Effectiveness and safety of simultaneous hybrid thoracoscopic and endocardial catheter ablation of lone atrial fibrillation
Introduction
With the development of new tools and advanced techniques, the treatment of lone atrial fibrillation (LAF) is moving toward a multidisciplinary approach involving cardiac surgeons and electrophysiologists (1). In the attempt to improve suboptimal results obtained by catheter ablation and surgical ablation (2-4), the so called hybrid approach has recently been introduced, which combines a thoracoscopic epicardial ablation with a percutaneous trans-septal procedure (5). Nonetheless, although a few papers have been recently published (6) as a result of a growing interest for this new method, reports investigating the safety and effectiveness of this procedure are limited.
Therefore, this study aims to report outcomes and complications of the hybrid endocardial epicardial technique for LAF.
Methods
Between 2009 and 2012, a cohort of 78 consecutive patients underwent ablation of LAF as a standard procedure using a thoracoscopic, hybrid epicardial-endocardial technique.
LAF was defined following the American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) Guidelines (7). Indication for minimally invasive surgery was based on the Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society (HRS/EHRA/ECA) Guidelines (8). Patients were referred for a hybrid procedure in cases of paroxysmal, persistent or long-standing persistent atrial fibrillation (AF) with left atrial (LA) volume index ≥29 mL/m2, after one or more failed catheter ablations or based on patient preference.
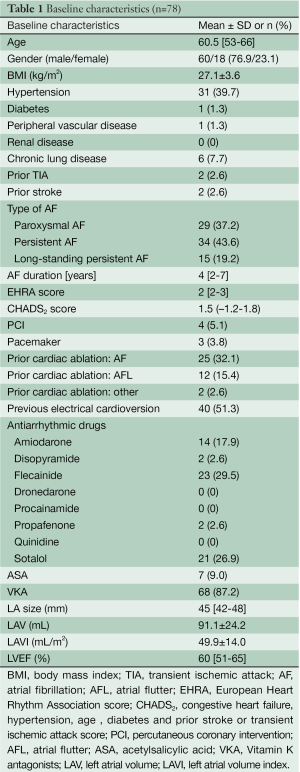
A transthoracic echocardiograph and a computed tomography (CT) scan were carried out preoperatively (pulmonary vein anatomy, coronary arteries), and all patients underwent a lung function test (spirometry). Exclusion criteria were: presence of atrial or left appendage thrombi, ‘giant’ left atrium (diameter >6.5 cm), significant coronary artery disease, and previous pulmonary or cardiac surgery. Patient characteristics are shown in Table 1.
Full table
All patients were followed up according to the HRS/EHRA/ECA expert consensus statement on catheter and surgical ablation of AF. Success was defined as no episode of AF, atrial flutter (AFL) or any atrial tachycardia (AT) lasting more than 30 seconds off antiarrhythmic drugs (AAD) after the three months blanking period (9).
Seven-day Holter monitoring (HM) was performed at 3 months, 6 months and 1 year and yearly thereafter. Monitoring was carried out with an external loop recorder (Del Mar Reynolds, Spacelabs Healthcare, Issaquah, WA, USA) and analyzed with Lifescreen Software (Del Mar Reynolds, Spacelabs Healthcare, Issaquah, WA, USA). For analysis, three rhythms were considered as postoperative AF: AF, AFL or AT lasting more than 30 seconds.
All patients reached 1-year follow-up. Median follow-up was 24 months [interquartile range (IQR) 12-36].
AADs were given postoperatively to all patients, and although we recommended discontinuation three months after ablation if the patient appeared to be AF free, their continued use was at the discretion of referring cardiologists. Warfarin was administered on postoperative day 2 with INR target of 2.5 and stopped after three months if the Holter recording showed a sinus rhythm (SR) or patients had a low thromboembolic risk (CHADS2 score
Echocardiography was carried out preoperatively and at follow-up appointments using a commercially available echocardiographic system (Philips iE33; Philips Medical Systems, Eindhoven, The Netherlands). LA diameter was measured in parasternal long-axis view. In the apical 4-chamber view, LA maximum volume was measured by the biplane area-length method and indexed to body surface area (LAVI). Left ventricular Ejection Fraction was calculated with the biplane Simpson’s method.
Surgery
All operations were performed by the same cardiac surgeon (M.L.M.). Surgery was carried out as previously described (5). In brief, after isolation of pulmonary veins (PVs), roof and inferior lines were created using the Coolrail linear pen (Atricure Inc, Westchester, OH, USA) or the Max 5 unidirectional bipolar pen (Atricure Inc, Westchester, OH, USA) to complete the box lesion. The endpoint was entrance and exit block for the created “box”.
A left femoral vein puncture was made, and a His bundle catheter (St Jude Medical, Inc, Minnesota, MN, USA) and a coronary sinus (CS) catheter (Medtronic, Inc, Minneapolis, MN, USA) were placed under fluoroscopic guidance. Through the right femoral vein, a single trans-septal puncture was made using transesophageal echocardiography (TEE) and fluoroscopy, and a long sheath 8F (SL0, St. Jude Medical Daig Division, Inc., Minnesota, MN, USA) was advanced into the LA. The patient was heparinized to keep the activated clotting time >300 seconds. During rapid ventricular pacing from the His catheter, contrast was injected through the long sheath in order to visualize the PVs and the LA. PVs were mapped with a suitably sized circular mapping catheter (Lasso, Biosense Webster, Inc, Diamond Bar, CA, USA) placed at the ostium of the PVs. Endocardial recordings at the PVs were employed to demonstrate entrance and exit block for each PV. Entrance block was defined as the absence of pulmonary vein potentials (PVPs). Exit block was defined as failure to capture when pacing from each dipole of the Lasso catheter with an output of 10 mA and 2.0 ms pulse width.
We identified the conduction gaps from the endocardium and ablated those with a 3.5 mm tip-catheter (ThermoCool, Biosense Webster, Inc., Diamond Bar, CA, USA) through the sheath in the LA. In most patients, the precise location of the linear lesions was visualized with the Coolrail probe (Atricure Inc, Westchester, OH, USA) in situ and using fluoroscopy.
In the case of new-onset left isthmus dependent flutter, a left isthmus line was made starting from the ablation line on the antrum of the left inferior PV and crossing the CS, using the Max 3 pen (Atricure Inc, Westchester, OH, USA) on the epicardial side. This line was completed by the electrophysiologist (EP) endocardially from the mitral annulus towards the CS with the Thermo Cool catheter (Thermo Cool, Biosense Webster, Inc., Diamond Bar, CA, USA).
IVC-to-SVC and SVC circumferential lesions were added when patients with persistent and long-standing persistent AF had a dilated right atrium. IVC circumferential isolation was performed in patients with a small portion of intrapericardial IVC to ensure that the SVC-IVC line would stop at an area of no conduction. The isolation of the SVC and the IVC was confirmed by the testing of conduction block across the ablation lines. Furthermore, if the patient had a history of typical right AFL or this arrhythmia became apparent during the procedure, we performed a cavo-tricuspid isthmus line (CTI) endocardially.
Moreover, in patients with CHADS2 score10 ≥1, or in the presence of a rapid firing coming from the left atrial appendage (LAA), and when the procedure was deemed safe, LAA exclusion/closure was performed under TEE guidance employing a stapler (Endo GIA, Covidien, Norwalk, CT, USA) or a clip (Atricure, West Chester, OH, USA). The absence of flow was confirmed by intraoperative TEE.
Statistical analysis
Normal values were expressed as mean ±1 standard deviation (SD), non-normal values as median and IQR, and categorical variables as percentages. The Mantel-Haenszel Chi Square was employed to establish differences among groupings. Statistical analysis was performed using SPSS release 12.0 (SPSS, Chicago, IL, USA). P values less than 0.05 were considered significant.
Results
Forty-one patients were in AF at the start of the procedure, and in all patients we achieved bidirectional block of all the PVs. In 9 patients (11%), we did not perform any other lesion because AF was not inducible. In 4 patients with inducible AF after PV isolation, we performed an additional roof line only. In 65 patients (83%), we created a box lesion epicardially. In 50 patients (64%), we were able to demonstrate endocardial entrance and exit block in the box during SR. Endocardial touch-up was carried out in 28 patients (36%) to complete the box lesions. Other surgical lines are shown in Table 2. At the end of the procedure, sixty-two patients (79%) left the operating room in SR.
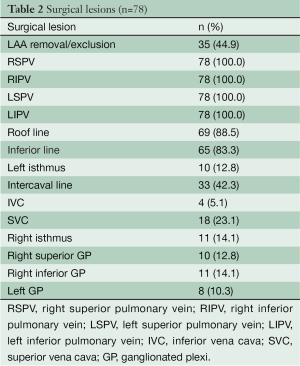
Full table
No death or conversion to cardiopulmonary bypass occurred. No patient demonstrated paralysis of the phrenic nerve. One patient (1%) underwent reoperation for bleeding, one (1%) suffered from bleeding not requiring reoperation, 2 (3%) had pneumonia and 2 (3%) underwent pacemaker implantation for complete atrio-ventricular block. Overall, the incidence of perioperative complications was 8% (n=6). Median hospital stay was six days (IQR 5.5-8).
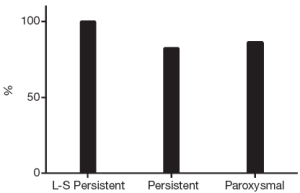
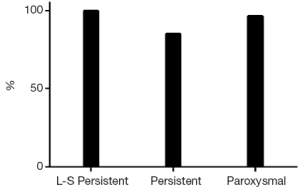
At the end of the follow-up there was a significant improvement in median EHRA score {1 [1-2], Pvs. baseline}. Sixty-eight patients (87%) were in SR with no episode of AF, AFL or AT lasting longer than 30 seconds and off ADD (Figure 1). Among patients with long-standing persistent AF, 15 (100%) were in SR and off AAD. Success rates were 82% (n=28) in persistent and 76% (n=22) in paroxysmal AF (P=0.08). The success rate on AAD (Figure 2) was 92% (n=72): 100% in long-standing persistent (n=15), 85% in persistent (n=29) and 97% in paroxysmal (n=28, P=0.72).
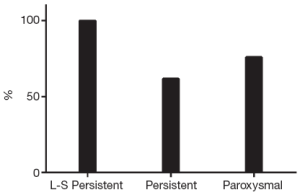
Ten patients (13%) underwent percutaneous ablation for recurrent AF or left AFL after the hybrid procedure. This means a single-procedure success rate (SR without AAD and/or redo procedure) of 74% (n=58): 100% (n=15) in long-standing persistent, 62% (n=21) in persistent and 76% (n=22, P=0.08) in paroxysmal AF (Figure 3).
Twenty-seven patients were on warfarin (35%) at the end of follow-up: 1 (1%) with long-standing persistent, 12 (15%) with persistent and 14 (18%) with paroxysmal AF (P
Finally, no patient died during the follow-up period. Furthermore, neither thromboembolic/bleeding events nor procedure-related complications occurred during follow-up.
Discussion
Pulmonary vein isolation (PVI), the cornerstone of percutaneous catheter ablation, has become a widely accepted therapy for symptomatic patients. However, catheter ablation of persistent and especially long-standing persistent AF remains challenging since PVI alone resulted in single procedural success rates as low as 21% in long-standing persistent AF (10,11).
On the other hand, although new technologies have allowed the creation of transmural lesions on a beating heart through alternative, less invasive incisions (12), efforts to emulate the highly successful cut-and-sew Maze procedure have been difficult. Indeed, results from AF ablation during concomitant cardiac surgery and minimally-invasive thoracoscopic epicardial ablation are still controversial (3,4).
The hybrid approach combines, in one step, a thoracoscopic epicardial ablation with a percutaneous catheter ablation procedure (13,14). The surgeon, through a thoracoscopy, can isolate the PVs and the posterior wall of the LA, whereas the endocardial “step” offers the possibility of evaluating the endpoints of the ablation adding an endocardial “touch-up” in case of incomplete epicardial PVI. In addition, the electrophysiologist can create lesions in regions which cannot be reached epicardially. Moreover, this technique may potentially decrease the complication rate of both surgical and catheter ablation procedures. Indeed, from the point of view of the surgeon, the risk of post-operative arrhythmias is reduced since ablation lines and lesion set can be controlled endocardially. Furthermore, the risk of injury is lower, since the electrophysiologist can make lesions in areas which cannot be easily reached by the surgeon (mitral isthmus, tricuspid isthmus). From the electrophysiology point of view, there is no risk of phrenic nerve and esophageal injury because these structures can be protected by the surgeon and the possibility of tamponade is low since the pericardium is open. In addition, by reducing the total number of endocardial ablations, the risk of embolism is potentially reduced (15).
Another potential advantage of the hybrid approach compared to percutaneous technique is the possibility of ablating the ganglionated plexi (GPs). However, although the autonomic nervous system seems to play an important role in AF, there is currently insufficient evidence to advocate a systematic GP ablation strategy (16-20). Finally, in the setting of a hybrid procedure, it is possible to perform extensive mapping to tailor the lesion set, whereas in the surgical approach there is the inability to precisely locate AF triggers and to map AT or re-entry arrhythmias known to occur during AF ablation (13).
However, even though the interest in this new technique has been growing, data on its effectiveness and safety are still scarce and most of the published studies have been limited in sample size, duration of follow-up and assessment of AF-associated symptomatology (6). We reported our experience with the hybrid epicardial-endocardial stand-alone ablation procedure using a thoracoscopic technique for the treatment of AF. At median follow-up of 24 months (IQR 12-36), the success rate on AAD was 92% (n=72): 100% in long-standing persistent (n=15), 85% in persistent (n=29) and 97% in paroxysmal (n=28). Sixty-eight patients (87%) were in SR with no episode of AF, AFL or AT lasting longer than 30 seconds and off ADD. Among patients with long-standing persistent AF, 15 (100%) were in SR and off AAD. Success rates were 82% (n=28) in persistent and 76% (n=22) in paroxysmal AF (P=0.08). The single-procedure success rate was 74% (n=58): 100% (n=15) in long-standing persistent, 62% (n=21) in persistent and 76% (n=22) in paroxysmal AF.
Our data compares favourably with Gehi et al. (19), who recently reported their experience with a pericardioscopic hybrid epicardial-endocardial technique. These authors showed a success rate of 63% after a single procedure and 70% including repeat ablation necessary in 6% of patients.
Zembala and co-workers reported 80% of patients in SR and off class II/III AAD one year after the pericardioscopic hybrid approach.
Moreover, our findings are comparable to those reported by Gelsomino et al. (6), who provided an overview of the thoracoscopic hybrid procedure for the treatment of stand-alone AF: success rate ranged from 86% to 92% and this percentage was even higher in long-standing persistent AF (81.8% to 100%).
Finally, the hybrid technique demonstrated, in our experience, a high degree of safety. No deaths occurred in the perioperative period and the incidence of complications was low (8%), with no patient requiring sternotomy and conversion to cardiopulmonary bypass. Furthermore, no deaths or procedure-related major complications occurred during the follow-up period.
Conclusions
Thoracoscopic hybrid epicardial endocardial technique proved to be effective in the treatment of LAF and to represent an important new option, suitable for treating stand-alone AF. Our findings need to be confirmed by further larger studies.
Acknowledgements
We gratefully thank Dr. Judith Wilson for the English revision of the manuscript.
Disclosure: Laurent Pison and Mark La Meir are consultants for Atricure. Other co-authors declare no conflict of interest.
References
- Stamou SC, Khabbaz KR, Mahmood F, et al. A multidisciplinary approach to the minimally invasive pulmonary vein isolation for treatment of atrial fibrillation. Ann Thorac Surg 2010;89:648-50. [PubMed]
- Oral H, Chugh A, Good E, et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 2007;115:2606-12. [PubMed]
- La Meir M, Gelsomino S, Lucà F, et al. Minimal invasive surgery for atrial fibrillation: an updated review. Europace 2013;15:170-82. [PubMed]
- Gelsomino S, La Meir M, Lucà F, et al. Treatment of lone atrial fibrillation: a look at the past, a view of the present and a glance at the future. Eur J Cardiothorac Surg 2012;41:1284-94. [PubMed]
- Pison L, La Meir M, van Opstal J, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol 2012;60:54-61. [PubMed]
- Gelsomino S, Van Breugel HN, Pison L, et al. Hybrid thoracoscopic and transvenous catheter ablation of atrial fibrillation. Eur J Cardiothorac Surg 2013. [Epub ahead of print]. [PubMed]
- Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 2011;57:e101-98. [PubMed]
- European Heart Rhythm Association (EHRA), European Cardiac Arrhythmia Scoiety (ECAS), American College of Cardiology (ACC), et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2007;4:816-61. [PubMed]
- European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360-420. [PubMed]
- Pisters R, de Vos CB, Nieuwlaat R, et al. Use and underuse of oral anticoagulation for stroke prevention in atrial fibrillation: old and new paradigms. Semin Thromb HemostSeminars in thrombosis and hemostasis 2009;35:554-9.
- Calkins H, Kuck KH, Cappato R, et al. HRS/EHRA/ECAS Expert consensus statement on catheter and surgical ablation of AF: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528-606. [PubMed]
- Sales VL, McCarthy PM. Minimally invasive surgery for atrial fibrillation. Tex Heart Inst J 2010;37:660-1. [PubMed]
- Mahapatra S, LaPar DJ, Kamath S, et al. Initial experience of sequential surgical epicardial- catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg 2011;91:1890-8. [PubMed]
- La Meir M, Gelsomino S, Lucà F, et al. Minimally invasive surgical treatment of lone atrial fibrillation: early results of hybrid versus standard minimally invasive approach employing radiofrequency sources. Int J Cardiol 2013;167:1469-75. [PubMed]
- Sauren LD, la Meir M, de Roy L, et al. Increased number of cerebral emboli during percutaneous endocardial pulmonary vein isolation versus a thoracoscopic epicardial approach. Eur J Cardiothorac Surg 2009;36:833-7. [PubMed]
- Schauerte P, Scherlag BJ, Patterson E, et al. Focal atrial fibrillation: experimental evidence for pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol 2001;12:592-9. [PubMed]
- De Ferrari GM, Schwartz PJ. Autonomic nervous system and arrhythmias. Ann N Y Acad Sci 1990;601:247-62. [PubMed]
- Jaïs P, Matsuo S, Knecht S, et al. A deductive mapping strategy for atrial tachycardia following atrial fibrillation ablation: importance of localized reentry. J Cardiovasc Electrophysiol 2009;20:480-91. [PubMed]
- Gehi AK, Mounsey JP, Pursell I, et al. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm 2013;10:22-8. [PubMed]
- Zembala M, Filipiak K, Kowalski O, et al. Minimally invasive hybrid ablation procedure for the treatment of persistent atrial fibrillation: one year results. Kardiol Pol 2012;70:819-28. [PubMed]