Quality of life after lung resection for lung cancer
Introduction
Advantages in the diagnosis and treatments of lung cancer have raised new important issues about patients perspectives in the decision making process. In this context is becoming mandatory to convey to the patient information about his residual quality of life (QoL) after the different treatments modalities.
In thoracic surgery, the use of QoL assessment has been certainly improved in the recent years, but its use in real practice remains unclear and underestimated. We are all aware about the raising interest and expectations of the patients during counseling about the impairment in their daily lifestyle and their growing needs of detailed comparison of different approaches in terms of QoL. Some patients may regard in-hospital postoperative complications as an acceptable risk, but are not ready to accept a long term disability in their lifestyle. Understanding the evolution of QoL after surgical treatment for lung cancer by the surgeon may give the patient the possibility to participate proactively to the difficult decision making process (1).
The aim of this paper is to expose our surgical community to most of the aspects of the delicate research of QoL after lung cancer surgery. The ESTS Quality of life and Patients Safety Working Group has the similar aim to promote collaboration between centers and develop guidelines in these relatively new fields of research.
Definition of QoL
The World Health Organization defined QoL as “‘individuals’ perceptions of their position in life in the context of their culture and value systems in which they live and in relation to their goals, expectations, standards and concerns” (2). Many other authors have tried to give a definition of QoL and the main characteristics consistently highlighted in these definitions are its subjectivity and multidimensionality.
Studying the outcomes of a treatment from the patient’s viewpoint is of crucial importance also for quality purposes and for the improvement of patient-centered care. Lung resection for cancer should aim at improving survival and symptoms without compromising the dignity of an acceptable QoL. The short and long-term effect of the resection on the QoL should be a mandatory information provided to the patient during the preoperative counseling. And the patient has the right to be informed about it.
QoL instruments and their use
A research about QoL after surgical treatment of lung cancer requires a detailed study design and the choice of an appropriate and validated tool, capable to investigate clinically significant parameters (3). Stages, types of surgery and the main endpoints may also affect the selection of the instrument.
Many authors have suggested that the selection of the QoL questionnaire should be performed among already validated tools in the field of interest (3,4). Important characteristics to be taken into account are: purpose/aim of the study, study population, measurement properties (reliability, validity and sensitivity), study design issues, scoring and data analysis.
In thoracic surgery we can distinguish between two types of questionnaires: generic and cancer specific. Table 1 shows the differences between the more broadly used questionnaires in our field. The peculiar feature of a generic survey is that it helps to compare our population with the healthy one. Intuitively, they cannot investigate symptom changes caused by specific treatments. One of the most used tool in this category is the Short Form 36 (SF36) (5).
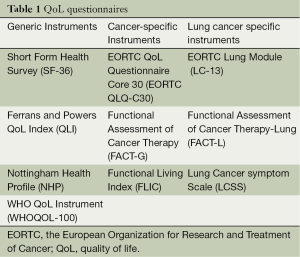
Full table
Cancer specific questionnaires study the effect of cancer and its treatment on the QoL. The widely used tool in oncology is the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire (EORTC QLQ-C30) (6). This questionnaire has been supported by several specific complementary modules, with the aim to be more responsive to changes in different subgroups. For instance, in our setting, the EORTC LC13 questionnaire for lung cancer has been useful to study specific symptoms like cough, haemoptysis, pain in chest and dyspnea (7).
However, so far, no specific validated questionnaire has been developed yet for lung cancer surgical population. That is why EORTC QoL Group has recently started an important research work to develop a revised lung cancer module. This should cover all QoL aspects relevant in the newly available diagnostic and therapeutic options and it should cover also all those QoL aspects that are relevant for patients with lung cancer but are missing in the previous version of LC13, like for instance, post-surgical symptoms.
Time of assessment
No guidelines have been developed in lung cancer setting about the best time to evaluate QoL after surgery. The preoperative values seem to have a crucial role in this type of research. In fact, it appears clear how difficult it is to define the effect of surgery on the life of a patient without a basal value for comparison. In studies dealing with Patient Reported Outcomes (8), a reasonably long term follow up after surgical intervention has been reported as desirable, since the patient may be biased in his judgment by factors such as presence of chest tube drainage limiting his mobility or by the news of cancer diagnosis (9). On the other hand, a shorter follow-up in QoL assessment may increase the response rate reducing the unsolved issue of drops-out in patient reported outcome measures (PROMs) research.
Mode of administration
Several studies have investigated the influence of the mode of administration of QoL questionnaires without finding any important effect (10,11). Recently, Gundy (12) reported only little effect on the reliability or the mean score of the EORTC QLQ-C30 between three different modes of administration (at home via mail, telephone interview and at the hospital clinic) with the possible exception of the emotional function scale. Other authors confirmed that the physical aspect of QoL is not sensitive to interviewer administration but the psychological aspect is (13).
QoL evolution after lung resection for cancer
A recent review (14) analyzed all the literature available to assess the impact of pulmonary resection on QoL of cancer patients. Surgery had a substantial effect on health-related quality of life (HRQOL). It has been demonstrated by many authors that, independently by the instrument used, patients submitted to pulmonary resection for lung cancer, experienced the most consistent decline in their health related QoL during the first trimester after surgery. The aspect of QoL mostly affected is the physical function (PF). This decline partly recovers in the next 3-12 months, but the standardized mean difference remain at medium relevance.
Compared to the general healthy population, patients with lung cancer waiting for the surgical treatment refer an impaired QoL in most of the subscales (15).
Predictors of decline
As a consistent proportion of lung cancer patients exhibit a significant postoperative worsening in their symptoms and emotional scales, many authors tried to identify factors associated with this decline in QoL. Predicting factors associated with residual QoL can help physicians involved in the care of lung cancer patients to refer patients to appropriate physical and emotional supportive programs either before or after surgery.
Extent of resection
Although surgery remains the gold standard for the treatment of lung cancer, recent technology improvements in radiotherapy and new biological targeted drugs have improved the survival rates of locally advanced cancer, which are considered medically inoperable. Most of the times, the only alternative to these therapies is pneumonectomy owing to the extent of the disease or the central location of the tumor. Pneumonectomy has been reported as the most consistent and strongest predictor of decline of QoL after surgery. Table 2 shows the main differences in the subscales of QoL after pneumonectomy compared to lobectomy in different studies.
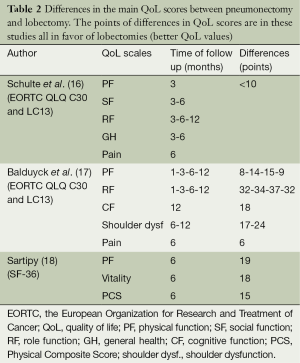
Full table
Schulte (16) et al. reported that patients submitted to pneumonectomy had significantly worse postoperative QoL values [statistical difference in PF at 3 months, social function (SF) at 3-6 months, role function (RF) at 3-6-12 months, general health (GH) at 3-6 months and pain at 6 months] compared to those submitted to lobectomy/bilobectomy.
This finding was confirmed by Balduyck et al. (17) In a cohort of 100 patients they found that the pneumonectomy group did not reach the baseline values in a 12-month follow-up period in PF, RF, pain, shoulder function and dyspnea scales. Leo et al. (19) estimated that almost 25% of survivors after pneumonectomy experienced an impaired overall QoL 6 months after the operation.
Moreover, Sartipy (18) identified the extent of resection as the strongest predictor of decline in the physical component of QoL scores after 6 months. The mental components however were not different after lobectomy or pneumonectomy. Similar results were described by Brunelli et al.: pneumonectomy patients had a significant lower Physical Composite Score (PCS) but similar Mental Composite Score (MCS) compared to lobectomy patients 3 months after the operation (20).
Age
Elderly people demonstrated to behave like younger counterparts in terms of self-reported outcomes after lung resections in many studies. Burfeind et al. (21) did not find any differences in QoL after lobectomy between patients older or younger of 70 years. Both groups experienced similar decline in PF, RF, SF, Global QoL increased pain in their chest and arm as well dyspnea at three months after surgery. More interesting, both groups returned to baseline at 6 months survey. All domains remained stables at 12 months except PF in the older group, which slightly decreased at the last follow-up. However, patient older than 70 experienced preoperatively less emotional impairment compared to the younger ones.
Ferguson et al. (22) reported similar QoL scores for older and younger patients despite an increased percentage of postoperative complications in the older group. No significant differences were found between the two groups but older patients had lower scores in PF, more fatigue, greater dyspnea and less depression. Age was found inversely related to physical functioning score and directly related to fatigue score and dyspnea score.
These results have been confirmed by Salati et al. (23). They found similar residual QoL values in elderly patients compared to younger ones 3 months after major lung resections. Conversely and in line with other reports, prior the operation, elderly patients had significant lower PCS (P=0.03) and PF (P=0.009) but higher MCS (P=0.08) and MH (P=0.02).
On the other hand, Schulte et al. (24) reported a failure to recover the preoperative EORTC QLQ C30 and LC13 scores in the elderly patients. The younger patients reported lower SF and RF at discharge compared to the elderly ones; however these scales recovered for younger patients up to 24 months scoring better compared to the older group. Younger patients exceeded preoperative level of global health after 24 months. Pain was always higher in the younger patients.
Others
Current smoking at the time of surgery has been reported to be associated with a poor postoperative QoL (25). Specific preoperative QoL scores were found to be correlated to a decline in most of the postoperative scores (26): Patients with higher preoperative scores of physical functioning and bodily pain and those with worse mental health score, were at high risk of relevant physical deterioration after surgery. Predictors of emotional worsening after treatment were lower ppoFEV1, higher SF and mental health.
Pompili et al. described an acceptable QoL in chronic obstructive pulmonary disease (COPD) patients after pulmonary lobectomy, which was similar to the residual QoL reported by non-COPD patients (27).
More than one analysis identified that the administration of adjuvant chemotherapy has a negative impact on the residual QoL of surgical patients (28,29). Möller et al. (29) described that adjuvant chemotherapy, along with the extent of resection and older age, was one of the factors significantly associated with worse physical scores 6 months after surgery. Paull et al. (28) identify exposure to postoperative chemotherapy as a risk factor for poor short and long-term QoL measured with Functional Assessment of Cancer Therapy (FACT-G) and Functional Assessment of Cancer Therapy-Lung (FACT-L).
Several studies have tried to investigate the association between objective functional parameters, traditionally used to select patients for operation, and residual QoL, without finding consistent results (15,20,22). However, Handy et al. (15) found in patients with carbon monoxide lung diffusion capacity (DLCO) <45% significantly worse values of the preoperative PF and RF and of the postoperative role functioning-physical and bodily pain compared to patients with higher DLCO values.
QoL after minimally invasive thoracic surgery
VATS procedures for treatment of lung cancer have demonstrated their superiority in terms of postoperative recovery and better tolerance of postoperative therapies compared to “open” surgery. Major studies have also reported equivalent 5-year survival and minor rates of postoperative complications after VATS lobectomy. However, few reports have been published so far reporting significant differences in terms of QoL between VATS and open lung surgery for cancer.
Handy et al. (30) compared QoL of 49 patients submitted to VATS lobectomy with 192 “open” procedures. He adopted the SF-36 preoperatively and 6 months after resection demonstrating a better QoL recovery after VATS lobectomy. Patients submitted to operation through thoracotomy reported a significantly worse physical functioning, role functioning-physical and social functioning compared to preoperative values. Postoperatively, VATS patients were either at baseline or better in all eight SF36 categories (physical functioning, role functioning-physical, role functioning-emotional, social functioning, bodily pain, mental health, energy, and GH). Obviously, we can speculate that other endpoints found by the authors at 6 months (less hospital readmission, less requirement of pain medication and improved functional outcomes) may have influenced in some ways the QoL results.
Most recently, Rizk et al. (31) reported similar physical component summary of SF-36 and pain scores after VATS and thoracotomy through the first 12 months after surgical resection. After thoracotomy the SF-36 mental composite score (MCS) scores were paradoxically even higher than after VATS surgery. The authors performed a prospective cohort study only in resections for stage I non-small cell lung cancer (NSCLC) with a generic survey and with only 59% of response rate at 12 months of follow-up. As many advances have been done in terms of minimally invasive treatment in lung cancer treatment, future properly powered studies will be needed to find differences in patients reported outcomes also for more advanced stages and using more specific validated questionnaires.
Cerfolio et al. (32) have recently collected QoL data from a cohort of 168 patients submitted to robotic anatomic pulmonary resections. Subjects were asked to fill the SF-12 pre-operatively and at both 3 weeks and 4 months postoperatively either at a clinic appointment or by mail. Further to objectives outcomes, patients reported better QoL scores compared with 318 propensity-matched patients who underwent lobectomy by rib and nerve-sparing thoracotomy. In particular, a higher mental QoL score was observed 3 weeks postoperatively in robotic patients compare to the thoracotomy group. A similar trend was observed for physical QoL score at 3 weeks without a significant statistical significance. At 4 months there was no difference between the two groups in terms of physical and mental QoL measured with SF-12 survey.
Although objective evidence seems to confirm that VATS lobectomy is non-inferior in terms of long-term cancer outcomes, larger scale properly powered studies are needed to verify the superiority of VATS in terms of QoL.
QoL and survival
Recent evidences in oncologic setting have associated patients reported outcomes to cancer free survival. This concept needs to be taken into account when evaluating the importance of establishing psychological and physical supporting programs.
Möller et al. (33) have analyzed the prognostic role of perioperative changes in QoL among a heterogeneous series of patients submitted to lung cancer surgery and using the SF-36 survey. They found that postoperative declines of at least 10% in the physical and mental component were associated with 18% and 13% higher risks of death, respectively. We confirmed this association (34) in a group of 131 consecutive patients submitted to pulmonary lobectomy for early stages NSCLC with a complete follow-up (median 40 months). The physical component of QoL was associated with overall and cancer-specific survivals. Patients with higher physical component of QoL (PCS >50) lived significantly longer than patients with lower score (PCS <50) independent of other confounding factors.
Of remarkable interest are the results from a multicenter study, which randomized high-risk operable patients to sublobar resection versus sublobar resections associated with brachytherapy. The authors included longitudinal QoL assessments (35) up to 24 months from the treatment and linked these results to the survival and adverse events rate. They found that poor baseline QoL scores were not predictive of worse overall or recurrence-free survival. They also found that VATS was associated with improvement in PF at 3 months, and improved dyspnea scores at 12 months.
QoL and patient centered-care
In the last years a growing debate has been originated in most of the International Societies: how to include patients’ preferences and acceptance of risks in the surgical decision algorithms. The importance of the informed consent and of a shared decision making process unfortunately came even from the expanded attitude to a “defensive” medicine. Actually, it is not so intuitive to insert the patient into this complex process (36,37): not all patients want to be involved in the treatments choice (38), not all patients possess the proper level of knowledge to get the sense of surgical risks or benefits. Moreover, a lack in the surgeon communications skills has been recognized at the base of certain level of misunderstanding during medical counseling (39). Training of health care professionals has only recently started to emphasize communication skills. The General Medical Council in UK has introduced a document focusing on the central role of a shared decision making process, empowering the patient beyond the doctor’s recommendations (40). In oncologic thoracic surgery, the British Thoracic Society has been the first to include the patient acceptance of risk as an integral part of the surgical risk assessment algorithm (41). The role of QoL in this patient-centered type of care is two-fold: first of all patients need to have complete information about their residual QoL after pulmonary resection in order to undertake the best decision for their cancer treatment. Secondly, how this shared decision should improve the postoperative QoL? Further studies are needed to address this issue. Recently, the European Society of Thoracic Surgeons (ESTS) has created a QoL and Patients Safety working group, which has among its tasks the one to check the present degree of knowledge and interest in QoL and patient-reported outcome (PRO) measures of the surgical community. The final aim is to promote the inclusion of QoL parameters among the outcome measures used for surgical audit and quality control.
Conclusions
Surgical treatment of NSCLC achieves the best results in terms of long-term survival. Advantages in surgical techniques and postoperative therapies have changed in the last decades the life expectancy of lung cancer survivors. But how these treatments affect the quality of the daily lifestyle of our patients is still object of investigations. The development of more specific surgical-related questionnaires may help the thoracic surgeons community to implement future research on QoL outcomes.
Acknowledgements
This paper has been developed thanks to the contributions of Nuria Novoa and Bram Balduyck as members of ESTS Quality of Life and Patient Safety Working Group.
Disclosure: The author declares no conflict of interest.
References
- Cykert S, Kissling G, Hansen CJ. Patient preferences regarding possible outcomes of lung resection: what outcomes should preoperative evaluations target? Chest 2000;117:1551-9. [PubMed]
- The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med 1995;41:1403-9. [PubMed]
- Fitzsimmons D, George S, Payne S, et al. Differences in perception of quality of life issues between health professionals and patients with pancreatic cancer. Psychooncology 1999;8:135-43. [PubMed]
- Juniper EF. Validated questionnaires should not be modified. Eur Respir J 2009;34:1015-7. [PubMed]
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [PubMed]
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994;30A:635-42. [PubMed]
- Brédart A, Razavi D, Robertson C, et al. Timing of patient satisfaction assessment: effect on questionnaire acceptability, completeness of data, reliability and variability of scores. Patient Educ Couns 2002;46:131-6. [PubMed]
- Montazeri A, Hole DJ, Milroy R, et al. Does knowledge of cancer diagnosis affect quality of life? A methodological challenge. BMC Cancer 2004;4:21. [PubMed]
- Weinberger M, Oddone EZ, Samsa GP, et al. Are health-related quality-of-life measures affected by the mode of administration? J Clin Epidemiol 1996;49:135-40. [PubMed]
- Perkins JJ, Sanson-Fisher RW. An examination of self- and telephone-administered modes of administration for the Australian SF-36. J Clin Epidemiol 1998;51:969-73. [PubMed]
- Gundy CM, Aaronson NK. Effects of mode of administration (MOA) on the measurement properties of the EORTC QLQ-C30: a randomized study. Health Qual Life Outcomes 2010;8:35. [PubMed]
- Cheung YB, Goh C, Thumboo J, et al. Quality of life scores differed according to mode of administration in a review of three major oncology questionnaires. J Clin Epidemiol 2006;59:185-91. [PubMed]
- Brunelli A, Pompili C, Koller M. Changes in quality of life after pulmonary resection. Thorac Surg Clin 2012;22:471-85. [PubMed]
- Handy JR Jr, Asaph JW, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. Chest 2002;122:21-30. [PubMed]
- Schulte T, Schniewind B, Dohrmann P, et al. The extent of lung parenchyma resection significantly impacts long-term quality of life in patients with non-small cell lung cancer. Chest 2009;135:322-9. [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life evolution after lung cancer surgery: a prospective study in 100 patients. Lung Cancer 2007;56:423-31. [PubMed]
- Sartipy U. Prospective population-based study comparing quality of life after pneumonectomy and lobectomy. Eur J Cardiothorac Surg 2009;36:1069-74. [PubMed]
- Leo F, Scanagatta P, Vannucci F, et al. Impaired quality of life after pneumonectomy: who is at risk? J Thorac Cardiovasc Surg 2010;139:49-52. [PubMed]
- Brunelli A, Socci L, Refai M, et al. Quality of life before and after major lung resection for lung cancer: a prospective follow-up analysis. Ann Thorac Surg 2007;84:410-6. [PubMed]
- Burfeind WR Jr, Tong BC, O'Branski E, et al. Quality of life outcomes are equivalent after lobectomy in the elderly. J Thorac Cardiovasc Surg 2008;136:597-604. [PubMed]
- Ferguson MK, Parma CM, Celauro AD, et al. Quality of life and mood in older patients after major lung resection. Ann Thorac Surg 2009;87:1007-12; discussion 1012-3. [PubMed]
- Salati M, Brunelli A, Xiumè F, et al. Quality of life in the elderly after major lung resection for lung cancer. Interact Cardiovasc Thorac Surg 2009;8:79-83. [PubMed]
- Schulte T, Schniewind B, Walter J, et al. Age-related impairment of quality of life after lung resection for non-small cell lung cancer. Lung Cancer 2010;68:115-20. [PubMed]
- Balduyck B, Sardari Nia P, Cogen A, et al. The effect of smoking cessation on quality of life after lung cancer surgery. Eur J Cardiothorac Surg 2011;40:1432-7; discussion 1437-8. [PubMed]
- Pompili C, Brunelli A, Xiumé F, et al. Predictors of postoperative decline in quality of life after major lung resections. Eur J Cardiothorac Surg 2011;39:732-7. [PubMed]
- Pompili C, Brunelli A, Refai M, et al. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg 2010;37:525-30. [PubMed]
- Paull DE, Thomas ML, Meade GE, et al. Determinants of quality of life in patients following pulmonary resection for lung cancer. Am J Surg 2006;192:565-71. [PubMed]
- Möller A, Sartipy U. Predictors of postoperative quality of life after surgery for lung cancer. J Thorac Oncol 2012;7:406-11. [PubMed]
- Handy JR Jr, Asaph JW, Douville EC, et al. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg 2010;37:451-5. [PubMed]
- Rizk NP, Ghanie A, Hsu M, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg 2014;98:1160-6. [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [PubMed]
- Möller A, Sartipy U. Associations between changes in quality of life and survival after lung cancer surgery. J Thorac Oncol 2012;7:183-7. [PubMed]
- Pompili C, Salati M, Refai M, et al. Preoperative quality of life predicts survival following pulmonary resection in stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;43:905-10. [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: Results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg 2015;149:718-26. [PubMed]
- Katz SJ, Hawley S. The value of sharing treatment decision making with patients: expecting too much? JAMA 2013;310:1559-60. [PubMed]
- Lim E. Patients' perspective in the surgical decision-making process. Thorac Surg Clin 2012;22:539-43. [PubMed]
- Hotta K, Kiura K, Takigawa N, et al. Desire for information and involvement in treatment decisions: lung cancer patients' preferences and their physicians' perceptions: results from Okayama Lung Cancer Study Group Trial 0705. J Thorac Oncol 2010;5:1668-72. [PubMed]
- Amalraj S, Starkweather C, Nguyen C, et al. Health literacy, communication, and treatment decision-making in older cancer patients. Oncology (Williston Park) 2009;23:369-75. [PubMed]
- General Medical Council. Consent: patients and doctors making decisions together. London: General Medical Council, 2008.
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65 Suppl 3:iii1-27. [PubMed]


