Management of neoplastic meningitis
Introduction
Neoplastic meningitis represents a spread of tumor cells into the subarachnoid space. Cancer cells may reach the leptomeninges through hematogenous spread, direct infiltration from tumor manifestations in the brain parenchyma or spread along the perineurium from cranial or spinal nerves. Based on the histological characterization of the underlying tumor, it is also referred to as leptomeningeal carcinomatosis, gliomatosis or lymphomatosis, respectively. Basically, all malignant tumors may cause neoplastic meningitis. However, similar to solid metastases to the CNS, there are several tumor entities which are much more frequently associated with leptomeningeal spread than others. Among the most common primary tumors associated with leptomeningeal dissemination are lung and breast cancer, melanoma as well as lymphoma and leukemia (1). Leptomeningeal spread is also observed in patients diagnosed with primary brain tumors such as medulloblastoma, germinoma or PNET whereas gliomas metastasize to the subarachnoid space less frequently. Neoplastic meningitis is found in approximately 5-10% of all patients with malignant tumors and is a condition frequently diagnosed in late stage cancer (2). Leptomeningeal tumor dissemination is associated with poor prognosis in patients with solid tumors and frequently accompanied with solid brain metastases in 50% of patients and even more frequently with extracranial metastases. The situation is different in patients with germ cell tumors of the CNS such as germinomas and in patients affected by medulloblastoma. Here, leptomeningeal disease is frequently found already at the time of initial diagnosis, not necessarily associated with poor prognosis, and therapy may still be curative.
It must be assumed that leptomeningeal tumor cell spread is rather underdiagnosed in the clinical setting since the available diagnostic procedures are not adequately sensitive to confirm the diagnosis in all cases (see below). Furthermore, diagnosis is not always forced in patients who have multiple systemic tumor manifestations in the absence of convincing treatment options.
Basically, there are two different types of leptomeningeal tumor manifestation: (I) solid tumor deposits; and (II) rather diffuse, non-adherent accumulation of tumor cells in the cerebrospinal fluid (CSF). A combination of both conditions is also found.
The clinical symptoms associated with neoplastic meningitis are symptoms due to increased intracranial pressure because of hydrocephalus such as nausea and vomiting, headaches and neck pain as well as confusion. Cranial nerve palsies resulting in diplopia, hemifacial weakness and radicular symptoms and signs like pain, paresthesia, paresis as well as loss of bladder or bowel control can also occur.
Neoplastic meningitis in patients with solid tumors has a poor prognosis. In the absence of treatment, median survival is typically in the range of 6-8 weeks. In patients with hematological neoplasms, the prognosis is better but still limited. Tumor-specific treatment such as radiotherapy or chemotherapy results in prolonged survival times which, however, are still restricted to a median of 2-8 months. Again, the prognosis is better for patients affected by lymphoma or leukemia. Furthermore, the disease course is somewhat more favorable in breast cancer patients which probably reflects the higher sensitivity of these tumor cells to irradiation and medical anti-tumor treatment. Still, it needs to be considered that the majority of patients affected by leptomeningeal disease do not die from tumor cell dissemination in the CSF but from systemic tumor progression. Negative prognostic factors associated with leptomeningeal tumor cell dissemination are low Karnofsky performance status, increased age, uncontrolled intracranial pressure as well as low glucose and high protein levels in the CSF (3-6).
Diagnostic procedures
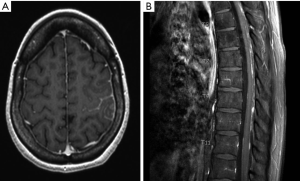
Patients with suspected neoplastic meningitis require a thorough diagnostic assessment with a detailed neurological examination as the first step. Here, particular attention should be paid to symptoms or signs caused by increased intracranial pressure as well as cranial nerve alterations. Symptoms evoked by extracranial tumor manifestations should also be taken into consideration. Imaging should comprise brain and spinal cord examination to allow for a comprehensive assessment of the subarachnoid space. Small tumor manifestations are typically only detected by MRI which is the gold standard (Figure 1). CT scans may just allow for the exclusion of significant hydrocephalus. Finally, CSF analysis is required to determine cell count, opening pressure as well as levels of protein, glucose and lactate. CSF cytology and immunohistochemical analyses may further help to confirm or exclude the presence of tumor cells. Markers such as alpha-fetoprotein or beta-HCG may be helpful in patients with (suspected) germ cell tumors. Here, CSF levels should always be compared with serum concentrations. Genomic alterations in tumor cells from the CSF can be detected by copy number analysis and may indicate their malignant origin (7). This approach however, has not yet reached clinical routine.
A recurrent challenge in the diagnosis of neoplastic meningitis which is suspected by clinical or imaging findings is negative CSF cytology despite advanced histological work-up (8). Here, flow cytometry which allows for an assessment of specific cell surface markers, e.g., epithelial-cell adhesion molecule (EpCAM) in patients with epithelial-cell cancers, may help to increase the diagnostic sensitivity (9-11). Monoclonal cell populations may be detectable by PCR, e.g., IgH rearrangements in lymphomas, and fluorescence in situ hybridization (FISH) can be used to detect chromosomal alterations (12). Such analyses, however, are often difficult to perform when only few cells are available and may be restricted to specialized laboratories. Another novel technique called “rare cell capture technology (RCCT)” uses anti-EpCAM antibody-covered magnetic nanoparticles to identify tumor cells. RCCT may increase the diagnostic sensitivity for tumor cell dissemination in the CSF but has not yet reached broad utilization (13).
Therapeutic approaches
There are only few prospective, randomized trials for patient with neoplastic meningitis. Thus, most therapeutic recommendations must be considered as low-level evidence and are mainly based on small clinical series, retrospective analyses or clinical experience. Because of the limited activity of the available therapeutic options, treatment goals must be carefully evaluated. Reduction of neurological symptoms and pain as well as limited life extension must be weighed against the side effects which are associated with any treatment. Accordingly, for some patients, best supportive care which aims at improving symptom control may be an appropriate approach. For those patients who are considered eligible for tumor-specific treatment, there are basically three therapeutic approaches which may be used as single treatment or in combination. There are no clear guidelines which treatments fits best for each patient. However, there is a consensus that the type of leptomeningeal tumor manifestation as assessed by MRI as well as the presence or absence of solid brain metastases and extracranial metastases defines the most appropriate therapeutic approach. Commonly, patients present with a combination of solid tumor manifestations in the leptomeninges and additional diffuse, non-adherent tumor cell spread. Accordingly, a combination of different therapeutic modalities is frequently required (Tables 1,2).
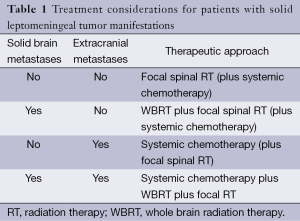
Full table
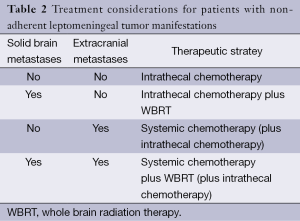
Full table
Radiation therapy
Craniospinal irradiation is only rarely performed because of significant bone marrow toxicity that can be associated with this approach. Irradiation of the entire neuroaxis is typically restricted to patients with leptomeningeal dissemination of primary brain tumors. Patients who are treated in such a way are rarely treated concomitantly with systemic chemotherapy and never with intrathecal therapy because of the increased risk of side effects. In most patients with neoplastic meningitis, whole brain radiation therapy (WBRT) which includes the subarachnoid space is used as a more focal approach (14). Typically, the chosen irradiation field involves the skull base as well as interpeduncular cisterns and the first cervical vertebrae (C1 and C2). WBRT is mostly administered in 3 Gy fractions to a total dose of 30-36 Gy. Focal spinal tumor manifestations are irradiated using a safety margin of one vertebral body above and below the lesion. Here, various fractionations are used, e.g., 5 × 2-3 Gy/week with a total dose of 30-36 Gy.
There are no controlled clinical trials assessing the activity of irradiation in patients with neoplastic meningitis. A retrospective series of patients with leptomeningeal carcinomatosis from non-small cell lung cancer (NSCLC) did not suggest a survival prolongation by WBRT (15). Still, radiation may help to restore CSF flow and reduce clinical symptoms. Therefore, it remains a treatment option for patients with leptomeningeal disease in the absence of other convincing therapeutic strategies.
Systemic chemotherapy and targeted therapy
Solid tumor manifestations in the brain probably respond to systemic therapy in a similar way as other systemic metastases (16,17). Accordingly, it can be assumed that solid leptomeningeal tumor lesions can be treated with systemic therapy. However, larger trials assessing this approach are largely lacking and the available evidence originates from a limited number of series (18,19). Whether the introduction of novel drugs within the last years such as pemetrexed, bevacizumab or tyrosine kinase inhibitors in patients with NSCLC improves the outcome of neoplastic meningitis remains unclear (20). Similar to parenchymal brain metastases, the best treatment for the primary tumor is also the best choice for the treatment of leptomeningeal disease. Many patients have already received one or more lines of systemic chemotherapy and therefore, when leptomeningeal carcinomatosis is diagnosed, only limited treatment options remain available. Another unresolved point remains the combination of systemic and intrathecal treatment. In breast cancer patients, the addition of intrathecal treatment to systemic chemotherapy did not result in improved outcome (21,22). Patients who suffer from non-adherent tumor cell dissemination in the CSF may benefit from systemic treatment provided that the chosen drug crosses the blood-CSF barrier at sufficient concentrations. Accordingly, promising results have been reported in patients who were treated with high-dose methotrexate (MTX) (19).
Targeted therapies using small molecule inhibitors have gained increasing interest during the last years. It has been reported that higher CSF levels of the EGFR inhibitor erlotinib may be achieved with an alternating dosing regimen compared to the standard schedule (23). Retrospective analyses suggest that erlotinib may improve the outcome of NSCLC patients with leptomeningeal carcinomatosis (24). Erlotinib may therefore be a valuable treatment option for patients with leptomeningeal carcinomatosis with tumor cells that harbor a sensitizing EGFR mutation. Compared to gefitinib, another EGFR inhibitor, erlotinib may achieve higher CSF concentrations and result in better control rates of leptomeningeal dissemination (25).
Intrathecal treatment
Intrathecal administration of drugs aims at efficiently targeting tumor cells in the CSF by circumventing the blood-CSF barrier while omitting systemic toxicity. However, drugs which are administered intrathecally probably have a limited penetration into solid leptomeningeal tumor deposits. Therefore, this approach is mainly restricted to patients who have non-adherent tumor cell spread in the CSF. Basically, treatment can be done following a lumbar puncture and subsequent drug injection. However, this approach has several drawbacks: repeated lumbar punctures are inconvenient for the patient and associated with an increased risk for misinjection and post puncture headache as well as complications such as infections or bleeding related to the procedure. Furthermore, distribution of the drug in the intra- and extraventricular CSF compartments may be insufficient (26). Therefore, it is recommended to place an intraventricular catheter system such as an Ommaya or Rickham reservoir. These devices allow for repeated injections and a better distribution of the drug. Among the drugs which are available for intrathecal treatment, MTX and cytarabine are most frequently used. Alternatively, thiotriethylenephosphoramide (thiotepa) has been approved in some countries. However, a retrospective analysis on the use of thiotepa in breast cancer patients with leptomeningeal carcinomatosis suggested only limited activity (27). MTX is typically given twice per week using single doses of 12-15 mg. No adaption to body weight or body surface area is required. In order to avoid systemic toxicity of MTX, folinic acid rescue should be started 6 h after the first injection and continued in 6 h intervals for 48 h. Cytarabine is also administered twice weekly using 40 mg per injection. MTX can be considered the standard of care for patients with neoplastic meningitis originating from solid tumors whereas cytarabine is more frequently used in patients affected by lymphoma or leukemia. In a randomized trial, neither MTX nor thiotepa displayed superior activity (28).
Liposomal cytarabine (DepoCyte®) is a sustained-release form of cytarabine. Liposomal cytarabine was compared with MTX in a controlled trial in patients with solid tumors and leptomeningeal carcinomatosis. Patients who were treated with liposomal cytarabine experienced a longer time until neurological progression. However, there was no significant difference in overall survival (29). In patients with leptomeningeal lymphomatosis, liposomal cytarabine resulted in higher response rates and improved quality of life compared to standard cytarabine (30) and is therefore a treatment option which may be considered for these patients. In a large retrospective analysis of breast cancer patients with leptomeningeal metastasis, liposomal cytarabine was similarly effective as MTX (31). Liposomal cytarabine requires only administration in 2-week intervals and may achieve more equal CSF distribution than non-liposomal cytarabine (32,33). In contrast, liposomal cytarabine is associated with an increased risk for radiculitis and arachnoiditis. The occurrence of arachnoiditis might be prevented by a prophylactic oral administration of dexamethasone (12 mg/day) starting with the first day of intrathecal treatment. A combination of radiation therapy and concomitant liposomal cytarabine administration has not been examined in controlled trials. Accordingly, there is a remaining concern that such a combination may result in neurotoxicity. Caution needs also to be taken in patients who are being treated with systemic chemotherapy that crosses the blood-brain barrier such as high-dose MTX or cytarabine. The addition of intrathecal treatment with liposomal cytarabine can result in significant neurotoxicity (34,35).
Data from a recent phase II study suggest that ventriculolumbar perfusion chemotherapy with MTX which represents continuous intraventricular perfusion through an intraventricular reservoir and drainage via lumbar catheter may allow for a better control of intracranial pressure and improved symptom control (36). These results, however, require confirmation in a larger trial.
There are no data available that support a combination of several drugs for intrathecal therapy. In contrast, data from rather old studies suggest no beneficial effect (37-39). Drugs which have already been used for intrathecal treatment include mafosfamide (40), topotecan (41) and etoposide (42). However, their administration must be considered as compassionate use. Emerging evidence exists for the use of intrathecal trastuzumab in patients with HER2/neu-positive breast cancer (43,44). Trastuzumab may be an active treatment which is overall well tolerated. The CD20 antibody rituximab has been administered intrathecally in patients with leptomeningeal lymphomatosis and was well tolerated. However, it remains to be determined whether this treatment approach results in a survival benefit (45).
At the beginning of treatment, WBC should be ≥3.000/μL and platelets ≥100.000/μL. Patients who are treated with MTX should have sufficient renal function. Otherwise, a close evaluation of potential toxicity is required. Intrathecal therapy should be performed without mixing drug solutions with other agents such as steroids. Overall, there are no clear guidelines on whether and how irradiation and intrathecal treatment should be combined. Because of the risk of side effects, a combined administration of both treatment modalities should only be done after a careful evaluation. Intrathecal treatment is mostly interrupted until irradiation has been completed or should be reduced to a one weekly administration. In most centers, irradiation is put on hold at the day of intrathecal treatment.
The duration of intrathecal therapy has not been standardized either. Clinical symptoms as well as CSF and MRI findings should be taken into account to decide whether the therapy should be continued or interrupted. Treatment aims at clearing the CSF from tumor cells within 2 weeks but may be continued on an individual basis. Early neurological and cytological responses may predict longer time-to-progression and overall survival (46). MRI responses are frequently difficult to determine and are of only limited help due to a lack of standardization with respect to response criteria (47). Clinical deterioration or continuous worsening of the CSF findings during therapy, e.g., an increasing number of tumor cells, requires an adaption of the treatment. Changing the therapeutic regimen to another drug or switching from chemotherapy to irradiation are the available options. Conversely, intrathecal therapy can be stopped when two subsequent CSF samples are free from tumor cells. No data are available which support the administration of a consolidation or maintenance therapy. In the case of tumor recurrence following prior clearance of the CSF, the same drug should be used as first choice.
Supportive therapy
Patients affected by neoplastic meningitis may suffer from various clinical symptoms as described above. Symptom relief is therefore a major goal of any chosen therapy. Here, steroids may help to decrease symptom burden similar to the situation of solid tumor manifestations in the brain (48). There are no particular treatment considerations for patients with leptomeningeal carcinomatosis available and dexamethasone in a dose of 4-16 mg/day may be considered a reasonable starting dose. Tapering should be considered whenever possible because of the manifold side effects associated with prolonged steroid use.
Patient who suffer from symptoms related to increased intracranial pressure because of hydrocephalus frequently benefit from ventriculoperitoneal shunting. Despite the risk of tumor cell dissemination from the CSF into the peritoneal cavity, this seems to occur only rarely (49). Seizures should be treated with an appropriate anticonvulsant. Drugs which do not interact with other compounds such as chemotherapeutic agents are preferred (50).
Outlook
Neoplastic meningitis remains a therapeutic challenge. The small number of controlled trials limits the available evidence for the different therapeutic options resulting in various non-standardized treatment regimens. Furthermore, there is a lack of data for specific tumor entities and clinical trials focusing on selected histological tumor types would be most helpful. The activity of many drugs which have become available within the last years remains largely unknown. A rigorous assessment of these drugs within clinical trials may help to define a better therapeutic management of patients affected by leptomeningeal tumor dissemination.
Acknowledgements
We thank Dr. Antonios Valavanis (Institute of Neuroradiology, University Hospital of Zurich) for providing MR images.
Disclosure: P Roth has received honoraria from MSD, Roche, Novartis and Molecular Partners for advisory board participation or lectures. M Weller has received research grants from Bayer, Isarna, MSD, Merck Serono and Roche and honoraria for lectures or advisory board participation from Isarna, Magforce, MSD, Merck Serono, Pfizer, Roche and Teva.
References
- Chamberlain MC. Neoplastic meningitis and metastatic epidural spinal cord compression. Hematol Oncol Clin North Am 2012;26:917-31. [PubMed]
- Chamberlain MC. Neoplastic meningitis. Oncologist 2008;13:967-77. [PubMed]
- Herrlinger U, Förschler H, Küker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci 2004;223:167-78. [PubMed]
- Chamberlain MC, Johnston SK, Glantz MJ. Neoplastic meningitis-related prognostic significance of the Karnofsky performance status. Arch Neurol 2009;66:74-8. [PubMed]
- Jaeckle KA. Neoplastic meningitis from systemic malignancies: diagnosis, prognosis and treatment. Semin Oncol 2006;33:312-23. [PubMed]
- Gwak HS, Joo J, Kim S, et al. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol 2013;8:599-605. [PubMed]
- Magbanua MJ, Sosa EV, Roy R, et al. Genomic profiling of isolated circulating tumor cells from metastatic breast cancer patients. Cancer Res 2013;73:30-40. [PubMed]
- Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 1998;82:733-9. [PubMed]
- Hegde U, Filie A, Little RF, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood 2005;105:496-502. [PubMed]
- Bromberg JE, Breems DA, Kraan J, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology 2007;68:1674-9. [PubMed]
- Subirá D, Simó M, Illán J, et al. Diagnostic and prognostic significance of flow cytometry immunophenotyping in patients with leptomeningeal carcinomatosis. Clin Exp Metastasis 2015;32:383-91. [PubMed]
- van Oostenbrugge RJ, Hopman AH, Arends JW, et al. Treatment of leptomeningeal metastases evaluated by interphase cytogenetics. J Clin Oncol 2000;18:2053-8. [PubMed]
- Nayak L, Fleisher M, Gonzalez-Espinoza R, et al. Rare cell capture technology for the diagnosis of leptomeningeal metastasis in sol [PubMed]
- Chang EL, Maor MH. Standard and novel radiotherapeutic approaches to neoplastic meningitis. Curr Oncol Rep 2003;5:24-8. [PubMed]
- Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol 2012;7:382-5. [PubMed]
- Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol 2011;22:2466-70. [PubMed]
- Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res 2007;13:1663-74. [PubMed]
- Bokstein F, Lossos A, Siegal T. Leptomeningeal metastases from solid tumors: a comparison of two prospective series treated with and without intra-cerebrospinal fluid chemotherapy. Cancer 1998;82:1756-63. [PubMed]
- Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol 1998;16:1561-7. [PubMed]
- Riess JW, Nagpal S, Iv M, et al. Prolonged survival of patients with non-small-cell lung cancer with leptomeningeal carcinomatosis in the modern treatment era. Clin Lung Cancer 2014;15:202-6. [PubMed]
- Boogerd W, Hart AA, van der Sande JJ, et al. Meningeal carcinomatosis in breast cancer. Prognostic factors and influence of treatment. Cancer 1991;67:1685-95. [PubMed]
- Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer 2004;40:2726-33. [PubMed]
- Grommes C, Oxnard GR, Kris MG, et al. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 2011;13:1364-9. [PubMed]
- Lee SJ, Lee JI, Nam DH, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol 2013;8:185-91. [PubMed]
- Lee E, Keam B, Kim DW, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 2013;8:1069-74. [PubMed]
- Sandberg DI, Bilsky MH, Souweidane MM, et al. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery 2000;47:49-54; discussion 54-5. [PubMed]
- Comte A, Jdid W, Guilhaume MN, et al. Survival of breast cancer patients with meningeal carcinomatosis treated by intrathecal thiotepa. J Neurooncol 2013;115:445-52. [PubMed]
- Grossman SA, Finkelstein DM, Ruckdeschel JC, et al. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol 1993;11:561-9. [PubMed]
- Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res 1999;5:3394-402. [PubMed]
- Glantz MJ, LaFollette S, Jaeckle KA, et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol 1999;17:3110-6. [PubMed]
- Le Rhun E, Taillibert S, Zairi F, et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol 2013;113:83-92. [PubMed]
- Phuphanich S, Maria B, Braeckman R, et al. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J Neurooncol 2007;81:201-8. [PubMed]
- Glantz MJ, Van Horn A, Fisher R, et al. Route of intracerebrospinal fluid chemotherapy administration and efficacy of therapy in neoplastic meningitis. Cancer 2010;116:1947-52. [PubMed]
- Jabbour E, O'Brien S, Kantarjian H, et al. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood 2007;109:3214-8. [PubMed]
- Ostermann K, Pels H, Kowoll A, et al. Neurologic complications after intrathecal liposomal cytarabine in combination with systemic polychemotherapy in primary CNS lymphoma. J Neurooncol 2011;103:635-40. [PubMed]
- Gwak HS, Lim HS, Shin SH, et al. Ventriculolumbar perfusion chemotherapy for the treatment of leptomeningeal carcinomatosis: a phase I study with pharmacokinetic data. Am J Clin Oncol 2013;36:491-9. [PubMed]
- Giannone L, Greco FA, Hainsworth JD. Combination intraventricular chemotherapy for meningeal neoplasia. J Clin Oncol 1986;4:68-73. [PubMed]
- Hitchins RN, Bell DR, Woods RL, et al. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol 1987;5:1655-62. [PubMed]
- Stewart DJ, Maroun JA, Hugenholtz H, et al. Combined intraommaya methotrexate, cytosine arabinoside, hydrocortisone and thio-TEPA for meningeal involvement by malignancies. J Neurooncol 1987;5:315-22. [PubMed]
- Blaney SM, Balis FM, Berg S, et al. Intrathecal mafosfamide: a preclinical pharmacology and phase I trial. J Clin Oncol 2005;23:1555-63. [PubMed]
- Groves MD, Glantz MJ, Chamberlain MC, et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol 2008;10:208-15. [PubMed]
- Chamberlain MC, Tsao-Wei DD, Groshen S. Phase II trial of intracerebrospinal fluid etoposide in the treatment of neoplastic meningitis. Cancer 2006;106:2021-7. [PubMed]
- Perissinotti AJ, Reeves DJ. Role of intrathecal rituximab and trastuzumab in the management of leptomeningeal carcinomatosis. Ann Pharmacother 2010;44:1633-40. [PubMed]
- Zagouri F, Sergentanis TN, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat 2013;139:13-22. [PubMed]
- Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007;25:1350-6. [PubMed]
- Fusco JP, Castañón E, Carranza OE, et al. Neurological and cytological response as potential early predictors of time-to-progression and overall survival in patients with leptomeningeal carcinomatosis treated with intrathecal liposomal cytarabine: a retrospective cohort study. J Neurooncol 2013;115:429-35. [PubMed]
- Chamberlain M, Soffietti R, Raizer J, et al. Leptomeningeal metastasis: a Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol 2014;16:1176-85. [PubMed]
- Roth P, Wick W, Weller M. Steroids in neurooncology: actions, indications, side-effects. Curr Opin Neurol 2010;23:597-602. [PubMed]
- Omuro AM, Lallana EC, Bilsky MH, et al. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology 2005;64:1625-7. [PubMed]
- Weller M, Stupp R, Wick W. Epilepsy meets cancer: when, why, and what to do about it? Lancet Oncol 2012;13:e375-82. [PubMed]


