AFP fragment and β-actin fragment were 475 bp and 759 bp respectively (Figure 2). In 52 patients, the AFP positive rates in HCC tissues were 76.9% (40/52). Among these patients, 25(69.4%) of 36 cases accompanied with serious cirrhosis had positive AFP mRNA in paratumor tissues, but no AFP mRNA expression was found in normal liver tissues of patients with liver cyst or hepatic hemangioma.
The expression level of AFP in HCC samples (58.4% ± 6.4%) was higher than that in paratumor tissues (30.2% ± 6.1%, P < 0.01). The expression level of AFP in different HCC groups is shown in Table 1. AFP expression in HCC with cirrhosis (65.6% ± 5.4%) was higher than that in HCC with out cirrhosis (48.2% ± 4.3%, P < 0.01). Nosigni-ficant differences in AFP expression were found between HCCs with and without metastasis (57.1% ± 6.4% vs 55.2% ± 7.8%, P > 0.05), as well as between HCCs with different capsule status or between large HCCs ( > 5 cm) and small HCCs ( ≤ 5 cm).
| Histological parameter | Cases | Positive tumor AFP mRNA | Positive paratumor AFP mRNA |
| Cirrhosisa | |||
| Yes | 36 | 88.8 (32/36) | 69.4 (25/36) |
| No | 16 | 50.0 (8/16) | 0.0 (0/16) |
| HBsAgb | |||
| (+) | 46 | 82.6 (38/46) | 52.2 (24/46) |
| (-) | 6 | 33.3 (2/6) | 16.7 (1/6) |
| Differentiation | |||
| Well | 18 | 77.8 (14/18) | 33.3(6/18) |
| Poor | 34 | 76.5 (26/34) | 55.9 (19/34) |
| Tumor size | |||
| ≤ 5 cm | 20 | 75.0 (15/20) | 25.0 (5/20) |
| > 5 cm | 32 | 78.1 (25/32) | 62.5(20/32) |
| Metastasis | |||
| Yes | 11 | 81.8(9/11) | 45.5(5/11) |
| No | 41 | 75.6 (31/41) | 48.5(20/41) |
| Capsule | |||
| Yes | 20 | 85.0 (17/20) | 40.0 (8/20) |
| No | 32 | 71.9 (23/32) | 53.1 (17/32) |
The HCC patients with severe cirrhosis had highest AFP positive rates in HCC tissues (88. 8%) and in paratumor tissues (69.4%) among these patients. But the HCC patients without cirrhosis had lower AFP positive rates in HCC tissues (50%) and were negative in all of paratumor tissues. A significant difference in AFP mR NA expression was found between the HCC patients with serum HBsAg (HCC tissues was 82.6%, paratumor was 52.2%) and without serum HBsAg. But, no significant di fference was found between the HCC with different differentiation, metastasis, or different capsule status (Table 2).
| Group | No. of cases | AFP gene expression -x±sx(%) | P |
| Total | |||
| HCC | 52 | 58.4 ± 6.4 | |
| Paratumor | 52 | 30.2 ± 6.1 | < 0.01 |
| Cirrhosis | |||
| Yes | 36 | 65.5 ± 5.4 | |
| No | 16 | 48.2 ± 4.3 | < 0.01 |
| Metastasis | |||
| Yes | 11 | 57.1 ± 6.4 | |
| No | 41 | 55.2 ± 7.8 | > 0.05 |
| Capsule | |||
| Complete | 20 | 53.1 ± 8.2 | |
| Incomplete | 32 | 51.8 ± 6.5 | > 0.05 |
| Tumor | |||
| ≤ 5 cm | 20 | 48.5 ± 5.3 | |
| >5 cm | 32 | 53.6 ± 8.3 | S > 0.05 |
AFP cDNA fragments were amplified from all HCC tissues of 32 patients with serum AFP > 20 μg/L, while from paratumor tissues of 14 HCC patients. Among 20 HCC patients with serum AFP ≤ 20 μg/L, there were 8 patients with AFP mRNA in HCC tissues. In other words, the positive rates of AFP mRNA had a significant difference between HCC patients with serum AFP > 20 μg/L (100%) and those with serum AFP ≤ 20 μg/L (40%, P < 0.01).
Human AFP gene is present on chromosome 4q11-21 and consists of 15 exons[4]. AFP is an oncofetal protein, and its gene expression is shown mainly by hepatocytes and endoderm cells of the visceral yolk sac in the early stages of fetal liver development, but repressed soon after birth. Reactivation of AFP gene expression is shown during massive liver necrosis and in hepatocarcinogenesis. Many experiments demonstrated that the major control of AFP production is probably at the level of gene transcription[5].
In the current study, RNA was extracted from the tissues by acid guanidinium thiocyanate-phenol-chloroform extraction method according to Chomczynski and Sacchi[3]. Because the amount of RNA was small when extracted from biopsy samples, we adopted this procedure for better yield of RNA in comparison with the CsCl gradient extraction of Chirgwin[6]. The portions of extracted RNA from surgically resected samples were electrophoresed through formaldehyde agarose gel, stained with ethidium bromide, and clear bands of 18S and 28S ribosomal RNA were observed. In this method, an amount of contaminating DNA in the prepared RNA was less than detectable level (Figure 1).
The results showed that the total positive rate of AFP mRNA in hepatoma tissues was 76.9%. It demonstrated that AFP gene was activated in HCC cells of most HCC patients. Among them, the positive rate of HCC patients with severe cirrhosis was highest (88.8%), while 69.4% in their paratumor tissues. But the HCC patien ts without cirrhosis had lower AFP mRNA levels: 50% in HCC tissues and negative in paratumor tissues. These results showed that the transcription levels of AFP mRNA were correlated to cirrhosis[7]. Koa and his colleagues found that the tumorgenesis rate of HCC patients with cirrhosis and serum AFP < 20 μg/L was 26%, but the patients with cirrhosis and AFP ≥ 20 μg/L was 46% in a five-year observation[8]. This suggested that the cells of paratumor tissues secreting AFP protein may be precarcinomatous cells. They were of some characteristics of embryonic hepatocytes or HCC cells, and expressed this embryonic antigen before genesis of HCC, Therefore, it aids earlier detection of HCC by assaying AFP mRNA in biopsy of HCC patients with cirrhosis[8]. We conclude that the expression levels of AFP mRNA in HCC tissues were higher than that in paratumor tissues. The activation of AFP gene in paratumor tissues may be related to precarcinomatous changes.
Muguti, et al[9] reported that the serum AFP level of HCC patients with serum HBsAg was higher than that of patients without HBsAg. They suggested that serum AFP levels had significant differences among different HBsAg titers. Lee also found that most of patients with serum HBsAg had high serum AFP levels and demonstrated that the motivate observation of AFP had great value for early diagnosis of HCC[10]. Our study suggested that the transcriptional activation of AFP in HCC tissues may be related to HBV infection[11-13].
In our experiment, the positive rate of AFP mRNA of patients with serum AFP ≤ 20 mg/L-was 40% in HCC tissues, and 15% in paratumor tissues. But, the posi tive rate of AFP mRNA of patients with serum AFP > 20 mg/L- was 100% in HCC tissues, and 68.8% in paratumor tissues. These results were supported by the reported paper[14]. Total po-sitive rate of HCC tissues was 76.9%, but that of serum AFP was 61.5%. These suggested that the activated AFP gene in transcription level was higher than that in translation level. Therefore, the activation of AFP gene included both gene transcription and translation levels.
On the other hand, our experiments certified that AFP gene re-expression had no relationship with tumor capsule status, differentiation and tumor mass size. Thus, although the motion of serum AFP level can predict the recurrence and metastasis of HCC patients after resection or treatment, there is no relationship between AFP mRNA expression in HCC tissues and recurrence and metastasis of HCC. In other words, AFP expression is not one of the reasons that result in the recurrence and metastasis of HCC.
Finally, gene therapy for HCC using retrovirus or adenovirus vectors carrying human AFP promoter for tissue specific expression of suicide genes or cytokines are now under investigation in some laboratories[15,16], including ours[17]. Quantitation of AFP transcripts in HCC shown in this study could provide a clue for selection of patients for such a targeted gene therapy, since expression of transduced genes could be expected in AFP-producing HCCs only.
Project supported by the National Natural Science Foundation of China, No.3900050.
Edited by Jing-Yun Ma
| 1. | Abelev GI. Alpha-fetoprotein in ontogenesis and its association with malignant tumors. Adv Cancer Res. 1971;14:295-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 671] [Cited by in F6Publishing: 707] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Hirohashi S, Shimosato Y, Ino Y, Kishi K, Ohkura H, Mukojima T. Distribution of alpha-fetoprotein and immunoreactive carcinoembryonic antigen in human hepatocellular carcinoma and hepatoblastoma. Jpn J Clin Oncol. 1983;13:37-43. [PubMed] [Cited in This Article: ] |
| 3. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40518] [Cited by in F6Publishing: 38830] [Article Influence: 1049.5] [Reference Citation Analysis (0)] |
| 4. | Dugaiczyk A, Harper ME, Minghetti PP. Chromosomal localization, structure, and expression of the human alpha-fetoprotein gene. Kroc Found Ser. 1985;19:181-188. [PubMed] [Cited in This Article: ] |
| 5. | Tilghman SM, Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci USA. 1982;79:5254-5257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 286] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294-5299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14827] [Cited by in F6Publishing: 17521] [Article Influence: 389.4] [Reference Citation Analysis (0)] |
| 7. | Di Bisceglie AM, Dusheiko GM, Paterson AC, Alexander J, Shouval D, Lee CS, Beasley RP, Kew MC. Detection of alpha-foetoprotein messenger RNA in human hepatocellular carcinoma and hepatoblastoma tissue. Br J Cancer. 1986;54:779-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 243] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Muguti G, Tait N, Richardson A, Little JM. Alpha-fetoprotein expression in hepatocellular carcinoma: a clinical study. J Gastroenterol Hepatol. 1992;7:374-378. [PubMed] [Cited in This Article: ] |
| 10. | Lee HS, Chung YH, Kim CY. Specificities of serum alpha-fetoprotein in HBsAg+ and HBsAg- patients in the diagnosis of hepatocellular carcinoma. Hepatology. 1991;14:68-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Songsivilai S, Dharakul T, Senawong S. Hepatitis B- and hepatitis C-associated hepatocellular carcinoma: evaluation of alpha-fetoprotein as a diagnostic marker. Asian Pac J Allergy Immunol. 1995;13:167-171. [PubMed] [Cited in This Article: ] |
| 12. | Wong CB, Attar BM, Shimoda SS. Marked episodic elevations of alpha-fetoprotein without hepatocellular carcinoma in a patient with hepatitis B. Am J Gastroenterol. 1995;90:1015-1016. [PubMed] [Cited in This Article: ] |
| 13. | Parkinson AJ, McMahon BJ, Zanis L, Lanier AP, Wainwright RB. Detection of alpha-fetoprotein and hepatitis-B surface antigen in blood spotted on filter paper: use as a screen for hepatocellular carcinoma in Alaska Natives. Arctic Med Res. 1996;55:123-128. [PubMed] [Cited in This Article: ] |
| 14. | Peng SY, Lai PL, Chu JS, Lee PH, Tsung PT, Chen DS, Hsu HC. Expression and hypomethylation of alpha-fetoprotein gene in unicentric and multicentric human hepatocellular carcinomas. Hepatology. 1993;17:35-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Kanai F, Lan KH, Shiratori Y, Tanaka T, Ohashi M, Okudaira T, Yoshida Y, Wakimoto H, Hamada H, Nakabayashi H. In vivo gene therapy for alpha-fetoprotein-producing hepatocellular carcinoma by adenovirus-mediated transfer of cytosine deaminase gene. Cancer Res. 1997;57:461-465. [PubMed] [Cited in This Article: ] |
| 16. | Mawatari F, Tsuruta S, Ido A, Ueki T, Nakao K, Kato Y, Tamaoki T, Ishii N, Nakata K. Retrovirus-mediated gene therapy for hepatocellular carcinoma: selective and enhanced suicide gene expression regulated by human alpha-fetoprotein enhancer directly linked to its promoter. Cancer Gene Ther. 1998;5:301-306. [PubMed] [Cited in This Article: ] |
| 17. | He P, Liu BB, Ye SL, Tang ZY. The cloning of the combined transcrip-tional regulatory sequence of human α - fetoprotein enhancer/albu-min promoter. Chin J Hepatol. 1998;6:136-138. [Cited in This Article: ] |