Identifying osteoporotic vertebral fracture
Why is it important to diagnose osteoporotic vertebral fractures?
Diagnosing an osteoporotic vertebral fracture is important in that it provides:
- A source for the patient’s symptoms;
- A marker of reduced bone strength;
- A predictor of further osteoporotic fracture.
Patient’s symptoms
As back pain is common in elderly patients, most vertebral fractures go unrecognized clinically with symptoms attributed to degenerative change. This is particularly so as osteoporotic vertebral fractures classically occur during normal day-to-day activities such as bending, walking or lifting relatively light objects. Three-quarter of patients with vertebral fracture do not seek medical attention (1) and even up to 2/3 of vertebral fractures may not result in notably severe symptoms (2).
Clinical assessment of vertebral fractures is generally poor and reliance is made on imaging studies for diagnosis. In addition to pain, osteoporotic vertebral fractures result in immobility with its accompanying problems of chest infection, muscle loss, inability to cope with daily activities and social isolation (3). Multiple vertebral fractures can result in loss of height, exaggerated thoracic kyphosis, poor self-image and loss of self esteem. As well as increased morbidity, increased mortality is also associated with vertebral fracture. For example, subjects with incident fracture have a 3-fold increased mortality risk over the ensuring 4 years compared to non-fractured counterparts, particularly due to pulmonary disease and cancer (4).
Marker of reduced bone strength
The presence of an osteoporotic or insufficiency fracture is the ultimate marker of reduced bone strength, overriding all other assessments in this respect. As dual energy X-ray absorptiometry (DXA) examination has become more widely used, not infrequently too much reliance has been placed on T-scores to make the diagnosis of osteoporosis. It is not uncommon to hear that “this patient does not have osteoporosis, since the DXA T-score is −1.4 even though the patient has got an unequivocal insufficiency vertebral fracture. To say this is not true. The presence of an insufficiency fracture, of which vertebral fracture is the most common, is a more absolute indication of reduced bone strength (i.e., osteoporosis) and an indication to treat irrespective than any bone densitometry measurement. Most patients with osteoporotic fracture have either osteopenia or even normal bone density since osteopenia is much more common than osteoporosis in females or males less than 90 years of age (5,6). DXA is a measure of bone density which, when reduced, is useful predictor of reduced bone strength and increased likelihood of fracture, though most osteoporotic fractures occur in patients in patients outside the osteoporotic range.
Harbinger of future osteoporotic fracture
An isolated osteoporotic vertebral fracture is usually the first osteoporotic fracture to occur and usually predates the occurrence of proximal femoral, radial, sacral or pelvic osteoporotic fractures (7). In this sense recognition and proper management following an osteoporotic vertebral fracture can prevent or delay the occurrence of subsequent osteoporotic fracture. Over an 8-year period, subjects with prevalent (i.e., pre-existing) vertebral fractures has a 5-fold increased risk of further vertebral fracture and a 3-fold increased risk if proximal femoral fracture (8). Similarly, during a 4-year period following an incident (i.e., new) vertebral fracture, postmenopausal women are 4 times more likely to develop a further vertebral fracture and twice as likely to develop a proximal femoral fracture as those without an incident vertebral fracture (8). The overall risk of further vertebral fracture is 20% in the year following incident fracture with relative risk being 4 times greater in those with severe rather than mild fractures and 3 times greater in those with multiple (>3) rather than single vertebral fractures (9). The clinical importance of osteoporotic fracture is recognized by the World Health Organization which defines “severe osteoporosis” as T-score −2.5 plus the presence of an osteoporotic fracture. Recognition is important not least because subjects with a vertebral fracture and a T-score of <2.5 appear to be those most likely to benefit from timely osteoporotic drug therapy. The presence of an isolated vertebral fracture may through alteration of spinal biodynamics precipitate additional adjacent vertebral fractures. Such fractures will further increase spinal kyphosis leading to even more vertebral fractures in a process known as “vertebral fracture cascade” (Figure 1).
Under-diagnosis of osteoporotic vertebral fracture in clinical settings
Despite the clear undisputed clinical relevance of vertebral fractures, these remain underdiagnosed in everyday clinical practice (10). Two main reasons account for this inadequacy. First, as mentioned, vertebral fractures frequently do not present as a clinically recognizable event. Second, many radiologically apparent vertebral fractures go unreported. Of patients aged more than 60 years attending Emergency Departments, about 1/6 had a moderate to severe vertebral fracture evident on lateral chest radiographs of which only about 1/2 were noted on radiology reports and fewer still received specific medical attention (11). Also when reporting vertebral fractures radiologists and clinicians should avoid using ambiguous terms “collapse”, “compression”, “loss of height”, “wedging” or “wedge deformity” and instead use the term mild, moderate or severe vertebral fracture.
Over-diagnosis of osteoporotic vertebral fracture in research settings
Given its clinical and biological relevance, the presence or absence of radiographically vertebral fracture is often understandably used an either an entry criterion or primary endpoint in cross-sectional interventional studies or is the primary variable assessed in cross-sectional observational studies. It is therefore crucial to have as reliable a method of diagnosing osteoporotic fractures as achievable possible. Over- or under-diagnosis of vertebral fractures on radiographs by an inexperienced reader can significantly skew research findings and considerably alter the outcome of clinical research trials. How vertebral fractures are diagnosed is a crucial element in the methodology of such trials and often an element, it seems, which is often not afforded due importance.
Definition of vertebral fracture
Vertebral fractures are compressive and are associated with a decrease, no matter how minor, in anterior (AH), middle (MH) or posterior (PH) vertebral height. As nearly all vertebral fractures are diagnosed on imaging studies, the diagnosis of vertebral fracture clearly depends on the sensitivity of that imaging study to detect changes consistent with vertebral fracture. Magnetic resonance imaging, because of its ability to visualize marrow oedema, seems to be able to detect recent vertebral fracture with greater sensitivity than any other imaging technique. However, because of cost and lack of availability, it is clearly not applicable to use magnetic resonance imaging (MRI) to detect vertebral fracture in everyday clinical practice.
Most vertebral fractures are diagnosed on radiography. Although there is no universal definition of what constitutes a vertebral fracture, it is now almost universally accepted that a vertebral fracture is diagnosed when there is an approximate 20% loss in vertebral body height relative to normal looking adjacent vertebra or relative to what one would expect normal vertebral height to be at that level. This same criterion is applied to other imaging modalities such as DXA and computed tomography. The 20% cut-off point is used to avoid inclusion of other non-fractures entities which lead to reduced vertebral body height in the absence of fracture.
Imaging modalities used to diagnose vertebral fracture
Spinal radiography
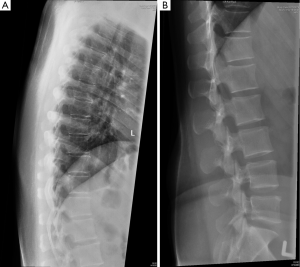
Radiography is quick, widely available and of low cost and is the best imaging modality to diagnose vertebral fracture. Lateral spinal radiography will usually suffice and should include a high quality standardized image of the C7-S1 vertebrae (Figure 2). The upper thoracic vertebral bodies are often not clearly seen on lateral thoracic spine radiographs though fortunately most osteoporotic fractures occur in the mid-thoracic and thoracolumbar regions below the T4 level. Typical effective radiation doses from a single lateral of the thoracic and lumbar spine small and in the order of 0.3 and 0.7 mSv respectively (12).
Generally no problem exists with diagnosing moderate to severe vertebral body fractures. The greatest difficultly with diagnosing vertebral fractures lies in the diagnosis of mild vertebral fracture due to several peculiarities of the spine, not due to fracture, though which may be misdiagnosed as a mild vertebral fracture. There are six common pitfalls that can be misdiagnosed as fracture as follows.
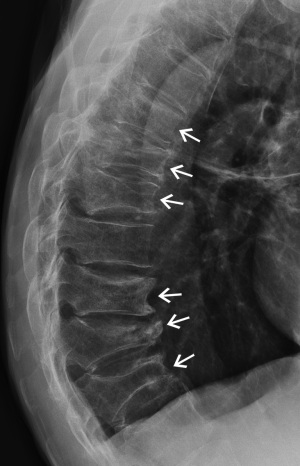
Physiological wedging
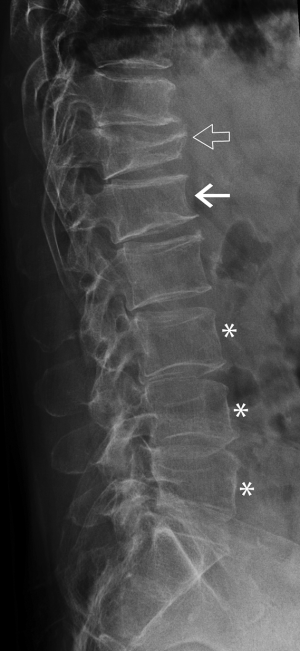
Mild anterior or posterior wedging is a normal physiological feature of thoracic and lumbar vertebral bodies as the spinal curvature moves from lordosis to kyphosis. All vertebrae are slightly wedged to a mild degree but this physiological wedging is greatest in the mid thoracic—upper lumbar region, particularly at L1 and L1 where the vertebrae are anteriorly wedged while the lower lumbar vertebrae (L4-L5) are usually posteriorly wedged (Figures 3,4).
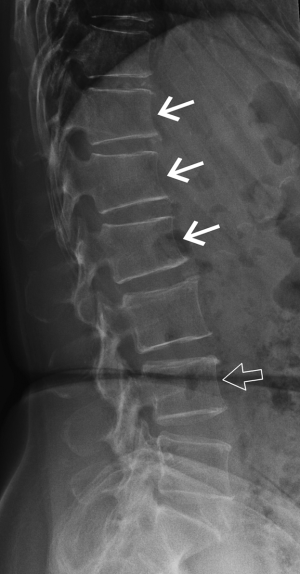
Short vertebral height (SVH)
SVH is a common feature that occurs with increasing age and with increasing degenerative change (Figure 4). Between about 30 and 70 years of age, the combined height of the anterior aspects of the vertebral bodies from T4-L5 decreases by about 1.5 mm per year while the combined middle and posterior heights decline by about 1.2 mm per year (13,14). SVH refers to reduction in vertebral height of up to about 20% expected height. Differentiating SVH from a mild vertebral fracture is probably the most contentious and difficult area in vertebral fracture diagnosis. The majority of evidence suggests that isolated SVH is not associated with low BMD or vertebral fracture (15). In a DXA vertebral fracture assessment (VFA) study in which comparison was made between 250 premenopausal and 1,350 postmenopausal women, the prevalence of SVH was approximately 35%. The prevalence was similar in pre- and post-menopausal women and was not associated with low lumbar spine BMD. Premenopausal women with SVH tended to be older and heavier than those without SVH, while postmenopausal women with SVH tended to have higher spine BMD than those without SVH. When reporting spine radiographs for vertebral fracture, one should consider the patient age and the degree of degenerative change present (Figure 4).
Scheuermann’s disease
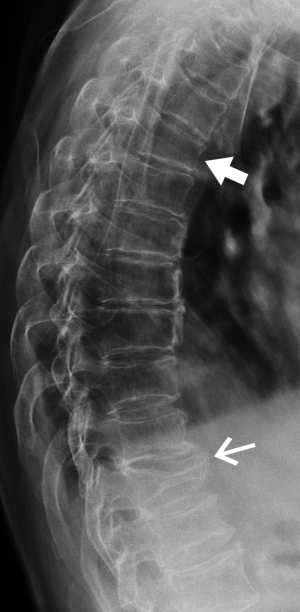
Scheuermann’s disease is an uncommon thoracolumbar spinal disorder characterized by endplate indentations, reduced disc height, reduced vertebral height, increased vertebral anteroposterior diameter and accelerated disc degeneration (Figure 5).
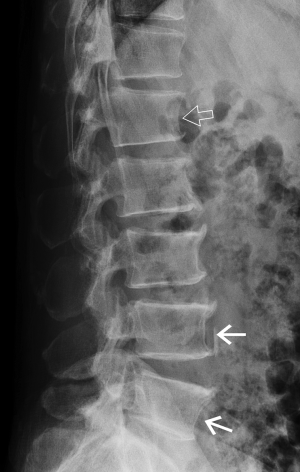
Degenerative scoliosis
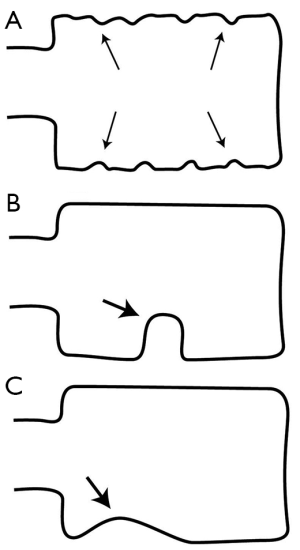
Degenerative scoliosis is common in the middle-aged or elderly spine and may lead to obliquity of vertebral bodies and side-to-side discrepancy in vertebral body height (Figure 6). On the lateral projection, this obliquity produces a spurious biconcave outline to the vertebral endplates, which may, if severe, be misinterpreted as a vertebral fracture. On the AP projection the vertebral bodies, particularly at the apex of the curve, will be shortened on the concave side and of normal height or even elongated on the convex side. This scoliotic wedging, providing it is predominantly one-sided and commensurate with the severity of scoliosis, should not be misinterpreted as a vertebral fracture.
Schmorl’s nodes
Schmorl’s nodes are discrete indentations of the endplates related to degenerative disc disease. Small Schmorl’s nodes are a common finding, being present in 40-75% of imaging studies, particularly in degenerative disease in the lumbar spine. Medium-sized or large Schmorl’s nodes occur much less frequently and may be misinterpreted as an endplate fracture (Figure 5). However, as opposed to endplate fractures, Schmorl’s nodes have a well-defined rounded contour with an intact sclerotic margin, and they do not involve the whole length of the endplate.
Cupid’s bow deformity
Cupid’s bow deformity is a reasonably common developmental endplate contour abnormality most frequently affecting the inferior, and less frequently the superior, endplates of the fourth and fifth lumbar vertebral bodies (16). This contour deformity may also involve the endplates of the more cephalad lumbar vertebrae and those of the thoracolumbar vertebrae. Cupid’s bow deformity most likely results from focal deficiency of the cartilage component of the endplate in the parasagittal regions of the vertebral body (Figure 5). This absence of the cartilage component focally impairs endochondral growth of the vertebral body leading to the characteristic concave endplate depressions seen radiographically. The shape of the resulting deformity on the anteroposterior projection resembles a “Cupid’s bow”. On the lateral projection, the posterior 2/3 of the inferior endplate are indented, simulating an endplate fracture depression.
Diagnosing and grading vertebral fractures on radiography
Methods have been developed to help try and standardize the diagnosis and grading of vertebral fracture. Although these methods were developed for radiography, they can also be applied DXA VFA, computed tomography (CT) or MRI. The semi-quantitative (SQ) or quantitative morphometic (QM) methods are the two most commonly used approaches.
SQ assessment
The most widely used SQ method was developed and refined by Genant et al. (17) (Figure 7). In this method, vertebral fractures are graded from 1 (mild) to 3 (severe). Grade 1 (mild) vertebral fracture corresponds to a ~20-25% reduction in AH, MH, and/or PH height compared to expected normal vertebral height. Grade 2 (moderate) vertebral fractures is a ~25-40% reduction in vertebral height while grade 3 (severe) vertebral fracture is a ~>40% reduction in vertebral height (Figure 8). An approximation of vertebral height reduction is used as height reduction is typically estimated visually rather than directly measured. Other morphologic changes such as end-plate buckling or bowing and disruption of cortical margins can be factored into the diagnosis of vertebral fracture. Applying these grades in research studies enables a spinal deformity index (SDI) to be assigned to each patient by summating the SQ scores for the T4 to L4 vertebrae (18). Incident fractures are defined as an increase of one grade or more on follow-up radiographs.
The SQ method is practically a very easy method to apply with excellent inter-reader reliability. For prevalent fractures, the agreement between each of three readers and a consensus reading yielded a kappa score of 0.84-0.87 for incident fractures and 0.86-0.96 for prevalent fractures and works best when performed by trained and experienced readers (19,20). SQ analysis can be applied both in clinical practice as well as research. Serial radiographs, if available, showed be viewed in a temporal order to best appreciate changing vertebral morphology. The severity of any vertebral fracture is also an important feature to note. It has been shown that the more severe the vertebral fracture, the greater the deterioration in bone architectural parameters and bone quality (21) which will increase fracture risk. Therefore, whether in clinical practice or clinical trials, the severity as well as the presence of a fracture should be reported. In the research setting, SQ analysis can be used as the sole method of assessment. Alternatively, radiographs can be first analyzed using a morphometic approach followed by SQ analysis by an expert reader of those vertebrae suspected of fracture.
Vertebral QM
This approach involved outlining the margins of each vertebral body from T4 to L4 by six points—one for each corner and one for the midpoint of the superior and inferior endplates (Figure 9). For vertebrae in which the endplate is not seen in profile, the mid-point of the ovoid upper and lower endplate contour is chosen. The reference point enable the AH, MH and PH vertebral height to be measured. Vertebral shape is defined by using vertebral height ratios. For example, AH/PH reflects anterior wedging, MH/PH reflects endplate concavity and PH/PH' of the adjacent normal vertebrae reflects posterior wedging (22). Vertebral fracture is defined as a reduction in one or more of the three vertebral height ratios (AH/PH, MH/PH, or PH/PH') of greater than 20% or three standard deviations (SD) from the mean of a reference population.
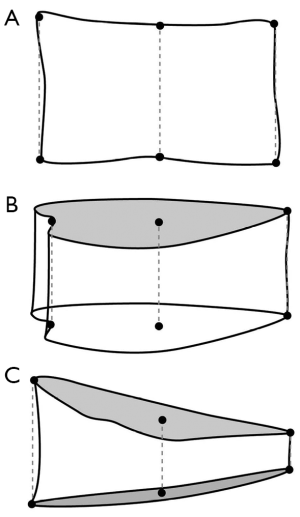
The application of QM in practice is often rather subjective with reference point placement dependent on observer experience, especially for the vertebral mid-points. QM relies solely on reference point placement and cannot take into account subtle alterations in radiographic projection. Vertebral QM is used in research, particularly in longitudinal studies and it is generally not used in the clinical setting. Its main advantages are that it can be performed by relatively inexperienced non-medical staff and it provides an objective measure vertebral height loss. There is good concordance between QM and SQ methods for the diagnosis of moderate or severe vertebral fractures. However, the concordance between both methods for detection of mild vertebral fractures is poor due mainly to false positive diagnoses by QM. As a result all vertebral fractures diagnosed by QM should be confirmed by an expert reader. While the reproducibility of QM is good in normal subjects (inter-observer coefficient of variation less than 2%), it is lower in those with osteoporotic fractures (inter-observer and intra-observer coefficient of variation of 5% and 6.3% for MH respectively) (23).
Algorithm-based qualitative (ABQ) assessment
The ABQ method, as the name implies, emphasizes a qualitative assessment of vertebral fracture and relies more on detection of vertebral endplate abnormalities related to fracture than loss of vertebral body height. The ABQ method categories vertebrae as either (I) normal; (II) osteoporotic fracture; or (III) non-osteoporotic deformity (i.e., SVH). The diagnosis of an osteoporotic vertebral fracture requires evidence of vertebral endplate fracture ± loss of expected vertebral height but with no minimum threshold for apparent reduction in vertebral height. If a fracture of the cortical margin in also visible radiographically, this provides clear-cut evidence that there is a fracture present and it is likely to be of recent origin. When one of more vertebral height (AH, MH or PH) is shorter than expected but without specific endplate abnormalities of fracture (altered texture below endplate due to microfractures), this is designated as non-osteoporotic deformity.
Dual energy X-ray absorptiometry (DXA)
DXA is widely employed to measure bone mineral density. DXA machines which incorporate fan beam technology and appropriate software which allow the acquisition of modest resolution images of the thoracic and lumbar spines to help identify vertebral fractures. Imaging vertebral fractures using DXA is known as “VFA” (Figure 10). VFA can be performed at the same time as bone densitometry with a lateral and frontal image of the spine being obtained in less than a minute. The vertebral bodies caudad to T7 can be routinely assessed while those between T7 and T4 are not consistently well seen. The advantages of VFA over radiography are convenience, less radiation, low cost. Combining prevalent vertebral fracture status with BMD enhances fracture risk prediction of both vertebral and non-vertebral fracture and this additional information can be incorporated into the FRAX model.
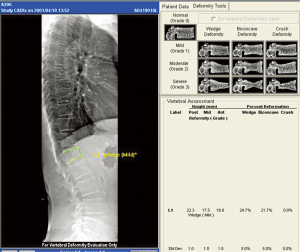
Since VFA is a digital technique, additional computational analysis is possible in the form of automated vertebral morphometry known as MXA. The vertebral body is automatically or manually demarcated by four or six reference points and vertebral body height, height ratios and average height calculated automatically. Although morphometic analysis is routinely undertaken on VFA, this alone is not recommended for fracture diagnosis. The ISCD recommends that visual inspection using the Genant SQ method be undertaken to diagnose and grade severity of vertebral fracture on VFA. SQ analysis of moderate and severe vertebral fractures on VFA compares well with radiography though only moderate correlation exists between VFA and radiography for diagnosis of mild vertebral fracture (24). VFA will have an increasing important role in the diagnosis of vertebral fractures and in estimating fracture risk.
Other imaging modalities used to diagnose vertebral fracture
Computed tomography (CT)
The ease with which midline sagittal reconstructions can be performed with multi-detector CT (MDCT) enables the thoracic or lumbar spine to be evaluated on all thoraco-abdominal CT studies being performed for alternative clinical indications. As thoraco-abdominal CT is a very common indication for CT in most hospitals, this should allow the fortuitous detection of many clinically unsuspected vertebral fractures. Volumetric CT datasets can also be used to analyses the macro- and micro-structure of vertebral bodies. Textural analysis and finite element analytical techniques applied to high resolution CT data can allow evaluation of vertebral bony architecture and strength although this has not yet used in an everyday clinical settling. The major limitations to the more widespread use of CT in VFA are cost, access to CT time, and radiation dose.
Magnetic resonance imaging (MRI)
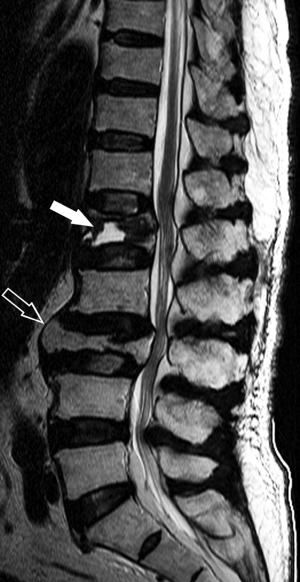
As the spine is the most common site for both skeletal metastases and osteoporotic fracture, even in patients with a known primary malignancy, 1/3 vertebral fractures will be osteoporotic and not metastatic in origin. MRI, because of its ability to visualize the bone marrow, is the most useful investigation for differentiating osteoporotic from neoplastic fracture (Figure 11). Applying specific imaging criteria, it is nearly always possible to distinguish reliably between osteoporotic and neoplastic vertebral fracture on imaging grounds without having to undertake percutaneous biopsy (25). MRI is also sensitive at distinguishing acute/subacute vertebral fractures from chronic vertebral fracture. This is clinically relevant as only acute/subacute vertebral fractures will benefit most from percutaneous vertebroplasty.
Conclusions
Vertebral fractures are important as they contribute significantly to patient symptom, provide a clear marker of impaired bone strength and a predictor of new vertebral and non-vertebral insufficiency fractures. Recognition and appropriate treatment of vertebral fracture at an early stage can reduce the risk of future fracture as well as reduced patient pain, deformity and suffering. Good technique in performing spinal radiographs, increased awareness and a high level of observer experience in image interpretation are keys to the reliable identification and reporting of vertebral fractures. A vertebral fracture is diagnosed when there is 20% or greater loss in expected vertebral body height. Several non-fractures vertebral deformities exist that may lead to misinterpretation of vertebral fracture by an inexperienced reader. All vertebral fractures should be reported in a clear and unambiguous manner noting the presence, site and severity of vertebral fracture. SQ analysis is the best method to use both in the clinical and research setting though quantitative vertebral morphometry can be complimentary to SQ in large longitudinal trials. MRI is able to diagnose vertebral fracture with greater sensitivity than other imaging techniques and can help determine fracture age as well as distinguish osteoporotic from neoplastic fracture.
Acknowledgements
Funding: The work described in this paper was partially supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. SEG_CUHK02).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Grigoryan M, Guermazi A, Roemer FW, Delmas PD, Genant HK. Recognizing and reporting osteoporotic vertebral fractures. Eur Spine J 2003;12:S104-12. [PubMed]
- Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ 3rd. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res 1992;7:221-7. [PubMed]
- Lips P, van Schoor NM. Quality of life in patients with osteoporosis. Osteoporos Int 2005;16:447-55. [PubMed]
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1999;159:1215-20. [PubMed]
- Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 2001;286:2815-22. [PubMed]
- Sanders KM, Nicholson GC, Watts JJ, Pasco JA, Henry MJ, Kotowicz MA, Seeman E. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone 2006;38:694-700. [PubMed]
- Meunier PJ, Delmas PD, Eastell R, McClung MR, Papapoulos S, Rizzoli R, Seeman E, Wasnich RD. Diagnosis and management of osteoporosis in postmenopausal women: clinical guidelines. International Committee for Osteoporosis Clinical Guidelines. Clin Ther 1999;21:1025-44. [PubMed]
- Black DM, Arden NK, Palermo L, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1999;14:821-8. [PubMed]
- Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E. Risk of new vertebral fracture in the year following a fracture. JAMA 2001;285:320-3. [PubMed]
- Delmas PD, van de Langerijt L, Watts NB, Eastell R, Genant H, Grauer A, Cahall DL. IMPACT Study Group. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res 2005;20:557-63. [PubMed]
- Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int 2000;11:577-82. [PubMed]
- Damilakis J, Adams JE, Guglielmi G, Link TM. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol 2010;20:2707-14. [PubMed]
- Diacinti D, Acca M, D'Erasmo E, Tomei E, Mazzuoli GF. Aging changes in vertebral morphometry. Calcif Tissue Int 1995;57:426-9. [PubMed]
- Masunari N, Fujiwara S, Nakata Y, Nakashima E, Nakamura T. Historical height loss, vertebral deformity, and health-related quality of life in Hiroshima cohort study. Osteoporos Int 2007;18:1493-9. [PubMed]
- Ferrar L, Jiang G, Armbrecht G, Reid DM, Roux C, Glüer CC, Felsenberg D, Eastell R. Is short vertebral height always an osteoporotic fracture? The Osteoporosis and Ultrasound Study (OPUS). Bone 2007;41:5-12. [PubMed]
- Chan KK, Sartoris DJ, Haghighi P, Sledge P, Barrett-Connor E, Trudell DT, Resnick D. Cupid's bow contour of the vertebral body: evaluation of pathogenesis with bone densitometry and imaging-histopathologic correlation. Radiology 1997;202:253-6. [PubMed]
- Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-48. [PubMed]
- Genant HK, Siris E, Crans GG, Desaiah D, Krege JH. Reduction in vertebral fracture risk in teriparatide-treated postmenopausal women as assessed by spinal deformity index. Bone 2005;37:170-4. [PubMed]
- Wu CY, Li J, Jergas M, Genant HK. Comparison of semiquantitative and quantitative techniques for the assessment of prevalent and incident vertebral fractures. Osteoporos Int 1995;5:354-70. [PubMed]
- Buehring B, Krueger D, Checovich M, Gemar D, Vallarta-Ast N, Genant HK, Binkley N. Vertebral fracture assessment: impact of instrument and reader. Osteoporos Int 2010;21:487-94. [PubMed]
- Genant HK, Delmas PD, Chen P, Jiang Y, Eriksen EF, Dalsky GP, Marcus R, San Martin J. Severity of vertebral fracture reflects deterioration of bone microarchitecture. Osteoporos Int 2007;18:69-76. [PubMed]
- Guglielmi G, Diacinti D, van Kuijk C, Aparisi F, Krestan C, Adams JE, Link TM. Vertebral morphometry: current methods and recent advances. Eur Radiol 2008;18:1484-96. [PubMed]
- Grados F, Roux C, de Vernejoul MC, Utard G, Sebert JL, Fardellone P. Comparison of four morphometric definitions and a semiquantitative consensus reading for assessing prevalent vertebral fractures. Osteoporos Int 2001;12:716-22. [PubMed]
- Diacinti D, Guglielmi G. Vertebral morphometry. Radiol Clin North Am 2010;48:561-75. [PubMed]
- Griffith JF, Guglielmi G. Vertebral fracture. Radiol Clin North Am 2010;48:519-29. [PubMed]