Published online Apr 15, 2001. doi: 10.3748/wjg.v7.i2.208
Revised: February 26, 2001
Accepted: March 1, 2001
Published online: April 15, 2001
AIM: To conduct a cohort study of 101 patients with hepatocellular carcinoma (HCC) presenting to a tertiary care medical referral center in Germany between 1997 and 1999.
METHODS AND RESULTS: Data were retrospectively analyzed by chart review. In 95 cases (72 males and 23 females) sufficient data were available for analysis. Twenty five (29%) of 85 patients were HBsAg or anti HBc positive, 21/85 (25%) were anti HCV positive, and 6/ 85 (7%) were positive for both HBV and HCV-markers. Age was significantly lower in HBV positive patients than in the other two groups. Thirty one (34%) of 90 patients had histories of alcohol abuse. In 79/94 (84%) patients, cirrhosis was diagnosed. Of these cirrhotic patients, 29/79 (37%) belonged to Child Pugh’s group (CHILD) A, 32/79 (40%) to CHILD B, and 18/79 (23%) to CHILD C. AFP was elevated in 61/91 (67%) patients. A single tumor nodule was found in 38/94 (40%), more than one nodule in 31/94 (34%), and 25/94 (26%) had a diffusely infiltrating tumor, i.e. the tumor margins could not be seen on imaging procedures. Portal vein thrombosis was present in 19/94 (20%). Imaging data consistent with lymph node metastases were found in 10/92 (11%), while distant metastases were found in 8/93 (9%). According to Okuda 28/94 (30%) were grouped to stage I, 53/94 (56%) were grouped to stage II, and 13/94 (14%) were grouped to stage III. Survival data were available for 83 patients. The Kaplan-Meier estimate for median survival was 84 months. Factors influencing survival were the Okuda score, the presence of portal vein thrombosis, and the presence of ascites. The presence of non complicated liver cirrhosis by itself, distant metastases, or infection with hepatitis viruses did not influence survival. AFP positivity by itself did not influence survival, though patients with an AFP value greater than 100 μg/L did experience shortened survival. Treatment besides tamoxifen or supportive care was associated with prolonged survival. The influence of therapy on survival was most pronounced in Okuda stage II patients. There was longer survival in those Okuda stage II patients who were treated with percutaneous ethanol injection.
CONCLUSION: Even in a low incidence area such as Germany, the majority of HCC is caused by viral hepatitis and therefore potentially preventable. Reflecting the high proportion of advanced stage tumors in our patients, the median survival was poor. Patients who received active therapy had a longer survival.
- Citation: Rabe C, Pilz T, Klostermann C, Berna M, Schild HH, Sauerbruch T, Caselmann WH. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma. World J Gastroenterol 2001; 7(2): 208-215
- URL: https://www.wjgnet.com/1007-9327/full/v7/i2/208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i2.208
Hepatocellular carcinoma (HCC) was responsible for the death of an estimated 609000 individuals 1998 representing 1.1% of total mortality in WHO member states[1]. The incidence varies with geographic[1] area since factors predisposing to HCC development are unevenly distributed across continents and sometimes even within countries or within populations[2,3].
The incidence of HCC is clearly linked to the rate of chronic HBV infection[4,5]. A large percentage of HCC patients show serological or histological markers of past contact with HBV[3]. Prospective studies have demonstrated that men chronically infected with HBV and expressing hepatitis B surface antigen (HBsAg) are about 70-fold more likely to develop HCC than their seronegative counterparts. Furthermore, even HBsAg-negative patients often exhibit serologic evidence, such as anti-HBc positivity, of past HBV-infection. Molecular mechanisms that account for that association are interaction of HBVproteins with signal transduction pathways, apoptosis, transcriptional machinery, and DNA repair[6]. Likewise, HCV infection predisposes to HCC[7]. While the molecular mechanisms of this association are not well understood at present, HCV proteins seem to interact with functions of the host cell in ways partially comparable to HBV proteins[8-12]. Examples include the capacity of HCV proteins for transactivation and their influence on apoptosis. The main difference between HCV and HBV related hepatocarcinogenesis is the lack of genomic integration of HCV sequences. It is interesting, however, that HCV frequently produces HCC only after liver cirrhosis has developed. This and the acquisition of HCV at a more advanced age may partially explain the older age at diagnosis and worse liver function compared to HBV-related liver cancer[13,14]. Therefore, HCC might be prevented by early antiviral therapy[15-17]. Besides hepatitis virus infection is associated with a large number of cases of HCC[18-23], liver cirrhosis itself regardless of etiology is a predisposing factor for HCC development[24,25]. In fact, most cirrhotics in Japan die from HCC[26]. Certain industrial[27], behavioral [28], or familial factors[29,30] also contribute to HCC development.
HCC can be cured by liver transplantation or liver resection[31-33]. However, due to the multifocal nature of the disease as well as coexisting conditions, only a small minority of patients can be treated surgically. This proportion of surgically treatable patients is likely to increase after institution of screening programs[34,35] that are based on the recognition of high risk groups[36] for the development of HCC. This is very important as the outcome of non-treatable patients remains poor[37,38].
While there is much data on the clinical characteristics and the outcome of patients with HCC in high risk countries in which the majority of HCC are viral hepatitis and/or aflatoxin-related[20-22,39], we know little about predisposing factors, clinical characteristics, and factors influencing outcome in low incidence areas such as northern Europe. Some of the data are contradictory with regard to factors predisposing to HCC development[18,40-43]. As incidence of HCC in many low-risk areas is rising albeit with local differences[44], presumably due to an increase in hepatitis virus-related liver cancer[45,46] and possibly rising rates of HCC development in women[47,48], an analysis of the present situation is needed to serve as a baseline for future refinements. The present study summarizes the data on a cohort of patients admitted to a hepatology unit at a tertiary medical referral center in Germany.
Our institution is an academic tertiary care center located in the western part of Germany that receives the majority of patients as referrals from other hospitals. Using computer based searching for patients that presented for the first time with a diagnosis of hepatocellular carcinoma between 1997-1999, we identified 101 patients . Their charts were retrospectively reviewed. In 95 patients enough data were available for comprehensive analysis. These cases were further analyzed.
Diagnosis of HCC was made either by biopsy, postmortem examination or by diagnosing a new liver lesion in the proper clinical context in the absence of a demonstrable extrahepatic tumor and observing the clinical course. Liver cirrhosis was diagnosed by histology or by clinical evidence (signs of portal hypertension on endoscopic examination or characteristic imaging features on ultrasound examination).
A diagnosis of alcohol abuse was made using the criteria of Kubicka et al[40]. In brief, alcohol abuse was defined as a chronic alcohol consumption of more than 60 g/day for both sexes or when a history of alcohol abuse was noted in the patients’ records.
Anti-HCV, HBsAg, Anti-HBc were measured using commercially available 2nd/3rd generation ELISA-based assays (Abbott Laboratories, Wiesbaden, Germany). Ultrasound examinations, CT scans, and chest X-rays were analyzed for the number of lesions, the size of the largest lesion, the approximate amount of liver volume taken up by the tumor, and the presence of extrahepatic lesions. The Okuda-score was calculated from imaging results and laboratory parameters. Patient survival was recorded by contacting the patients’ primary care physician by mail or telephone.
For statistical analysis data were described by median and range. The number of patients with a specific trait was listed along with the number of patients for whom enough data was available to asses the absence or presence of this trait. Comparisons of continuous data between groups were performed using the Mann-Whitney-U-test (two groups) or the Kruskal-Wallis-test (more than two groups). Relationship between groups of nominal/ordinal data were explored using the chi-squared test, if necessary in its modification as the Fisher’s exact test. To analyze survival data Kaplan-Meier-analysis was performed and survival differences between groups tested using the Log-rank-test. The Kaplan-Meier estimate for median or mean survival was reported depending on patient number.
Patient characteristics are summarized in Tables 1 and 2. Median patient age was 63 years (Range: 31-85 years) for men and 67 years (Range 42-83 years) for women. There was no statistically significant difference in age between men and women (U-test). The three most frequent concomitant diseases were diabetes mellitus in 32/95 (34%), arterial hypertension in 16/95 (17%) and nephrolithiasis in 6/95 (6%) patients. Apart from nephrolithiasis that was only diagnosed in men there was no predominance of one sex in these associated conditions. There was no difference in body mass index between those patients who suffered from diabetes and those who did not have diabetes. 24/32 (75%) Patients with diabetes suffered from liver cirrhosis.
| Variable | Number/Total | Percentage |
| Sex | ||
| Male | 72/95 | 76% |
| Female | 23/95 | 24% |
| Markers of past or present HBV infection | ||
| Yes | 25/85 | 29% |
| No | 60/85 | 71% |
| Markers of HCV infection | ||
| Yes | 21/85 | 25% |
| No | 64/85 | 75% |
| Markers of both HBV and HCV-infection | ||
| Yes | 6/85 | 7% |
| No | 79/85 | 93% |
| History of alcoholism | ||
| Yes | 31/90 | 34% |
| No | 59/90 | 66% |
| Cirrhosis | ||
| Yes | 79/94 | 84% |
| No | 15/94 | 16% |
| Child score (only cirrhotics) | ||
| A | 29/79 | 37% |
| B | 32/79 | 40% |
| C | 18/79 | 23% |
| AFP elevated | ||
| Yes | 61/91 | 67% |
| No | 30/91 | 33% |
| Variable | Median (Range) |
| Age | 64 (31-85) years |
| GPT | 32 (10-244) U/L |
| AP | 214 (2-1490) U/L |
| Bilirubin | 1.5 (0.4-14.4) mg/dL |
| Prothrombin time acc. to Quick | 83 (25-122)% |
| Albumen | 3.4 (1.9-4.6) g/dL |
| AFP | 38 (0.9-831000) μg/L |
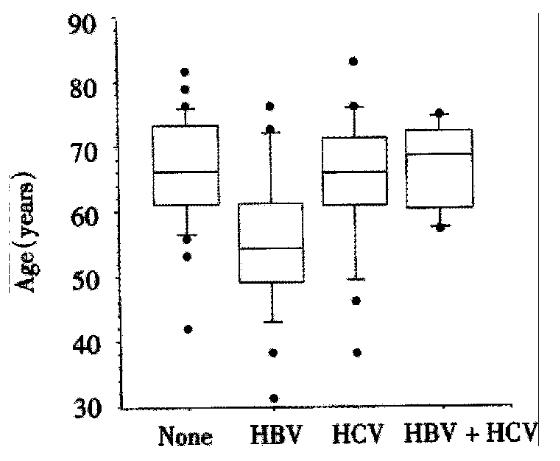
Hepatitis viruses Twenty-five/85 (29%) of HCC patients had markers of past or present HBV infection. Another 21/85 (25%) were infected by the hepatitis C virus (HCV), while 6/85 (7%) of patients had markers of infection with both viruses. There was a significant difference in age in patients with HBV, HCV, HBV/HCV coinfection, and those without hepatitis (P < 0.01, Kruskal-Wallis-test). The median age (55 years, range 31-76 years) of patients with markers of HBV-infection was more than ten years lower than the median ages of patients without hepatitis (66, range 42-82 years), with HCV-infection (66, range 38-83 years), or with HBV/HCV-coinfection (69, 57-75 years). This is depicted in Figure 1. Interestingly, only 1/21 patients with HCV infection and none of the patients with HCV/HBV coinfection did not suffer from liver cirrhosis, while 2/24 patients with HBV-infection and 7/33 patients without hepatitis did not have cirrhosis.
Alcoholism Thirty-one/90 (34%) of HCC patients in our cohort were alcoholics. Age of alcoholics was not statistically different than that of patients with H C C from other causes ( P > 0.05, U test). Comparing the presence of alcoholism with the presence of viral markers, there was no apparent positive correlation between the presence of alcoholism and presence of markers indicating past or present HBV or HCV-infection. Indeed, only 9/81 patients were both alcoholics and had markers of hepatitis B or C, while 21/81 patients were alcoholics without markers of hepatitis and 39/81 patients had hepatitis but no alcoholism. 12/81 patients suffered neither from hepatitis nor alcoholism. Therefore it seems likely that a negative association between hepatitis and alcoholism exists in our cohort. This is confirmed by a borderline significant Fisher’s exact test (P < 0.05) for this inverse relationship.
Cirrhosis Seventy-nine/94 (84%) of HCC patients suffered from liver cirrhosis. The biggest percentage (40%) of these patients could be classified as CHILD-Pugh B, while 37% and 23% could be classified as CHILD A and C respectively. Age of cirrhotics was identical to that of non-cirrhotic patients (P > 0.05, U test).
Tumor characteristics Tumor characteristics are listed in Table 3. 61/91 (67%) of patients had a positive AFP-test. The diameter of the largest tumor nodule in AFP positive tumors was significantly larger (P < 0.05, U-test) than that of AFP negative tumors. A single tumor nodule was observed in 38/94 patients, while the remaining tumors were multinodular, with a diffuse growth pattern in 25/94. 20/89 tumors were smaller than 3 cm, 31/89 were between 3 and 5 cm, and 38/89 were larger than 5 cm. An involvement of both liver lobes was noted in 49/93 cases. In 19/94 cases an associated portal vein thrombosis was diagnosed. Ascites was seen during ultrasound in 43/95 patients. It was not possible to further classify ascites as malignant or due to liver cirrhosis retrospectively. Lymph node metastases were seen in 10/92 patients, while distant metastases (mainly lung metastases) were diagnosed in 8/93 patients.
| Variable | Number/Total | Percentage |
| Type of tumor growth | ||
| One nodule | 38/94 | 40% |
| > 1 Nodule | 31/94 | 34% |
| Diffuse | 25/94 | 26% |
| Size of largest nodule | ||
| < 3 cm | 20/89 | 22% |
| 3 cm-5 cm | 31/89 | 35% |
| > 5 cm | 38/89 | 43% |
| Involvement of both liver lobes | ||
| Yes | 49/93 | 53% |
| No | 44/93 | 47% |
| Portal vein thrombosis | ||
| Yes | 19/94 | 20% |
| No | 75/94 | 80% |
| Lymph node metastases | ||
| Yes | 10/92 | 11% |
| No | 82/92 | 89% |
| Distant metastases | ||
| Yes | 8/93 | 9% |
| No | 85/93 | 91% |
| Presence of ascites | ||
| Yes | 43/95 | 45% |
| No | 52/95 | 55% |
| Tumor stage according to Okuda | ||
| I | 28/94 | 30% |
| II | 53/94 | 56% |
| III | 13/94 | 14% |
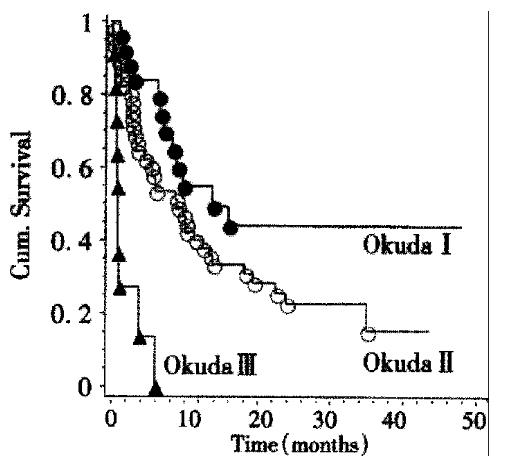
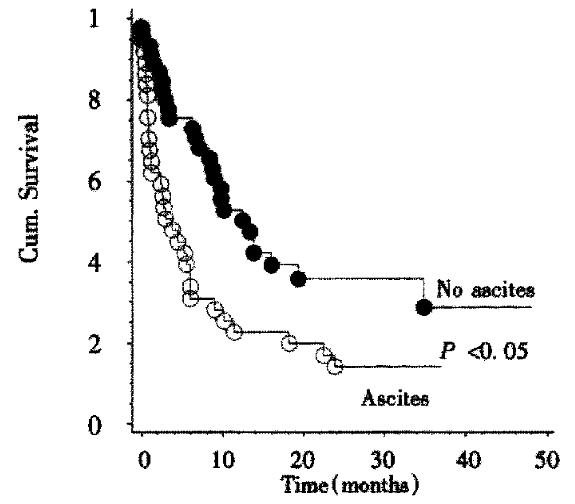
Survival data were available for 83 patients. The Kaplan-Meier estimate for median survival for all patients was 8.4 months. Survival curves according to Okuda stage are shown in Figure 2. There is a highly significant (P < 0.001, Log-rank-test) relationship between tumor stage according to Okuda and patient survival. Kaplan-Meier-estimates for mean survival were 13.8 months for Okuda stage I, 9 months for Okuda stage II, and 1 month for Okuda stage III. By survival analysis other single factors influencing survival could be demonstrated: A significant (P < 0.02, Log-rank test) influence on survival could be shown for the presence of portal vein thrombosis. The Kaplan-Meier estimate for mean survival in the absence of portal vein thrombosis was 10 months while the estimate for mean survival in the presence of portal vein thrombosis was 2.4 months. Likewise, patient survival was significantly shorter (P < 0.004, Log-rank test) if ascites was present. The Kaplan-Meier estimates for median survival were 13 months in the absence and 3.8 months in the presence of ascites (Figure 3). The CHILD score was significantly correlated with survival of cirrhotic patients: CHILD A patients (n = 27) survived a median time of 14 months, CHILD B patients (n = 29) survived a median time of 6.6 months, while CHILD C patients (n = 13) survived a median time of 0.7 months. This influence of CHILD stage on survival was significant ( P < 0.01, Log-rank-test) . CHILD stage was significantly correlated to Okuda score (P < 0.01, Chi-squared test).
No difference in survival could be found for patients with or without hepatitis (P = 0.8, Log-rank test), AFP-positive versus AFP-negative patients (P = 0.11, Log-rank test), the presence of distant metastases (P = 0.22, Log-rank test), and for the presence or absence of liver cirrhosis (P = 0.95, Log-rank test).
While AFP positivity by itself was not associated with lower survival, AFP-levels greater than 100 ug/L (n = 33) were associated with a median survival of 3 months, while lower AFP-levels (n = 46) were associated with a survival of 12.5 months (P < 0.01, Log-rank test).
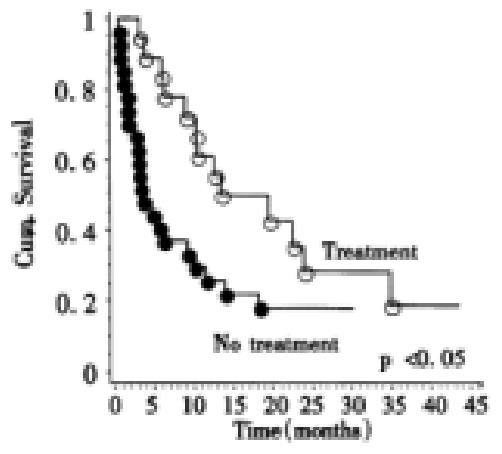
To investigate the influence of therapy on survival, the patients were divided in a group that received active therapy (17 percutaneous ethanol injection, 8 transarterial chemoembolisation, 11-I-131-Lipiodol administration, 2 resection, 2 octreotide for more than 4 weeks) and a group that received either supportive care (n = 36) or tamoxifen treatment (n = 13), that has recently been shown to yield no survival benefit compared to placebo. These patients were then stratified according to Okuda score. Only Okuda stage I or II patients were included. When survival was compared between both stratified groups overall there were significant differences (P < 0.04, log-rank test) in survival between treated and untreated patients. For Okuda stage I the Kaplan Meier estimate for mean survival in the treatment group (n = 13) was 9 months, while the estimate for mean survival in the untreated group (n = 12) was 12 months. For Okuda stage II the Kaplan-Meier estimate for mean survival for the treatment group (n = 19) was 19 months while the estimate for mean survival for the untreated group (n = 27) was 7 months (Figure 4). While the use of percutaneous ethanol injection was not significantly related to increased survival overall(P = 0.18, Log-rank test); in Okuda stage II tumors there was better survival for patients receiving this treatment modality (P < 0.02, Log-rank test). When only patients were analyzed who received either supportive therapy (n = 36) or tamoxifen treatment (n = 13), no difference for survival times could be found between tamoxifen treated patients and those who received supportive therapy.
The age and sex distribution of our patient cohort closely reflected published data from other series. Interestingly, there was a high prevalence of diabetes mellitus in our patients. Other studies on HCC also reported a high prevalence of diabetes mellitus[49-51]. The body mass index of diabetic patients was similar to that of their non-diabetic counterparts as was the number of subjects with liver cirrhosis. The high prevalence of diabetes mellitus in our series can be interpreted as a consequence of the high prevalence of liver cirrhosis in HCC patients overall. Cirrhosis is associated with impaired glucose tolerance and diabetes in 20%-30% of cases[52,53].
The presence of liver cirrhosis in 84% of our patients is well within the range of cirrhosis rates reported in the literature (Table 4). All but three of the patients with viral hepatitis were cirrhotics. The other HCCs can most likely be explained by the high prevalence of alcoholism in our patients. As alcoholism was not associated with viral hepatitis in our patients, alcohol abuse may have been an independent risk factor for HCC development. Liver cirrhosis was fairly advanced in most of our HCC patients as evidenced by the high proportion of CHILD B and CHILD C liver cirrhosis.In our study 29% of HCC had evidence of past or present HBV-infection, 25% had evidence of HCV-infection, and 7% had evidence of both HBV and HCV-infections. This is comparable to infection rates in northern Europe and some more recent studies from southern Europe (Table 4) that show that a rising proportion of hepatocellular carcinoma is HCV related. This is similar to Asian countries where one can also observe a clear relationship of HCC and hepatitis B and C (Table 4). The lower age of HCC-patients with HBV infection compared to that of HCC-patients with HCV-infection and HCC-patients without HBV/HCV-infection or with HBV/HCV-coinfection may be explained by two hypotheses: Firstly, HBV may have been acquired earlier in life than HCV. Secondly, HBV might be a more potent oncogenic stimulus than HCV. There is some evidence to support this latter hypothesis: HBV has been identified as a risk factor for HCC in individuals that are not cirrhotic. This may mean that the many interactions of HBV proteins with the host cell[6] and especially the transactivating function of HBV proteins coded by integrated viral sequences[54-56] can cause cancer regardless of cirrhosis development. Indeed, two of our 24 patients with HBV infection did not have cirrhosis. The older age of HBV/HCV coinfected patients compared to patients with HBV monoinfection might also be related to specific modes of transmission that occur only at an older age. It is a very interesting observation that the majority of HCC even in a low-risk area for viral hepatitis such as Germany are related to viral hepatitis and, therefore, potentially preventable. An important factor in preventing liver cancer in Germany will be an effective reduction or eradication of HBV infection through general vaccination programs. This approach has already proved successful in Asia. Another possibility of preventing HCC could be interferon treatment of HCV infection. Although there is still some controversy if this may prevent HCC development, it seems very likely that successful interferon therapy can prevent progression to liver cirrhosis and will therefore reduce HCC incidence. HCV-related HCC occurs following the development of liver cirrhosis in the majority of cases. In our patients there was only one patient with HCV infection but without liver cirrhosis.
| Author | Number | Cirrhosis | HBV | HCV | HBV + HCV (%) | Country |
| Northern/Central Europe | ||||||
| Van Roey | 154 | 60 | 52 | 55 | NA | Belgium |
| Widell | 95 | NA | 29 | 17 | NA | Sweden |
| Kubicka | 268 | 74.6 | 35.1 | 26.9 | 10 | Germany |
| Petry | 100 | 89.5 | 22 | 37 | 0 | Germany |
| Peters | 86 | 90 | 49 | 37 | NA | Germany |
| This study | 95 | 84 | 29 | 25 | 7 | Germany |
| Southern Europe | ||||||
| Kuper | 333 | NA | 58 | 12 | 3 | Greece |
| Stroffolini | 1148 | NA | 12 | 71 | 5 | Italy |
| Asia | ||||||
| Kubo | 330 | NA | 17 | 26 | 53 | Japan |
| Shiratori | 205 | NA | 11 | 71 | 13 | Japan |
| Zhang | 113 | NA | 63 | 11 | NA | China |
| Chuang | 128 | NA | 77 | 20 | NA | China |
Two thirds of our patients had a positive AFP-test. This is similar to the results of other German studies[41,43] and illustrates that other tumor markers are needed to detect early HCC in AFP-negative cases. This is emphasized by significantly larger tumor nodules in AFP positive cases. Patients with large tumor nodules may not be good candidates for liver transplantation or resection, the only curative therapies available for HCC.
As detailed in Table 3, most of the tumors were in an advanced stage at presentation. 70% of tumors were Okuda stage II or III. Involvement of both liver lobes was predominant in this study. there was also a high rate of portal vein thrombosis. The tumor stages in our patients were more advanced than the tumor stages in other German series, in which the percentage of Okuda stage II and III tumors ranged from 43% to 61%[40,41,57].
It is surprising, however, that despite locally advanced tumors, we detected only a relatively small rate of nodal or extranodal metastases. This may indicate that extrahepatic tumor spread in HCC does not generally present a major problem although it may become highly relevant in individual cases especially after liver transplantation.
Overall outcome was poor. Reflecting the large number of advanced tumors and the minuscule number of surgically treated patients in our study, the Kaplan-Meier estimate for median survival was only 8.4 months. This is similar to German results reported by Petry et al[41] and within the scope of survival results for medically treated patients reported by other authors[38,58]. Other authors had also observed the clear-cut relationship of survival to the Okuda stage of the tumor.
AFP positivity was not related to survival. However, when only tumors with AFP levels greater than 100 ug/L were compared to tumors with lower or negative AFP levels, survival was shortened for the patients with the higher AFP levels. This may reflect a larger tumor burden in the patients with higher AFP levels as evidenced by significantly larger tumor nodules in AFP positive patients. This relationship of decreased survival in patients with an AFP level greater than 100 ug/L had also been described by others[57]. The relation of CHILD stage to survival was expected since the CHILD stage reflects some of the same variables that are measured by the Okuda score. The disappointing survival of CHILD C cirrhotics with HCC (median survival of less than one month) might suggest that these patients are not treated but offered symptom-targeted supportive care. However, this has not been tested prospectively.
Comparing survival of tamoxifen treated patients to patients receiving supportive therapy, there was no survival benefit for tamoxifen treated patients. This matches results from prospective studies[59,60] that proved tamoxifen to be ineffective in HCC. Therapy other than tamoxifen was related to increased survival. As small tumors in cirrhosis have a relatively good prognosis[61], it was therefore important to assess the influence of therapy on survival without the confounding effect of Okuda stage. The analysis of treatment groups stratified by Okuda stage confirmed that treated Okuda stage II patients survived longer. Ethanol injection (PEI) was associated with improved survival in Okuda stage II patients. A favorable impact of PEI on survival was previously reported in the literature[62].
One has to be cautious in interpreting retrospective data such as ours. It may be possible that patients were offered therapy because they were in a better clinical state, even though their Okuda stages were identical. Prospective studies must address the effectiveness of therapy in HCC.
As HCC is mostly a problem confined to the liver, with most patients dying from liver related complications such as liver failure or variceal bleeding, an effective approach to HCC treatment may consequently be any therapy aimed at local tumor control such as PEI, high-frequency thermocoagulation, or laser induced thermoco-agulation[63,64] to prevent portal vein thrombosis and parenchymal displacement.
Our data show the majority of HCC seen at our institution to be related to viral hepatitis. Therefore, to reduce the incidence of HCC, the prevalence of viral hepatitis must be controlled through vaccination programs and possibly through antiviral treatment. As only about two thirds of HCC were AFP positive, additional tumor markers must be sought to identify patients with HCC early in their disease when they might still benefit from curative surgical interventions. Survival was poor, but therapy (in our series mostly TACE, ethanol injection, and 131I-Lipiodol treatment) was associated with improved survival.
We thank Profs. Dr. Dr. F. Bidlingmaier and Klingmüller, Institute for Clinical Biochemistry, University of Bonn, for help in obtaining some of the AFP measurements. We are also indebted to Prof. Dr. B. Matz, Institute of Medical Microbiology, Section of Virology, University of Bonn, for his help in obtaining the hepatitis status for some of our patients.
Edited by Ma JY
| 1. | WHO. World Health Report. Geneva: WHO 2000; . [Cited in This Article: ] |
| 2. | Blakely T, Bates M, Garrett N, Robson B. The incidence of hepatocellular carcinoma in New Zealand. N Z Med J. 1998;111:471-474. [PubMed] [Cited in This Article: ] |
| 3. | Blakely TA, Bates MN, Baker MG, Tobias M. Hepatitis B carriage explains the excess rate of hepatocellular carcinoma for Maori, Pacific Island and Asian people compared to Europeans in New Zealand. Int J Epidemiol. 1999;28:204-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1838] [Cited by in F6Publishing: 1721] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 5. | Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 19] [Reference Citation Analysis (0)] |
| 6. | Rabe C, Caselmann WH. Interaction of Hepatitis B virus with cellular processes in liver carcinogenesis. Crit Rev Clin Lab Sci. 2000;37:407-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Caselmann WH, Alt M. Hepatitis C virus infection as a major risk factor for hepatocellular carcinoma. J Hepatol. 1996;24:61-66. [PubMed] [Cited in This Article: ] |
| 8. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 931] [Cited by in F6Publishing: 895] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 9. | Ray RB, Steele R, Meyer K, Ray R. Hepatitis C virus core protein represses p21WAF1/Cip1/Sid1 promoter activity. Gene. 1998;208:331-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 129] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Ray RB, Lagging LM, Meyer K, Ray R. Hepatitis C virus core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype. J Virol. 1996;70:4438-4443. [PubMed] [Cited in This Article: ] |
| 11. | Rüster B, Zeuzem S, Roth WK. Hepatitis C virus sequences encoding truncated core proteins detected in a hepatocellular carcinoma. Biochem Biophys Res Commun. 1996;219:911-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Ray RB, Steele R, Meyer K, Ray R. Transcriptional repression of p53 promoter by hepatitis C virus core protein. J Biol Chem. 1997;272:10983-10986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 217] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Tanabe G, Nuruki K, Baba Y, Imamura Y, Miyazono N, Ueno K, Arima T, Nakajyou M, Aikou T. A comparison of hepatocellular carcinoma associated with HBV or HCV infection. Hepatogastroenterology. 1999;46:2442-2446. [PubMed] [Cited in This Article: ] |
| 14. | Lee CM, Lu SN, Changchien CS, Yeh CT, Hsu TT, Tang JH, Wang JH, Lin DY, Chen CL, Chen WJ. Age, gender, and local geographic variations of viral etiology of hepatocellular carcinoma in a hyperendemic area for hepatitis B virus infection. Cancer. 1999;86:1143-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 15. | Okanoue T, Itoh Y, Minami M, Sakamoto S, Yasui K, Sakamoto M, Nishioji K, Murakami Y, Kashima K. Interferon therapy lowers the rate of progression to hepatocellular carcinoma in chronic hepatitis C but not significantly in an advanced stage: a retrospective study in 1148 patients. Viral Hepatitis Therapy Study Group. J Hepatol. 1999;30:653-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Iino S. Relationship between infection with hepatitis C virus and hepatocellular carcinoma in Japan. Antivir Ther. 1998;3:143-146. [PubMed] [Cited in This Article: ] |
| 17. | Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124-1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 387] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Widell A, Verbaan H, Wejstål R, Kaczynski J, Kidd-Ljunggren K, Wallerstedt S. Hepatocellular carcinoma in Sweden: its association with viral hepatitis, especially with hepatitis C viral genotypes. Scand J Infect Dis. 2000;32:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Chiesa R, Donato F, Tagger A, Favret M, Ribero ML, Nardi G, Gelatti U, Bucella E, Tomasi E, Portolani N. Etiology of hepatocellular carcinoma in Italian patients with and without cirrhosis. Cancer Epidemiol Biomarkers Prev. 2000;9:213-216. [PubMed] [Cited in This Article: ] |
| 20. | Zhang JY, Dai M, Wang X, Lu WQ, Li DS, Zhang MX, Wang KJ, Dai LP, Han SG, Zhou YF. A case-control study of hepatitis B and C virus infection as risk factors for hepatocellular carcinoma in Henan, China. Int J Epidemiol. 1998;27:574-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Tsukamoto T, Hamba H, Shuto T, Okuda T, Tamori A, Kuroki T. High prevalence of infection with hepatitis B and C viruses in patients with hepatocellular carcinoma in Japan. Hepatogastroenterology. 1999;46:357-359. [PubMed] [Cited in This Article: ] |
| 22. | Kuper HE, Tzonou A, Kaklamani E, Hadziyannis S, Tasopoulos N, Lagiou P, Trichopoulos D, Stuver S. Hepatitis B and C viruses in the etiology of hepatocellular carcinoma; a study in Greece using third-generation assays. Cancer Causes Control. 2000;11:171-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Shiratori Y, Shiina S, Imamura M, Kato N, Kanai F, Okudaira T, Teratani T, Tohgo G, Toda N, Ohashi M. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C- viral infection in Japan. Hepatology. 1995;22:1027-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 230] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Dürr R, Caselmann WH. Carcinogenesis of primary liver malignancies. Langenbecks Arch Surg. 2000;385:154-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | del Olmo JA, Serra MA, Rodríguez F, Escudero A, Gilabert S, Rodrigo JM. Incidence and risk factors for hepatocellular carcinoma in 967 patients with cirrhosis. J Cancer Res Clin Oncol. 1998;124:560-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Kato Y, Hamasaki K, Aritomi T, Nakao K, Nakata K, Eguchi K. Most of the patients with cirrhosis in Japan die from hepatocellular carcinoma. Oncol Rep. 1999;6:1273-1276. [PubMed] [Cited in This Article: ] |
| 27. | Døssing M, Petersen KT, Vyberg M, Olsen JH. Liver cancer among employees in Denmark. Am J Ind Med. 1997;32:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 28. | Mukaiya M, Nishi M, Miyake H, Hirata K. Chronic liver diseases for the risk of hepatocellular carcinoma: a case-control study in Japan. Etiologic association of alcohol consumption, cigarette smoking and the development of chronic liver diseases. Hepatogastroenterology. 1998;45:2328-2332. [PubMed] [Cited in This Article: ] |
| 29. | Donato F, Gelatti U, Chiesa R, Albertini A, Bucella E, Boffetta P, Tagger A, Ribero ML, Portera G, Fasola M. A case-control study on family history of liver cancer as a risk factor for hepatocellular carcinoma in North Italy. Brescia HCC Study. Cancer Causes Control. 1999;10:417-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Strohmeyer G, Niederau C, Stremmel W. Survival and causes of death in hemochromatosis. Observations in 163 patients. Ann N Y Acad Sci. 1988;526:245-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, Nashan B, Kubicka S, Maschek H, Tusch G. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol. 1999;17:324-331. [PubMed] [Cited in This Article: ] |
| 32. | Ringe B, Wittekind C, Weimann A, Tusch G, Pichlmayr R. Results of hepatic resection and transplantation for fibrolamellar carcinoma. Surg Gynecol Obstet. 1992;175:299-305. [PubMed] [Cited in This Article: ] |
| 33. | Klintmalm GB. [International registry of liver tumors in liver transplantation. A registry update on hepatocellular carcinoma (HCC)]. Zentralbl Chir. 2000;125:642-646. [PubMed] [Cited in This Article: ] |
| 34. | McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, Dunaway E, Williams J. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 264] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Wong LL, Limm WM, Severino R, Wong LM. Improved survival with screening for hepatocellular carcinoma. Liver Transpl. 2000;6:320-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, Farinati F, Naccarato R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132-2137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 37. | Pawarode A, Voravud N, Sriuranpong V, Kullavanijaya P, Patt YZ. Natural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patients. Am J Clin Oncol. 1998;21:386-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Sangro B, Herráiz M, Martínez-González MA, Bilbao I, Herrero I, Beloqui O, Betés M, de-la-Peña A, Cienfuegos JA, Quiroga J. Prognosis of hepatocellular carcinoma in relation to treatment: a multivariate analysis of 178 patients from a single European institution. Surgery. 1998;124:575-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Chuang WL, Chang WY, Lu SN, Su WP, Lin ZY, Chen SC, Hsieh MY, Wang LY, You SL, Chen CJ. The role of hepatitis B and C viruses in hepatocellular carcinoma in a hepatitis B endemic area. A case-control study. Cancer. 1992;69:2052-2054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 40. | Kubicka S, Rudolph KL, Hanke M, Tietze MK, Tillmann HL, Trautwein C, Manns M. Hepatocellular carcinoma in Germany: a retrospective epidemiological study from a low-endemic area. Liver. 2000;20:312-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Petry W, Heintges T, Hensel F, Erhardt A, Wenning M, Niederau C, Häussinger D. [Hepatocellular carcinoma in Germany. Epidemiology, etiology, clinical aspects and prognosis in 100 consecutive patients of a university clinic]. Z Gastroenterol. 1997;35:1059-1067. [PubMed] [Cited in This Article: ] |
| 42. | Van Roey G, Fevery J, Van Steenbergen W. Hepatocellular carcinoma in Belgium: clinical and virological characteristics of 154 consecutive cirrhotic and non-cirrhotic patients. Eur J Gastroenterol Hepatol. 2000;12:61-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Peters M, Wellek S, Dienes HP, Junginger T, Meyer J, Meyer Zum Büschendfelde KH, Gerken G. Epidemiology of hepatocellular carcinoma. Evaluation of viral and other risk factors in a low-endemic area for hepatitis B and C. Z Gastroenterol. 1994;32:146-151. [PubMed] [Cited in This Article: ] |
| 44. | La Vecchia C, Lucchini F, Franceschi S, Negri E, Levi F. Trends in mortality from primary liver cancer in Europe. Eur J Cancer. 2000;36:909-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227-3230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 313] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 46. | Law MG, Roberts SK, Dore GJ, Kaldor JM. Primary hepatocellular carcinoma in Australia, 1978-1997: increasing incidence and mortality. Med J Aust. 2000;173:403-405. [PubMed] [Cited in This Article: ] |
| 47. | Bethke BA, Schubert GE. Primary hepatic cancer and liver cirrhosis. Autopsy study covering fifty years. Hepatogastroenterology. 1984;31:211-214. [PubMed] [Cited in This Article: ] |
| 48. | Rimkus K, Dhom G. The epidemiology of primary liver cancer in a West German population: the Saarland. J Cancer Res Clin Oncol. 1986;111:248-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | Nagasue N, Kohno H, Tachibana M, Yamanoi A, Ohmori H, El-Assal ON. Prognostic factors after hepatic resection for hepatocellular carcinoma associated with Child-Turcotte class B and C cirrhosis. Ann Surg. 1999;229:84-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Braga C, La Vecchia C, Negri E, Franceschi S. Attributable risks for hepatocellular carcinoma in northern Italy. Eur J Cancer. 1997;33:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Nagasue N, Kohno H, Chang YC, Taniura H, Yamanoi A, Uchida M, Kimoto T, Takemoto Y, Nakamura T, Yukaya H. Liver resection for hepatocellular carcinoma. Results of 229 consecutive patients during 11 years. Ann Surg. 1993;217:375-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 232] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Petrides AS, Schulze-Berge D, Vogt C, Matthews DE, Strohmeyer G. Glucose resistance contributes to diabetes mellitus in cirrhosis. Hepatology. 1993;18:284-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Petrides AS. [Hepatogenic diabetes: pathophysiology, therapeutic options and prognosis]. Z Gastroenterol. 1999;Suppl 1:15-21. [PubMed] [Cited in This Article: ] |
| 54. | Kekulé AS, Lauer U, Meyer M, Caselmann WH, Hofschneider PH, Koshy R. The preS2/S region of integrated hepatitis B virus DNA encodes a transcriptional transactivator. Nature. 1990;343:457-461. [PubMed] [Cited in This Article: ] |
| 55. | Caselmann WH, Meyer M, Kekulé AS, Lauer U, Hofschneider PH, Koshy R. A trans-activator function is generated by integration of hepatitis B virus preS/S sequences in human hepatocellular carcinoma DNA. Proc Natl Acad Sci USA. 1990;87:2970-2974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Caselmann WH. Trans-activation of cellular genes by hepatitis B virus proteins: a possible mechanism of hepatocarcinogenesis. Adv Virus Res. 1996;47:253-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Allgaier HP, Deibert P, Olschewski M, Spamer C, Blum U, Gerok W, Blum HE. Survival benefit of patients with inoperable hepatocellular carcinoma treated by a combination of transarterial chemoembolization and percutaneous ethanol injection--a single-center analysis including 132 patients. Int J Cancer. 1998;79:601-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 58. | Schöniger-Hekele M, Müller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in Austria: aetiological and clinical characteristics at presentation. Eur J Gastroenterol Hepatol. 2000;12:941-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Liu CL, Fan ST, Ng IO, Lo CM, Poon RT, Wong J. Treatment of advanced hepatocellular carcinoma with tamoxifen and the correlation with expression of hormone receptors: a prospective randomized study. Am J Gastroenterol. 2000;95:218-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Riestra S, Rodriguez M, Delgado M, Suárez A, González N, de la Mata M, Diaz G, Miño-Fugarolas G, Rodrigo L. Tamoxifen does not improve survival of patients with advanced hepatocellular carcinoma. J Clin Gastroenterol. 1998;26:200-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Ebara M, Hatano R, Fukuda H, Yoshikawa M, Sugiura N, Saisho H. Natural course of small hepatocellular carcinoma with underlying cirrhosis. A study of 30 patients. Hepatogastroenterology. 1998;45 Suppl 3:1214-1220. [PubMed] [Cited in This Article: ] |
| 62. | Giorgio A, Tarantino L, Mariniello N, de Stefano G, Perrotta A, Aloisio V, Voza A, Finizia L, Alaia A, Del Viscovo L. Percutaneous ethanol injection under general anesthesia for hepatocellular carcinoma: 3 year survival in 112 patients. Eur J Ultrasound. 1998;8:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Vogl TJ, Mack M, Straub R, Eichler K, Engelmann K, Roggan A, Zangos S. [Percutaneous interstitial thermotherapy of malignant liver tumors]. Rofo. 2000;172:12-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Grasso A, Watkinson AF, Tibballs JM, Burroughs AK. Radiofrequency ablation in the treatment of hepatocellular carcinoma--a clinical viewpoint. J Hepatol. 2000;33:667-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |