Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities
Background
Modulation of the immune response to elicit antitumor activity has been well established in the setting of malignant melanoma and renal cell carcinoma. High-dose interleukin-2 had been the mainstay for management of advanced disease in these clinical settings. Discovery of immune checkpoints that regulate the immune response has led to development of strategies that can be positively exploited to impact T cell activity and generate clinically relevant antitumor activity. Antibodies blocking cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death-1 (PD-1) have been approved for the treatment of advanced malignant melanoma and an anti-PD-1 antibody has been approved for squamous non-small cell lung cancer (NSCLC) (1-10).
Generation of an antitumor immune response is a complex multi-step process—recognition of the tumor antigen in the context of self-human leukocyte antigen (HLA) molecules by T cells constitutes the first step. Fine-tuning of the immune response then ensues and involves interactions between molecules expressed on the T cells and antigen presenting cells (APCs). CD28, a stimulatory checkpoint expressed on T cells, binds to the ligands CD80 and CD86 (B7-1 and B7-2) on APCs and results in stimulation of T cells. CTLA-4 is an inhibitory checkpoint protein that is expressed on the surface of activated T cells that also binds to the B7 family of molecules expressed on APCs and inhibits the T cells. CTLA-4 binds to the B7 molecules with a higher affinity than CD28, resulting in loss of co-stimulation through CD28 (11). Ipilimumab, an anti-CTLA-4 antibody, binds to CTLA-4 and blocks its interaction with B7 molecules, preventing T cell inactivation (1). PD-1 is also an immune inhibitory checkpoint expressed on the surface of activated T cells. Interaction between PD-1 and its ligands, programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2), expressed on APCs on some normal cells and tumor cells, leads to T cell inactivation. Additionally, PD-L1 expressed on T cells can interact with the B7 family of molecules expressed on APCs and results in the T cells switching off. In contrast to CTLA-4-mediated inhibition that is a central event, PD-1-mediated inhibition can occur peripherally in the tumors, providing a potential mechanism for adaptive immune resistance (11). Anti-PD-1 antibodies, nivolumab and pembrolizumab, can bind to the PD-1 receptor, blocking its interaction with PD-L1/L2 to prevent T cell inactivation (2,3). Both checkpoint inhibitor pathways have a mechanism of action that is not limited to one tumor or tissue type (11).
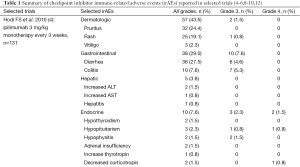
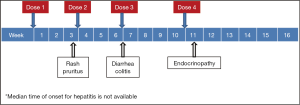
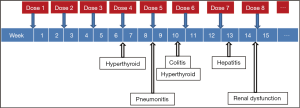
While unrestrained T cell activation with immune checkpoint blockade has been shown to translate into antitumor responses, it can also manifest as toxicity in the form of autoimmune breakthrough or immune-related adverse events (irAEs) (Table 1). “Select adverse events” allude to toxicities that have an autoimmune etiology and require careful monitoring and specific management strategies. These toxicities have varying time to onset and include dermatologic adverse events in the form of rash and pruritus, gastrointestinal adverse events in the form of diarrhea and colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency (Figures 1,2). Specific treatment algorithms have been developed to guide the treating physician to mitigate these autoimmune toxicities. These toxicities are reversible if treated promptly and appropriately, but can lead to high-grade adverse events, including death, if unrecognized. Treatment consists of immunosuppression using corticosteroids and other agents such as tumor necrosis factor-alpha antagonists and mycophenolate mofetil, depending on severity (13-15).
The use of immune checkpoint blockade so far has been limited to a relatively small fraction of physicians involved in the treatment of malignant melanoma (16). With the proof of principle and efficacy established in this disease process, these agents are being extensively investigated in other malignancies including lung cancer, renal cell carcinoma, gastric cancer, bladder cancer, ovarian cancer, and hematologic malignancies. Early results from some of these investigations are extremely encouraging and will likely lead to more indications in addition to the approved indications for the treatment of malignant melanoma and squamous NSCLC (12). It is therefore essential that the oncology community be aware of the irAEs to recognize them in a timely fashion and be well-versed with their management. We discuss the select adverse events and their management at our institution based on the established algorithms.
Management of common irAEs
Education and communication between patients, caregivers, and the clinical team is vital for timely recognition and successful management of irAEs. The most common adverse events reported in patients receiving ipilimumab are fatigue, diarrhea, pruritus, rash and colitis (1,4,5). Adverse events in >20% of patients receiving PD-1 inhibitors include fatigue, rash, pruritus, cough, diarrhea, decreased appetite, constipation, and arthralgia (2,3,6-10). Treatment-related irAEs with PD-1 inhibitors are predominantly grade 1 or 2 in severity, and can be managed with algorithms developed for irAEs observed with ipilimumab (13-15). Prior ipilimumab exposure does not appear to impact the safety profile of currently approved PD-1 inhibitors (6,8,9). There were no drug-related deaths in a phase I study of 89 patients with advanced melanoma refractory to CTLA-4 inhibition treated with pembrolizumab; four (4.5%) patients discontinued due to immune-related or special interest adverse events, and fatigue was the only grade 3 to 4 event reported in more than one patient (6). In a phase III study of nivolumab that included patients with advanced melanoma who had previously received ipilimumab, no treatment-related deaths were observed (9).
General principles for the optimal management of irAEs include early recognition and judicious use of immunosuppression which based on the severity of the event. Clinical presentations, suggested laboratory and radiologic investigations, and decision considerations for continuation of immunotherapy for selected irAEs are discussed below and summarized in Table 2. While not directly related, it is important to note that if prolonged immunosuppression is expected, patients must also receive appropriate antibiotic prophylaxis to prevent opportunistic infections (13-15).
Dermatologic toxicity
Dermatologic toxicities, such as rash and pruritus, occur in approximately 50% of patients treated with ipilimumab. The median time to onset of moderate, severe, or life-threatening immune-mediated dermatitis in patients treated with ipilimumab in one phase III trial was 3.1 weeks and ranged up to 17.3 weeks from treatment initiation (4). Rashes are often mild, appearing after the first or second dose. Rare cases of severe rashes such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are reported in <1% of patients (1,4,5). PD-1 inhibitor data to date shows dermatologic toxicity of all grades in up to 37.4% of patients (2,3,6-10). Workup should include a physical exam for signs and symptoms of reticular, maculopapular, and erythematous rash, usually on the trunk or extremities.
Mild to moderate (grade 1 to 2) dermatologic toxicity, defined by the Common Terminology Criteria for Adverse Events (CTCAE) as a maculopapular rash with or without symptoms (e.g., pruritus, burning, tightness) covering up to 10-30% of body surface area and limiting instrumental activities of daily living (ADL) (17), can usually be treated for symptomatic relief with topical corticosteroid ointments and does not require interruption in immune checkpoint inhibitor therapy (13-15). For grade 2 toxicity, defined in the ipilimumab Risk Evaluation and Mitigation Strategy (REMS) program as a diffuse, non-localized rash that is ≤50% of skin surface (13), it is recommended to withhold ipilimumab and treat with topical corticosteroids with consideration for systemic corticosteroids at 0.5 mg/kg/day prednisone or equivalent if there is no improvement in symptoms within 1 week (13-15). Our institutional recommendation is to continue ipilimumab or PD-1 inhibitor therapy if patients have involvement of <30% of body surface area, are asymptomatic, or toxicity can be managed with topical corticosteroid creams and antipruritics, such as hydroxyzine and diphenhydramine. For patients with 10-30% of body surface area involvement that is symptomatic, ipilimumab or PD-1 inhibitor therapy is held and consideration is given to initiation of steroids at 0.5-1 mg/kg prednisone or equivalent for control of symptoms.
Severe (grade 3 to 4) toxicity may require admission to the hospital and a formal dermatology consultation with consideration of skin biopsy for rashes that show signs of blistering, full thickness dermal ulceration, or necrotic, bullous, or hemorrhagic changes. Immune checkpoint inhibitor therapy should be permanently discontinued and systemic corticosteroids initiated at 1-2 mg/kg/day prednisone or equivalent for these patients. In the pivotal phase III melanoma trial, patients who received ipilimumab and developed dermatitis had complete resolution of symptoms with high-dose corticosteroids at a median dose of 60 mg/day prednisone or equivalent administered for up to 14.9 weeks followed by a taper (4). Steroids should be tapered over 1 month following improvement of symptoms to mild severity (13-15).
Vitiligo was reported to occur in both CTLA-4 and PD-1 inhibitor clinical trials. Toxicity can be permanent but does not require interruption of immune checkpoint inhibitor therapy or toxicity treatment (1-10). Oral mucositis and dry mouth are more frequently reported with PD-1 inhibitors. Oral candidiasis may be considered, especially if a patient has been on corticosteroids for the management of other irAEs (13-15).
Diarrhea/colitis
Diarrhea and colitis may present approximately 6 weeks into immune checkpoint inhibitor therapy and appears to be dose-dependent with ipilimumab (1,4,5). Diarrhea at any grade was reported in approximately 30% of 511 patients treated with ipilimumab in a phase III melanoma trial. Less than 10% of patients had severe grade 3 or 4 diarrhea, defined as ≥7 stools above baseline, fever, ileus, or peritoneal signs. Of the 511 patients, five (1%) developed intestinal perforation, four (0.8%) died as a result of complications, and 26 (5%) were hospitalized for severe enterocolitis (4). In PD-1 inhibitor trials, diarrhea and colitis were observed to be less frequent. The incidence of grade 3 or 4 immune-mediated colitis, defined as requiring use of corticosteroids with no clear alternate etiology, occurred in 1-2% of patients (2,3,6-10). Patients who had significant diarrhea/colitis during ipilimumab treatment have subsequently been treated with PD-1 inhibition without developing diarrhea/colitis (6,9).
For mild (grade 1) symptoms, defined as <4 stools above baseline per day, clinical algorithms recommend continuing immune checkpoint inhibitor therapy with symptomatic treatment, without initiation of corticosteroids (13-15). Our institutional recommendations include stool studies testing for Clostridium difficile infection, lactoferrin, and ova and parasites in addition to a baseline complete blood count (CBC) with differential, complete metabolic panel (CMP) and magnesium and phosphorus levels for electrolyte repletion in this group of patients. We also recommend avoiding reflex treatment with antidiarrheal agents (e.g., loperamide, diphenoxylate/atropine) that could potentially mask higher-grade toxicity. Our supportive care recommendations include adequate oral hydration, bland diet, closer monitoring, and follow-up depending on the results of stool studies, especially for patients on concomitant medications with potential to mask toxicity (e.g., opioids). Budesonide may be considered in selected cases but is not recommended as standard prophylaxis given no statistically significant difference in the incidence of diarrhea and colitis with or without budesonide in a phase II, double-blind, placebo-controlled study with patients treated with ipilimumab (18).
For moderate (grade 2) symptoms, defined as 4 to 6 stools above baseline per day, abdominal pain, or blood or mucus in stool, infection must be ruled out with a Clostridium difficile test, ova and parasites, and a stool culture. Ipilimumab REMS toxicity management recommends withholding immune checkpoint inhibitor therapy and initiating systemic corticosteroids at 0.5 mg/kg/day prednisone or equivalent if symptoms persist for >1 week (13). Our institutional recommendations are in line with the REMS recommendation to hold immune checkpoint inhibitor therapy, but we prefer not to use reflex steroids for stable patients until results from the stool studies are available. We also prefer endoscopic evaluation with flexible sigmoidoscopy to prove autoimmune colitis if symptoms persist >1 week, prior to initiating steroids.
For severe (grade 3 or 4) toxicity, defined as ≥7 stools above baseline per day, peritoneal signs consistent with bowel perforation, ileus or fever, immune checkpoint inhibitor therapy should be permanently discontinued. Ipilimumab REMS toxicity management recommends initiation of systemic corticosteroids at 1-2 mg/kg/day prednisone or equivalent once bowel perforation is ruled out (13). Our institutional recommendations are to admit these patients for observation and intravenous hydration, obtain stool studies, and defer initiation of steroids if the patient is clinically stable until stool studies are available (usually 24 hours). Gastroenterology evaluation with flexible sigmoidoscopy is preferred prior to committing these patients to high-dose steroids. Clinically unstable patients are initiated on high-dose steroids immediately at the time of admission; our preference is methylprednisolone 125 mg intravenously every day for 3 days to evaluate response to steroids, followed by a slow prednisone taper starting at 1-2 mg/kg over at least 1 month. In phase III ipilimumab clinical trials, patients with grade 3 to 5 enterocolitis were treated with high-dose corticosteroids with a median duration of treatment of 2.3 weeks for up to 13.9 weeks (4,5). The duration of high-dose corticosteroid treatment for patients receiving nivolumab and pembrolizumab in clinical trials has ranged from 7 days to up to 2.4 months with complete resolution of symptoms in a majority of the patients (2,3,6-10). If there is no improvement in symptoms after 5-7 days of high-dose steroids, our institutional recommendations require consideration for infliximab at a dose of 5 mg/kg in keeping with standard REMS management after ruling out bowel perforation or sepsis (13). Infliximab may be repeated 2 weeks after the first dose if high-grade symptoms persist despite continuing steroids. Mycophenolate mofetil may need to be considered for selected patients. Empiric antibiotics should be considered for patients who present with fever or leukocytosis; prophylactic antibiotics should be administered to patients on long-term immune suppression. Rare cases resulting in bowel perforation may require colostomy (13-15).
Hepatotoxicity
Both CTLA-4 and PD-1 inhibitors can cause autoimmune hepatotoxicity that manifests as increased transaminases and total bilirubin, usually with a median onset approximately 8-12 weeks after initiation of treatment. The incidence of grade 2 hepatotoxicity was 2.5% and grade 3-5 events was 2% in a phase III ipilimumab clinical trial (4). The incidence of immune-mediated hepatitis, defined as a requirement for corticosteroids and no clear alternate etiology, was <5% in PD-1 inhibitor clinical trials (2,3,6-10). In a phase III trial with 268 advanced melanoma patients treated with nivolumab, grade 2 to 3 hepatitis occurred in three (1.1%) patients. Liver function tests returned to grade 1 within 4-15 days of initiation of corticosteroids, however hepatitis did recur in two of the three patients (9). Of the 411 patients treated with pembrolizumab in a clinical trial database, hepatitis occurred in 0.5% of patients with complete resolution following administration of corticosteroids (2,6,7).
Hepatic function should be monitored prior to each dose of ipilimumab, nivolumab or pembrolizumab (1-3,13-15). If an increasing trend in liver function tests is noted, evaluation should be carried out to rule out other infectious, non-infectious, and malignant causes such as progression of disease. We recommend laboratory testing for antinuclear antibodies (ANA), smooth muscle antibody (SMA), CBC with differential, CMP, direct and indirect bilirubin, and gamma-glutamyl transferase (GGT). If hepatotoxicity is suspected, the frequency of liver function test monitoring should increase to every 3 days. Computed tomography (CT) scans and liver biopsy may be considered depending on severity.
For grade 2 hepatotoxicity, defined as an aspartate aminotransferase (AST) or alanine transaminase (ALT) >2.5 times but ≤5 times the upper limit of normal (ULN) or total bilirubin >1.5 times but ≤3 times ULN, further therapy should be held and corticosteroids initiated at 0.5-1 mg/kg/day prednisone or equivalent and continued until improvement in toxicity to grade 0 or 1. Steroids should be tapered over 1 month and immunotherapy may resume (13-15).
For grade ≥3 hepatotoxicity, defined as AST or ALT >5 times ULN or total bilirubin >3 times ULN, immunotherapy should be discontinued permanently, liver function tests should be monitored daily, and a hepatology or gastroenterology consultation and liver biopsy should be considered. Patients should be hospitalized for an AST or ALT >8 times ULN and receive methylprednisolone 125 mg intravenously daily. Additional immunosuppression with mycophenolate mofetil 500 mg orally every 12 hours may need to be initiated if no response is elicited after 3-5 days of steroid therapy. Patients receiving mycophenolate mofetil should also receive appropriate antibacterial and antiviral prophylaxis. Other hepatotoxins such as alcohol or acetaminophen should be avoided (13-15).
Endocrinopathies
Immune checkpoint inhibition can cause autoimmune breakthrough events in the form of endocrinopathies. The incidence of endocrinopathy is reported in <10% of patients treated with CTLA-4 and PD-1 inhibitors in clinical trials (1-10). Given that the presentation for endocrinopathy can be insidious, the true incidence may be underreported due to non-specific symptoms that may mimic other causes such as brain metastasis, sepsis, or progression of disease. Infectious and non-infectious causes should be ruled out in suspected cases; unless an alternate etiology is identified, signs or symptoms of endocrinopathies should be considered immune-mediated.
The most common endocrinopathies reported with immune checkpoint inhibitor therapy are hypophysitis and hypothyroidism (1-10). It is recommended to check thyroid function prior to treatment with immune checkpoint inhibitors. Additional laboratory testing for cortisol, adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), follicle stimulating hormone (FSH), growth hormone (GH), prolactin, and testosterone is indicated for suspected immune-mediated endocrinopathy. Radiographic pituitary gland imaging may be warranted with magnetic resonance imaging (MRI) of the brain with special attention to the pituitary gland.
Hypophysitis can present as fatigue, headaches, and visual field defects. Diagnosis is based on levels of pituitary hormones [ACTH, thyroid stimulating hormone (TSH), FSH, LH, GH and prolactin] and radiographic imaging showing an enlarged pituitary, with or without necrosis (12-14). Hypophysitis was reported in 0.5% (2/411) of patients in the initial pembrolizumab clinical trial database with a time to onset of up to 1.7 months, similar to the incidence and onset of hypophysitis reported in ipilimumab clinical trials (2,6,7). The ipilimumab REMS toxicity management recommendation is to withhold immune checkpoint inhibitor therapy and initiate high-dose corticosteroids at 1 mg/kg prednisone or equivalent daily for grade ≥2 toxicity (13). Our institutional recommendation is methylprednisolone 125 mg intravenously daily or dexamethasone 6 mg every 6 hours intravenously for 3 days with a switch to oral prednisone 1-2 mg/kg daily after improvement of symptoms. We prefer a formal endocrinology consultation and follow-up for longitudinal hormone replacement and monitoring. Immune checkpoint inhibitor therapy should be permanently discontinued for severe or life-threatening grade 3 or 4 toxicity (13-15). For patients with existing hypophysitis due to ipilimumab, pembrolizumab may be administered if patients are stable on physiologic hormone replacement therapy (6)
Hypothyroidism was reported in approximately 2% of patients treated with ipilimumab and up to 8.3% of patients with treated with PD-1 inhibitors; the time to onset ranged from 0.7 weeks to 19 months in PD-1 inhibitor trials (2,3,6-10). Hypothyroidism is diagnosed if TSH level is increased with a low free T4 level, whereas hypophysitis presents with a low TSH and low free T4. Immune checkpoint inhibitor therapy may be continued without interruption with appropriate levothyroxine replacement (13-15).
The incidence of primary hyperthyroidism has been lower than hypothyroidism for both CTLA-4 and PD-1inhibition (1-10). In a phase III nivolumab trial with 268 advanced melanoma patients, grade 1 or 2 hypothyroidism and hyperthyroidism occurred in 8% and 3% of patients, respectively, with a time to onset of thyroid dysfunction ranging from 24 days to 11.7 months from initiation of therapy (9). If TSH is decreased, we recommend observation and close monitoring with continuation of immune checkpoint inhibitor therapy. Hyperthyroidism may represent acute thyroiditis secondary to immune activation for which a short period of high-dose steroids (1 mg/kg prednisone or equivalent) may need to be considered for symptomatic patients. Most patients subsequently become hypothyroid and need long-term hormone replacement (13-15).
Other endocrinopathies include severe or life-threatening adrenal insufficiency (usually secondary to hypopituitarism), characterized by hypotension, dehydration, hyponatremia, and hyperkalemia that may mimic sepsis syndrome. The incidence of severe or life-threatening hypopituitarism is reported in <2% of patients treatment with immune checkpoint inhibitor therapy (1-10). Adrenal insufficiency requires immediate hospitalization and management with intravenous corticosteroids after sepsis is ruled out. Corticosteroids should be initiated at 60-80 mg prednisone daily or equivalent and tapered over 1 month. Long-term steroid replacement with hydrocortisone is usually required. If primary or secondary hypoadrenalism is suspected, ACTH and cortisol levels need to be checked and endocrinology consultation considered for interrogation of the pituitary-adrenal axis. Repeat laboratory testing in 1-3 weeks and/or imaging in 1 month should be considered for follow-up of all patients treated for suspected endocrinopathies (13-15).
Management of less frequent irAEs
Other organ systems can be affected after treatment with immune checkpoint inhibitors. Although the incidence of events in other organ systems is low, and the management is in the form of immunosuppression with steroids, some of these events merit mention.
Pneumonitis
Immune-mediated lung injury that manifests as pneumonitis can occur with both CTLA-4 and PD1-inhibitors (1-10). As with other irAEs, the clinical presentation can be deceptive and non-specific, therefore complaints of new cough or dyspnea in patients treated with these agents warrants evaluation with pulmonary function tests and radiographic imaging (e.g., CT scan). Bronchoscopy may be considered to rule out other etiologies including infections prior to treatment with corticosteroids. The overall incidence of grade 3-4 pneumonitis observed with nivolumab and pembrolizumab is <1% (2,3,6-10). In a clinical trial database of 411 patients treated with pembrolizumab, the median time to onset of pneumonitis was 5 months. While most cases can be managed effectively using high-dose corticosteroids with a slow taper, fatal events have been reported (2,6,7).
For grade 2 pulmonary symptoms requiring medical intervention or limiting instrumental ADLs, admission to the hospital and pulmonary consultation is warranted. Our institutional recommendations are in keeping with REMS algorithms with initiation of methylprednisolone 1 mg/kg/day intravenously or oral equivalent until improvement to mild severity with a taper over 1 month following treatment (13-15).
For grade 3 or 4 pulmonary symptoms that are severe or life-threatening, including new or worsening hypoxia, limiting self-care ADL, oxygen requirements, and respiratory compromise requiring urgent intervention, immunotherapy should be permanently discontinued and methylprednisolone 2-4 mg/kg/day intravenously should be administered until improvement to mild severity. Steroids should be tapered over at least 6 weeks in this setting with consideration of additional immunosuppressive therapy if symptoms persist after 48 hours, worsen, or recur on steroid taper (13-15).
Asymptomatic elevation of amylase and lipase
Elevation of amylase and lipase has been observed with both CTLA-4 and PD-1 inhibitors (1-10). The phenomenon of asymptomatic increase in amylase and lipase without overt pancreatitis has especially been described with nivolumab and pembrolizumab and does not require holding therapy; grade 3-4 toxicities that are symptomatic require treatment to be held (1-10). New onset diabetes with diabetic ketoacidosis and pancreatic insufficiency has been documented and may warrant endocrinology/gastroenterology consultation as indicated (13-15).
Renal insufficiency
CTLA-4 and PD-1 inhibitors have been associated with renal insufficiency (1-10). Nephritis has been reported in <1% of patients in a pembrolizumab clinical trial database, with one case of grade 2 autoimmune nephritis, and two cases of interstitial nephritis with renal failure confirmed by biopsy (one grade 3 and one grade 4). The onset of nephritis was 11.6 months after the initiation of treatment. All patients recovered with high-dose corticosteroids at a dose of ≥40 mg/day prednisone or equivalent followed by a taper (2,6,7).
The incidence of renal dysfunction with nivolumab is reported to be <1%; elevated creatinine was reported in up to 22% of patients. Grade 2 or 3 immune-mediated nephritis or renal dysfunction occurred in 0.7% (2/268) of patients treated with nivolumab in a phase III study (9). It is important to monitor patients for elevated serum creatinine prior to and periodically during treatment. Management of renal irAEs at our institution is as per clinical algorithms for PD-1-associated renal adverse events in published trials. For grade 1 toxicity, defined as an increased creatinine up to 1.5 times above baseline, creatinine should be monitored at least once a week without interruption of immunotherapy. If serum creatinine worsens to grade 2 or 3, defined as a creatinine above 1.5 times baseline up to 6 times ULN, creatinine should be monitored at least every 2-3 days, immunotherapy should be withheld, and methylprednisolone 0.5-1 mg/kg/day intravenously or equivalent should be initiated until resolution of symptoms to grade 1 or below, followed by a taper over 1 month. Grade 4 toxicity, defined as life-threatening symptoms or creatinine >6 times ULN, warrants daily monitoring of creatinine, permanent discontinuation of therapy, consideration for nephrology consultation and biopsy, and high-dose corticosteroids with methylprednisolone 1-2 mg/kg/day intravenously or equivalent with a taper over at least 1 month (8-10).
Ophthalmologic disorders
Ophthalmologic disorders such as episcleritis, conjunctivitis, and uveitis occur in <1% of patients treated with ipilimumab and may be treated with topical corticosteroids; more severe events require ophthalmologic evaluation and systemic steroids (1,4,5).
Rare irAEs
Rare disorders reported in ≤1% of patients include red cell aplasia, thrombocytopenia, hemophilia A, Guillain-Barre syndrome, myasthenia gravis, posterior reversible encephalopathy syndrome, aseptic meningitis, and transverse myelitis (1-3).
Discussion
Immune checkpoint inhibitors, ipilimumab, pembrolizumab and nivolumab, have shown significant clinical benefit in several malignancies and are already approved for advanced melanoma and squamous NSCLC, marking the advent of immune-oncology. Based on their mechanism of action, these agents can exert toxicities that are unlike conventional cytotoxic chemotherapy. Since immune checkpoint inhibitors are not selective to tumor or tissue type, there is a substantial effort underway to explore their efficacy and role in the management of malignancies other than currently approved indications. Preliminary data is encouraging and there are several other immune checkpoint inhibitors in development with impressive clinical activity that are likely to be approved. It is therefore important that the oncology community acclimate to the nuances of immune-oncology therapeutic modalities that may potentially gain acceptance for the treatment of several malignancies.
Although the irAEs profiles of the three approved agents may differ slightly, they share the clinical presentation of symptoms and general principles guiding their management. It is extremely important to make the distinction that immunotherapy is not chemotherapy; the irAEs observed with immunotherapy have a completely different underlying mechanism compared to toxicity observed with chemotherapy. The irAEs can present in an insidious and unpredictable fashion, therefore the clinical team, as well as the patient, have to be educated and aware of the potential toxicities so that they are reported early, generate appropriate level of suspicion and prompt investigation. If identified early, the irAEs are almost always reversible with the initiation of immunosuppression. If they go unrecognized, these events can lead to significant morbidity, organ dysfunction, and even death. As opposed to cytotoxic chemotherapy, the tenet of ‘more is better’ does not necessarily fit the bill for immunotherapy. The idea is to ‘take the brakes off’ the immune effector T cells and fine-tune the balance between increased antitumor activity and autoimmunity. While some patients may not incur any toxicity and experience a response, others may have irAEs and not respond. The appearance of irAEs is indicative of the immune status and if no antitumor response is elicited at that heightened level of activation, then ‘taking further brakes off’ by continuing therapy is unlikely to translate into more benefit justifying the rationale to discontinue. The current recommendations for the management of irAEs are presented and summarized in Table 2.
Several questions remain unanswered with the current level of insight into immune checkpoint inhibitors and will likely get resolved as clinical experience evolves with more widespread use in off protocol and clinical trial settings. Based on the mechanism of action, previous clinical trials typically excluded patients with underlying autoimmune disorders (e.g., Crohn’s disease, ulcerative colitis, rheumatoid arthritis). It is not clear if the presence of an existing autoimmune disorder constitutes an absolute contraindication. Similarly, it is not known how or if patients on chronic immunosuppression will respond. There is paucity of data regarding the safety and efficacy of these agents in patients with severe organ dysfunction (e.g., patients requiring dialysis for renal insufficiency) since they were also excluded from early studies.
Studies to identify biomarkers and other factors predictive of response and resistance to immunotherapy, and combination trials of immunotherapy with chemotherapy, targeted therapy, or multiple immune-modulators are underway to further define the role of this treatment modality for cancer. Early studies suggest that combination therapy with dual immune checkpoint inhibition (CTLA-4 plus PD-1) may increase efficacy, but at the cost of increased toxicity (19). The addition of granulocyte-macrophage colony-stimulation factor (GM-CSF) to CTLA-4 inhibition has been shown to prolong survival with fewer irAEs in an early study for the treatment of melanoma, however, this needs to be validated (20). Correlation between the expression of PD-L1 on tumors and response to PD-1 inhibitors has not been confirmed and remains an area of active investigation.
Discovery of immune checkpoint inhibitors has afforded an unprecedented opportunity for the development of effective treatment options for some malignancies. It is expected that these agents will be incorporated in the management of other tumor types. It is therefore imperative that the clinical teams including physicians, first responders, nurses, pharmacists as well as the patients become familiar with the irAEs and their management.
Acknowledgements
None.
Footnote
Conflicts of Interest: Jeryl Villadolid declares no conflict of interest. Asim Amin received honorarium from BMS for speaker’s bureau and advisory board; participated in clinical trials sponsored by Merck and BMS.
References
- Yervoy® [package insert]. Princeton, NJ, USA: Bristol-Myers Squibb Company, 2013. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2265ef30-253e-11df-8a39-0800200c9a66, accessed June 2015.
- Keytruda® [package insert]. Whitehouse Station, NJ, USA: Merck & Co., Inc., 2015. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9333c79b-d487-4538-a9f0-71b91a02b287, accessed June 2015.
- Opdivo® [package insert]. Princeton, NJ, USA: Bristol-Myers Squibb Company, 2015. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f570b9c4-6846-4de2-abfa-4d0a4ae4e394, accessed June 2015.
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [PubMed]
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [PubMed]
- YERVOY™ (ipilimumab) US Prescribing Information: Risk Evaluation and Mitigation Strategy (REMS). Princeton, NJ, USA: Bristol-Myers Squibb Company, 2011. Available online: https://www.hcp.yervoy.com/pages/rems.aspx, accessed April 2015.
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691-7. [PubMed]
- Fecher LA, Agarwala SS, Hodi FS, et al. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist 2013;18:733-43. [PubMed]
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines) Melanoma. Version 3. 2015. Available online: www.nccn.org, accessed April 2015.
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf, accessed April 2015.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009;15:5591-8. [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [PubMed]
- Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 2014;312:1744-53. [PubMed]