Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung
Introduction
Metastases to lungs from various malignancies have generally been regarded as incurable and ultimately fatal (1,2). Systemic chemotherapy has played a major palliative role in keeping cancer-related symptoms and disease progression under control for a limited time, after which these tumors generally become refractory to chemotherapy. Long-term survival with chemotherapy for metastatic lung disease is extremely rare (2). A select group of patients develop lung metastases that are limited in number and extent, and are amenable to surgical or locally ablative techniques such as stereotactic body radiotherapy (SBRT) (2-4). In others with widespread disease, effective chemotherapy with near complete response could result in limited lung metastases (2,3). This state of limited metastases was coined “oligometastasis” in the 1990s when radiation planning and delivery were experiencing major technical advances (5). Patients with oligometastasis have been considered candidates for curative treatments because prolonging survival can be expected (6-8).
With the advent of improved 3-dimensional computed tomography (CT) based radiation treatment planning and more precise dose delivery methods, treatments using radiation have taken a leap forward in offering a more curative and less toxic approach in the management of cancers overall. The dose escalation coupled with high doses of radiation delivered per fraction in a short overall treatment time using high degrees of anatomic targeting accuracy results in an improved therapeutic ratio while minimizing radiation-associated early and late pulmonary toxicity. SBRT utilizes a large number of non-opposing beams with anatomic targeting using stereotactic localization and/or image guidance. Improved reproducibility in patient set-up and targeting accuracy facilitates the use of large fraction, ablative radiation doses resulting in high local control (LC) rates.
Many reports are now available on the use of SBRT for oligometastatic lung disease, although patient cohorts in these studies are heterogeneous with respect to cancer types and selection criteria (2-4,9-11). SBRT can either be done for patients with new overt oligometastatic disease (patients not suitable for chemotherapy/surgery), or after the chemotherapy options have been exhausted. Furthermore, the extent of oligometastatic disease varies in patients included in different studies. For example, an early study by the University of Rochester included patients with five or fewer lesions, not necessarily confined to the thorax (2). Kyoto University uses criteria of one or two pulmonary metastases, tumor diameter <4 cm, locally controlled primary tumor, and no other metastatic sites (12). Duke University’s criteria are stage IV cancer (any histology) with 1 to 5 metastases, with each metastasis ≤10 cm or ≤500 mL in volume on standard imaging (4).
The University of Rochester started using SBRT for oligometastasis in 2001 and has previously published survival and tumor control data showing 2-4 years overall survival (OS) rates of 50% and 28% and progression-free survival (PFS) rates of 26% and 26% respectively. Most of these patients were treated with a 10-fraction regimen using 4-6 Gy daily. As the outcomes of SBRT with less protracted regimes of five or fewer fractions were published by other institutions, our policy changed from ten-fraction SBRT to five-fraction SBRT using larger daily fraction sizes of 8-12 Gy. The present retrospective study was carried out to analyze the survival and tumor control and failure patterns for oligometastatic lung metastases treated with five fractions of SBRT among patients with chemorefractory disease or who were not candidates for chemotherapy or surgical resection.
Methods
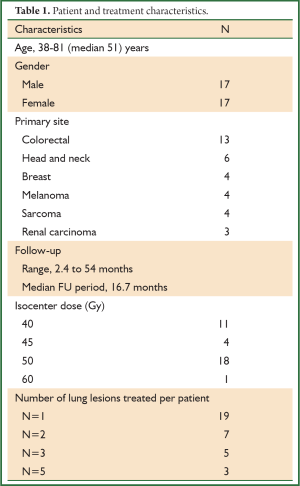
Between January 2008 and December 2011, thirty-four patients with oligometastatic cancer to the lungs who were considered refractory to (n=28) or ineligible for (n=6) chemotherapy were treated with SBRT. The 17 male and 17 female patients’ ages ranged from 38 to 81 years with a median age of 51 years (Table 1). The study was approved by the University of Rochester Medical Center Research Subjects Review Board.
Full table
The inclusion criteria of this study included patients with one to five lung metastases, age >18, KPS >70%, tumor diameter (on CT) <5 cm, locally controlled primary tumor, and no other active metastatic sites. Patients with primary non-small cell lung cancer were not included [as patients with separate nodules within the same lung are defined as T3 (same lobe) to T4 disease (same lung, different lobes)]. The work up included contrast enhanced CT of the thorax and upper abdomen and FDG-PET. Patients were followed with CT or PET-CT every 3-6 months. Patients with no progression of treated lesions who developed new radiographically apparent oligometastatic lesions on follow-up imaging were allowed to undergo repeat cycle(s) of SBRT for new lesions (13).
SBRT technique
The SBRT techniques that have been described in detail in previous publications from our group are briefly summarized here (2). All patients undergoing initial CT simulation required immobilization with a vacuum cushion device. All patients were treated with the Novalis ExacTrac system (BrainLab Inc.). The ExacTrac patient positioning platform using infrared reflecting body fiducial markers monitored by two ceiling mounted infrared cameras was used for patient positioning and real-time monitoring. Respiratory motion was minimized by using relaxed expiratory breath hold techniques (in most patients) or shallow breathing (in patients with poor lung function). Patients also underwent a CT in the set-up position, which was fused to the planning CT, prior to treatment and after the second fraction to ensure three-dimensional set-up accuracy. The gross tumor volume (GTV) was delineated using CT and fused PET imaging when needed. The use of arcs and non co-planner beams was encouraged. Dose volume histograms (DVH) were calculated for the lung (defined as total lung minus GTV), heart, esophagus, spinal cord, and liver. The planning target volume (PTV) was defined as a 7 mm circumferential and 11 mm superior-inferior expansion of the GTV (with no expansion for CTV) (2,3,13). The 80% isodose line encompassed the PTV, with isocenter dose defined as 100% of the prescribed dose. The prescribed target dose was determined based on the DVH of normal (uninvolved) lung and surrounding organs. The median prescription dose was 50 Gy in five fractions (range, 40-60 Gy) to isocenter with 80-100% isodose covering 95% of PTV. Patients were required to have 1,000 mL of tumor free lung, with a volume of lung receiving >20 Gy (V20) less than 25%. The spinal cord maximum was required to be <4.5 Gy/fraction. Care was taken so that hot spots (i.e., >80% isodose) occurred solely within the GTV. The dose for smaller peripheral tumors was mostly 50-60 Gy and the dose for larger central tumors was mostly 40-50 Gy.
Outcomes/statistics
The primary end point was tumor LC and secondary end points included regional control as well as OS. Actuarial tumor control and survival were calculated using the Kaplan-Meier actuarial survival analyses. OS was defined from date of completion of SBRT until death or last follow-up. Patient LC was scored as an event if any treated lesion grew by ≥20%, based on the Response Evaluation Criteria In Solid Tumors (RECIST) criteria or a local failure was confirmed pathologically. LC was analyzed per patient, meaning that if a patient had more than one lesion treated, progression of any of the treated lesions was considered a local failure. LC was analyzed by tumor size; among patients with more than one lesion, treated tumor size represents the largest lesion treated. Among patients who underwent repeat courses of SBRT for new lesions(s), only the LC of the index lesion(s) was considered in this study. STATA version 9.2 was used for all data analysis.
Results
The primary cancer sites among the 34 patients included colorectal (n=13), head and neck (n=6), breast (n=4), melanoma (n=4), sarcoma (n=4) and renal carcinoma (n=3). Follow-up ranged from 2.4 to 54 months (median 16.7 months) (Table 1). Nineteen patients had one lesion treated, seven patients had two lesions, five patients had three lesions, and three patients had five lesions treated with SBRT.
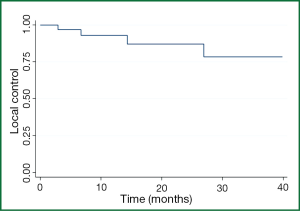
The 1-, 2-, and 3-year patient LC rates for all comers were 93%, 88%, and 80% respectively (Figure 1) with 1-, 2-, and 3-year OS of 62%, 44%, and 23% respectively. Four patients had lung metastases recur locally within 12 months; only one patient developed a local recurrence beyond 24 months (at 26 months), although only 12 patients were alive with follow-up beyond two years.
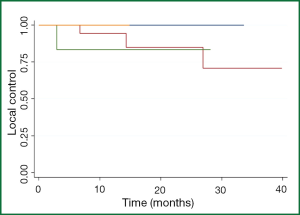
Among the 25 patients with maximal lesion size of 1- <2 cm, the 1- and 2-year patient LC rates were 95% and 88%. Among the seven patients with maximal lesion size of 2- <3 cm, the 1- and 2-year patient LC rates were 86% and 86%, and not significantly different than for patients with smaller lesions (Figure 2). Only one patient was treated with a maximal lesion size of >3 cm and only one patient was treated with a maximal lesion size <1 cm (neither of whom experienced a local recurrence. All five patients with local recurrences had colorectal cancer. Gender (P=0.30), previous treatment with chemotherapy (P=0.95), radiation dose (0.26), and nodule size (P=0.97) were not statistically significant on univariate analysis. A multivariate analysis was not done because of the small number of events. Symptomatic pneumonitis (grade ≥2) was not seen in any patient. Post-radiation fibrotic changes and consolidation occurred in 26 of the 34 patients.
Discussion
Metastatic disease to lung is one of the most common life threatening complications of cancer (2) and has been regarded as an incurable condition (1). However, patients with oligometastatic lung metastases have been considered candidates for curative treatment because of prolonged tumor LC rates and OS. Improved imaging now allows detection of tumor metastases at a smaller size and effective systemic therapy allows for potential ‘downstaging’ widely metastatic disease to an oligometastatic state, and thus provides an opportunity for local therapy as consolidation for patients with minimal bulk metastases (2). Surgical pulmonary metastatectomy in suitable patients with oligometastases is recognized as a potential curative treatment, and published data reveal a 5-year survival rate in these patients to be 20-40% (14). Alternatively, SBRT has also been used as a curative treatment of oligometastasis especially in patients who are not eligible candidates for surgery, either because of medical comorbidities, or because central lesions and/or multiple lesions would require a more extensive surgery than the patient could tolerate. The International Registry of Lung Metastases (IRLM) (14) reported the results of pulmonary complete resection in a large number of patients with lung metastases showing a 2-, 5-, and 10-year survival rate of 70%, 36%, and 26% respectively. In one of the largest published series on SBRT comprising of 175 patients (311 lesions), Siva et al. (15) has shown encouraging results with an OS rate of 54.5%. The IRLM study (14) also reported that a disease-free interval of more than 36 months and single metastasis were good prognostic factors. In our current study, gender (P=0.30), previous treatment with chemotherapy (P=0.95), radiation dose (0.11), and nodule size (P=0.97) were not statistically significant on univariate analysis. Symptomatic pneumonitis requiring treatment or hospitalization was not seen in any of the patients treated with SBRT.
Several reports have been published regarding the outcomes of SBRT for metastatic lung tumors, but no standard treatment regimens have been defined with respect to the optimal dose and fractionation schedules. From published studies, the dose-fractionation of SBRT varies from 40-60 Gy in 3-10 fractions. Our institution had been using 5 Gy ×10 from the inception of SBRT at the University of Rochester in 2001, but we recently changed the dose to 8-12 Gy in five fractions (2). Japanese studies have shown the correlation of dose effect with improved LC rates. With regards to the biologic effective dose, assuming an alpha/beta ratio of ten, (BED10), Hamamoto et al. (16) have reported rather poor LC of 25% at two years using 48 Gy in four fractions (105.6 Gy10) where as another report by Norihisa et al. (12) showed that LC rate of 43 metastatic lung tumors was 90% at two years with 60 Gy in five fractions (132 Gy10). A recent multi-institutional phase I/II study by Rusthoven et al. (17) reported a 2-year LC of 96% by 48-60 Gy in three fractions (124-180 Gy10) for 63 metastatic lung lesions. Similarly, McCammon et al. (18) showed the dose-LC relationship of SBRT for 246 lesions (primary or metastatic) by using a regimen of 54-60 Gy in three fractions (151-180 Gy10) achieving LC of 89% at three years.
Our earlier institutional report (2) showed a LC of 83% with 5 Gy fractions for total doses of 50 to 60 Gy, whereas a subsequent report showed LC of 87% at two and six years (3). In the current study, SBRT was delivered to a median dose of 50 Gy (range, 40-60 Gy) in five fractions with 1- and 2-year LC rates of 93% and 87% for all patients. Local progression occurred in four patients within 12 months and the other 30 patients had excellent LC and remained locally NED to date except one patient with primary colon cancer who failed locally at 26 months.
Onishi et al. (19) concluded that BED10 of >100 Gy at isocenter is preferable for treatment of primary lung cancer to achieve an optimal OS rate. For SBRT for pulmonary metastases, the BED10 of published dose-fractionation schedules ranges from 70-162 Gy, with the 2-year survival ranging from 33% to 84% in various studies (11,12,20,21). Norihisa et al. (12) have reported a 2-year survival rate of 84% in their study, whereas Lee et al. (11) have reported a 2-year survival rate of 68% from their study. Onimaru et al. (20) and Wulf et al. (21) reported survival rates of 49% and 33% at two years. The median and OS in present series was 16 months and 62%, 44%, and 23% at one, two, and three years, respectively.
When comparing dose fractionation schemes, it is important to recognize that different institutions prescribe dose differently and use different methodologies to plan and deliver SBRT. The dose can be prescribed to a point (i.e., isocenter), volume (i.e., GTV or PTV), or isodose line. Also, the PTV margins vary from institute to institute depending upon set up accuracy. Furthermore, defining the PTV reflects a difference in CT scanning with regards to free breathing vs. breath holding and fast vs. slow scan times (12). Also, some utilize 4-D scanning and definition of an ITV. Difference in dose calculation by taking in to account tissue heterogeneity corrections would affect margin dose in lung tumors (12). Lastly, differences in planning approaches (fixed vs. arcing beams; 3-D conformal vs. IMRT vs. VMAT) may also be relevant.
The primary cancer site seems to have a significant effect on outcomes of patients treated with SBRT. Milano et al. (22) reported earlier results from our institution using 50 Gy in ten fractions with 2-year LC of all lesions being 77%, concluding that metastatic tumors originating from the pancreas, biliary, liver, or colon were associated with poorer LC. Hamamoto et al. (16) also reported LC of 25% at two years and attributed the poor outcome to a large proportion of metastatic tumors from the colon (67%). Similarly Kim et al. (23) have also reported a poor outcome with 3-year LC of 52.7% using 39-51 Gy in three fractions. Takeda et al. (24) compared outcomes of primary lung tumors with metastases treated by SBRT showing a LC of 94% vs. 72% at two years (P<0.05). The present study also showed poor outcome with colorectal cancers, as all of the local failures were seen in this group.
In many studies, tumor size plays a significant role in predicting the LC, as various studies have shown a trend for improved LC with smaller size of the tumor and interval tumor volume (ITV <17 mL, i.e., approximately 3 cm in diameter) (23). A study by McCammon et al. (18) showed better LC in smaller tumors with GTV <8.9 mL (P=0.003). Kim et al. (25) reported that tumors <2.5 cm were associated with higher LC than tumors >2.5 cm; 100% vs. 82.3% in patients with primary or metastases lung tumors. Oh et al. (1) also reported that tumors <2.5 cm have better LC 98.3% vs. 77.8% (P<0.01). Our current study did not show a statistically significant effect of tumor size on patient LC, albeit with a relatively narrow range of size for most patients and a heterogeneous patient population.
Weaknesses of our study include the small retrospective nature, with a diverse population, in terms of primary site and histology. Because the majority of patients were treated with the same dose (50 Gy in five fractions), and the dose range was not large, we could not adequately analyze a dose-response relationship. Nevertheless, we are able to report promising LC and survival outcomes in this cohort of patients with oligmetastatic disease of the lung. Our conclusion is that SBRT for oligometastatic cancer to the lungs is effective and well tolerated for nonsurgical/chemorefractory patients.
Acknowledgements
The authors thank Ms. Laura Finger for editorial assistance.
Disclosure: The authors declare no sources of funding or conflicts of interest.
References
- Oh D, Ahn YC, Seo JM, et al. Potentially curative stereotactic body radiation therapy (SBRT) for single or oligometastais to the lung. Acta Oncol 2012;51:596-602. [PubMed]
- Okunieff P, Petersen AL, Philip A, et al. Stereotactic Body Radiation Therapy (SBRT) for lung metastases. Acta Oncol 2006;45:808-17. [PubMed]
- Milano MT, Katz AW, Zhang H, et al. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys 2012;83:878-86. [PubMed]
- Salama JK, Hasselle MD, Chmura SJ, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer 2012;118:2962-70. [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [PubMed]
- Singh D, Yi WS, Brasacchio RA, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys 2004;58:3-10. [PubMed]
- Kavanagh BD, McGarry RC, Timmerman RD. Extracranial radiosurgery (stereotactic body radiation therapy) for oligometastases. Semin Radiat Oncol 2006;16:77-84. [PubMed]
- Yang JC, Abad J, Sherry R. Treatment of oligometastases after successful immunotherapy. Semin Radiat Oncol 2006;16:131-5. [PubMed]
- Nagata Y, Negoro Y, Aoki T, et al. Clinical outcomes of 3D conformal hypofractionated single high-dose radiotherapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2002;52:1041-6. [PubMed]
- Hof H, Herfarth KK, Münter M, et al. Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 2003;56:335-41. [PubMed]
- Lee SW, Choi EK, Park HJ, et al. Stereotactic body frame based fractionated radiosurgery on consecutive days for primary or metastatic tumors in the lung. Lung Cancer 2003;40:309-15. [PubMed]
- Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys 2008;72:398-403. [PubMed]
- Milano MT, Philip A, Okunieff P. Analysis of patients with oligometastases undergoing two or more curative-intent stereotactic radiotherapy courses. Int J Radiat Oncol Biol Phys 2009;73:832-7. [PubMed]
- Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg 1997;113:37-49. [PubMed]
- Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: a systematic review. J Thorac Oncol 2010;5:1091-9. [PubMed]
- Hamamoto Y, Kataoka M, Yamashita M, et al. Local control of metastatic lung tumors treated with SBRT of 48 Gy in four fractions: in comparison with primary lung cancer. Jpn J Clin Oncol 2010;40:125-9. [PubMed]
- Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. [PubMed]
- McCammon R, Schefter TE, Gaspar LE, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2009;73:112-8. [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [PubMed]
- Onimaru R, Shirato H, Shimizu S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys 2003;56:126-35. [PubMed]
- Wulf J, Haedinger U, Oppitz U, et al. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: a noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004;60:186-96. [PubMed]
- Milano MT, Katz AW, Schell MC, et al. Descriptive analysis of oligometastatic lesions treated with curative-intent stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:1516-22. [PubMed]
- Kim MS, Yoo SY, Cho CK, et al. Stereotactic body radiation therapy using three fractions for isolated lung recurrence from colorectal cancer. Oncology 2009;76:212-9. [PubMed]
- Takeda A, Kunieda E, Ohashi T, et al. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011;101:255-9. [PubMed]
- Kim H, Ahn YC, Park HC, et al. Results and prognostic factors of hypofractionated stereotactic radiation therapy for primary or metastatic lung cancer. J Thorac Oncol 2010;5:526-32. [PubMed]