Periprosthetic fractures around the femoral stem: overcoming challenges and avoiding pitfalls
Introduction
Periprosthetic fractures around the femoral stem after THA represent a significant and growing technical challenge for orthopaedic surgeons, requiring proficiency in both THA and trauma care. The incidence of such fractures continues to rise as the number of patients undergoing primary and revision THA increases (1,2). Historically, these fractures have been treated using a simple algorithm based upon the Vancouver classification. Fractures around a well-fixed stem are treated with osteosynthesis, whereas fractures with femoral stem loosening require revision arthroplasty and those with poor bone stock must also be augmented with a bone graft. While there are reports of good outcomes using this paradigm (3-17), the literature suggests a higher rate of failure for osteosynthesis of fractures around a well-fixed stem compared with revision of fractures around a loose stem (18-36). This may seem paradoxical; however, these injuries are plagued by significant biologic and mechanical challenges. Additionally, many of these fractures may merely be the sequelae of more complex underlying pathology, such as unrecognized osteolysis or weakening of bone stock (1,37-40). Surgeons have employed a variety of constructs in an attempt to overcome these difficulties, including cables, wires, bands, clamps, locking and non-locking plates, and allograft struts; and in recent years some focus has shifted to using minimally disruptive surgical techniques. While there is no general consensus as to the best technique for operative fixation, we will review the various options that have been used in practice to achieve optimal results.
The optimal treatment for Vancouver type B fractures is controversial. This is largely a consequence of the available literature, which mostly includes small to medium sized heterogeneous case series with little comparative evidence (4). While open reduction and internal fixation (ORIF) remains the mainstay of treatment for fractures around a well-fixed stem, there are patients who would likely benefit from alternative management. The goals of this review are to discuss the appropriate methods for evaluation and treatment of type B fractures and situations in which alternative management may provide a more suitable long-term solution.
Diagnosis
As with most other orthopedic injuries, clinical history and exam are paramount. The overwhelming majority of patients with periprosthetic fractures (90-95%) present with minimal or even no history of trauma, most commonly with the clinical onset of pain (1,41). Risk factors for fracture, including osteoporosis or other bony abnormalities, should be identified. It is of particular importance to assess the premorbid functional status of the joint, as worsening pain or dysfunction of the hip prior to injury is suggestive of preexistent implant loosening (2,40,41). Pain around the thigh experienced with initiation of ambulation or rising from a chair are indicative signs of femoral stem loosening, whereas groin pain of a similar nature suggests acetabular loosening. In all cases, suspicion of periprosthetic joint infection (PJI) should be thoroughly ruled out. A previous study demonstrated that the PJI rate in patients with periprosthetic fractures can be as high as 11.6% (42). Unfortunately, erythrocyte sedimentation rate and C-reactive protein are not reliable diagnostic markers in the setting of periprosthetic fracture given the increased inflammation associated with the fracture. False positives rates have been reported to be 31% and 43%, respectively (41,42).
Following clinical evaluation, high-quality anteroposterior and lateral radiographs are used to assess characteristics of the fracture (location, pattern, and bone quality), as well as the status of the prosthesis. Most importantly, the X-rays should be carefully examined for signs of femoral stem loosening. This includes assessing for continuous radiolucency around the prosthesis interfaces (bone-metal or bone-cement-metal) and signs of osteolysis (2,41). Lucent zones of 1-2 mm may occur around stable implants; thus, it is critical to compare current X-rays with previous radiographs. This comparison also allows for an appraisal of component migration, which is usually seen as subsidence (>10 mm) or varus tilt of the stem (41,43-45). In the setting of an acute fracture, a focal split of the cement mantle is not in itself indicative of loosening, but this must be closely examined. In addition to the femoral component, it is important to evaluate the stability of the acetabular component; in one study of nearly 900 periprosthetic fractures treated using revision THA, over 62% of acetabular cups were found to be loose (38).
Confirming prosthesis stability on radiograph alone is difficult, and as many as 20% of loose femoral stems go unnoticed. This limitation of radiographic sensitivity has been found repeatedly in studies that compared radiographic and intraoperative assessment of stability (23,46-49). Some authors have theorized that underestimation of stem loosening is largely to blame for high failure rates seen with ORIF of periprosthetic femur fractures where the stem is thought to be well fixed, as revision arthroplasty would have been a more appropriate option (23,34). This highlights the importance of intraoperative testing for component stability. If a surgeon considers that the stem is likely stable, the fracture site is most often approached directly and the potential for morbidity with hip arthrotomy and dislocation for stability testing can be avoided (34). Whenever possible, indirect methods for testing intraoperative stability without the need for exposing the joint should be employed. Depending on the fracture pattern and exposure, the distal implant can be tested for translation relative to the femur (41). If this is not possible, fluoroscopy can be utilized for dynamic testing in the operating room. For all cases in which the stability of the implant remains in question, surgical dislocation should be performed for further evaluation. This is especially true for patients with premorbid hip pain or dysfunction and fractures that occurred with minimal trauma, as these are often signs of a more complex pathology relating to the implant itself (1,34,38-40).
Classification
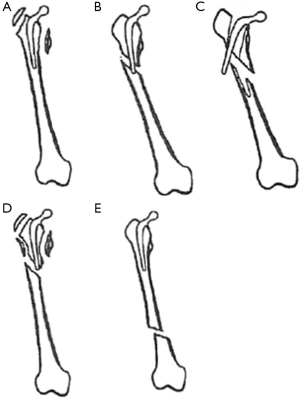
The widely accepted classification system for postoperative periprosthetic fractures of the femur, developed by Duncan and Masri in 1995, stratifies patients according to the location of the fracture, stability of the prosthesis, and quality of bone stock (Figure 1) (50). Commonly called the Vancouver classification, it has proved to be quite practical due to its reliability, high validity, and its established treatment algorithm (47-50). Fractures are categorized by three types based on level. Type A fractures are located at the proximal metaphysis, and are further subdivided based on involvement of the greater trochanter (AG) and lesser trochanter (AL). Type B fractures include those around or just below the stem. These also are sub-classified: type B1 fractures occur around a well-fixed stem, type B2 fractures occur around a loose prosthesis but with good bone stock, and type B3 fractures are seen in cases with a loose stem and poor bone stock or significant comminution. Finally, type C fractures occur well below the stem. Other classifications used historically in the literature include the Bethea (fracture pattern), Johansson (fracture location), Mont and Mar (fracture location and pattern), and Beals and Tower (fracture location/prosthetic interphase disruption) systems, among others (21,51-53).
While the Vancouver system is quite effective in most cases, its major fault lies in its total reliance on preoperative radiographic evaluation of stem stability to distinguish type B1 and type B2 fractures. In principle, type B1 fractures are assumed to be the result of traumatic injuries in relatively normal bone, although some osteoporosis may still be present. On the contrary, type B2 and B3 fractures are byproducts of pathological interfaces between the bone-cement-prosthesis or bone-prosthesis. It is critical that type B2 and B3 fractures be identified so that the pathologic prostheses may be addressed. Unfortunately, not all of these pathologic fractures can be recognized based upon radiographic loosening of the femoral stem, and this may result in incorrect classification. For example, as high as 20% of loose stems are missed on preoperative X-ray evaluation, and many surgeons fail to adequately test stability in the operating room. Also, a well-fixed stem does not always indicate a clear lack of pathology, and the fracture event could accelerate impending mechanical failure (38,54).
The Coventry classification, developed by Ninan et al. in 2007, stratifies fractures into “happy hips” or “unhappy hips” based upon multiple criteria to evaluate for signs suggestive of femoral stem pathology, not just radiograph alone. “Unhappy hips” include previously established loosening in patients already scheduled for revision surgery, worsening hip pain or dysfunction, fracture with minimal trauma, or clear signs of loosening on radiograph (40). Just like type B1 and B2 fractures, “happy hips” can be managed with fracture fixation, whereas “unhappy hips” require revision. Using multiple modalities to assess the status of the femoral component should reduce the likelihood that a loose femoral prosthesis is left in place.
Epidemiology
The incidence of periprosthetic femur fractures is expected to increase dramatically, as the prevalence of primary and revision THA and life expectancy continue to rise (1,2). Reports on the incidence of periprosthetic fracture after primary THA have ranged from 1-2.3%, to as high as 1.5-7.8% after revision THA (55-60). Such fractures are now the third most common cause of reoperation for THA and account for 6% of cases in Sweden (34). According to the Swedish National Hip Arthroplasty Registry [1979-2000], the breakdown of periprosthetic fractures based on the Vancouver system was as follows: 4% were type A fractures, 86% type B fractures, and 10% type C fractures. Following primary THA, the majority (70%) of type B fractures occurred around a loose stem (type B2), whereas fractures after revision surgery are more commonly (51%) around a well-fixed prosthesis (type B1) (34). Most (60%) of the available literature reports on type B1 fractures around cemented prostheses, although the risk for fracture is higher in cementless implants (1,41,61). Osteoporosis has been identified in nearly 60% of patients with a periprosthetic fracture, and thus female gender (52-70% of fractures) and advanced age (mean age ranging from 60-77 years) are often reported as risk factors (1,41,62). In one study, femoral stems were determined to have been loose at the time of fracture in 70% of cases following primary THA and 44% after revision THA (34). Other risk factors include those that affect bone quality, such as inflammatory arthropathy, previous hip fracture, bony deformity (Paget’s), and the presence of osteolysis. Technical factors, such as revision surgery, press-fit implants, and malposition of implants also place patients at increased risk for periprosthetic femur fractures (34,46,62,63). The great majority (about 85%) of fractures were the result of falls from sitting or standing, with “spontaneous fractures” and high-energy trauma accounting for only 10% and 5% of cases, respectively (1,61,64). One study found that 37% of such fractures were considered “spontaneous” following revision THA (34).
Finally, periprosthetic femoral fractures commonly occur in elderly patients with multiple comorbidities. The reported 1-year mortality following operative treatment of periprosthetic fractures is high (9-17%), similar to that of hip fractures (16.5%); and two studies reported a substantially higher mortality rate for such fractures when treated by osteosynthesis compared with revision arthroplasty (30-32% vs. 10-12%) (1,34,38,65-67). This difference has been attributed to immediate full weight bearing and improved mobilization of patients undergoing revision arthroplasty with a long-stem prosthesis, which prevents deconditioning and reduces morbidity and mortality (38,65,66). On the contrary, extramedullary constructs require a minimum period of non- or partial weight bearing to prevent mechanical failure (64,66). Additionally, some studies have shown less need for reoperation after immediate revision arthroplasty (34,39). Given that definitive treatment is more critical in geriatric patients for whom revision surgery in the future may not be feasible, revision arthroplasty has gained support as a viable and sometimes preferred alternative in select patients (39,65-68).
Pathogenesis
It is a well-known concept that implantation of rigid orthopedic hardware within a long bone creates a “stress riser”, or an area of high stress concentration. The stem tip clearly plays a role in determining where a periprosthetic femoral fracture may occur; Beals and Tower determined that 75% of fractures occur at the stem tip (21,69). Biomechanical studies have demonstrated that only loose stems act as “stress risers”, whereas well-fixed stems have not been shown to cause such an effect (69,70). A loose femoral stem that has subsided into varus malalignment would impinge on the lateral cortex of the femur (71). In addition to a loose stem, the “stress riser” effect is clearly dependent on cortical density, as peak stress was found to increase as cortical thickness decreased (69,70). Defects in cortical bone stock can occur from cortical perforation during canal preparation, stem impingement, old screw holes, or pre-existing bone loss (71). In canines, cortical perforation reduced bone strength by 44% in an intact femur (72). Therefore, a high-risk framework for developing a periprosthetic fracture is a loose stem with deficient cortical bone, such as in osteoporotic bone. Additionally, cementless implants do not have a stable mantle around the stem as they do not immediately become fully osseointegrated into host bone, and may initially act as “stress risers”. Thus, periprosthetic fractures around cementless femoral stems commonly occur only 0.5 years after insertion, compared with 6.6 years for cemented implants (21). After bony ingrowth, there is greater fixation for the femoral implant and periprosthetic fractures should be less common (69,70).
Even in patients at high risk for developing periprosthetic fracture, a fracture will not occur unless a stress is applied that exceeds the strength of the bone. Biomechanical studies on osteoporotic cadaveric femurs with implanted prostheses have demonstrated how various applied loads lead to resultant periprosthetic fracture patterns. In one study, a torsional load consistently yielded a peritrochanteric fracture (Vancouver A), an anterior load produced a supracondylar distal femur fracture (very distal Vancouver C), and a lateral load led to a fracture line near the tip of the stem (Vancouver B) (73). These fracture loads were also applied to similar cadaveric femurs without an implant for comparison. While an anterior load still yielded a supracondylar fracture, the results of a lateral load were inconsistent: two specimens demonstrated fractures in the supracondylar area, a third one in the mid-shaft, and one final fracture in the peritrochanteric region. The maximal stress load at failure, or bone strength, was also compared between the implant and implant-free models, as well. For a laterally applied load, 32% less force (4,692 vs. 6,931 N, P<0.05) was required to produce a fracture at the stem tip of an implanted femur. Of note, the implants were reported to be loose in 3 out of 4 specimens. Other studies argue that low-energy torsional force, which can be caused by occasional overload during daily activities in the presence of a loose stem, is commonly responsible for “spontaneous” Vancouver B fractures (74,75). A low torsional load (<50 N) applied to cadaveric femurs with loose implants resulted in long spiral fractures of the proximal femur, whereas cadaveric femurs with well-fixed implants yielded more distal fractures after application of a greater torsional load (>100 N) (75). This evidence supports the concept of a reduced fracture threshold near the tip of the prosthesis, particularly if the stem is loose.
In accordance with the above discussion, any pathologic process that weakens bone stock will greatly increase the risk for periprosthetic fracture. Osteolysis is a particularly precarious threat for late periprosthetic fracture, as it results in both endosteal resorption of bone as well as aseptic loosening of the stem (62,71). Some studies suggest that as high as 75% of all revision arthroplasties are the result of underlying osteolysis, and the impact of this process may be growing (34,76). The mechanism of osteolytic disease involves an inflammatory response to wear debris, primarily mediated by macrophages, which can weaken bone strength (76). In addition to osteolysis, revision arthroplasty is associated with a greatly increased risk of periprosthetic fracture, and the rate of fracture increases proportionally with the number of revisions (34,55,59). Patients undergoing revision THA often have large deficiencies in bone stock either from disuse osteopenia, previous surgery (e.g., windowing), infection, or osteolysis (62,71). Revision may require the use of longer or larger stems that increase the risk for fracture. For instance, long, straight revision stems can impinge on the anterior cortex of a bowed femur (71). Other conditions that can affect bone stock include osteoporosis, inflammatory arthropathy, trauma, and tumors (62,77). Bethea et al. suggested that a loose femoral stem can result in bone resorption independent of osteolysis, possibly as a result of increased motion at the bony interface (51,62). The effect of bone quality on periprosthetic fracture risk highlights the potential value of reconstructing cortical bone support using strut bone graft (69).
Ideal Vancouver B1 fractures, by definition, show no signs of femoral loosening and largely have intact cortical bone. Consequently, no “stress riser” is present to increase the risk for fracture. Although some degree of osteoporosis or acquired bone deficiency does not preclude type B1 classification, most cases with severely deficient bone stock often present with loose femoral components (78-80).
Prevention
Successful prevention of periprosthetic femoral fractures requires special considerations, both intraoperatively and in follow-up. First, preparation of the canal must be performed with considerable care and bony defects should either be grafted or bypassed with a long stem (81-83). Furthermore, 70% of periprosthetic fractures occur in patients with loose stems, of which 27% were known to be loose prior to injury (34). Clearly, revision procedures should be handled expeditiously to avoid further complication. However, as much as 80% of femoral stem loosening is clinically silent (84). Thus, more routine radiographic follow-up may be warranted to foster early detection of osteolysis and aseptic loosening (34,82). Routine standardized monitoring appears to have the potential for cost-savings in that acute care of periprosthetic fractures is considerably more expensive than scheduled revision of aseptic loose femoral stems (85).
Management
Management of type B periprosthetic fractures is typically operative unless surgical intervention is strictly prohibited due to medical contraindication. Poor union, exceedingly long hospital admission, delayed mobilization, and, most importantly, high mortality, have plagued non-operative management (53,56,86-88). The mainstay of operative treatment for periprosthetic fractures around a well-fixed stem is ORIF; however, it is critical to identify patients for whom osteosynthesis may result in poor outcomes. Clearly, fractures around femoral stems that are loose should be treated using revision THA. Additionally, strong consideration should be made for revision in patients with a history of worsening hip pain or dysfunction prior to the fracture, or femur fracture with minimal trauma. Consideration for revision can also be made for patients with difficult to treat fracture patterns (transverse or short oblique fractures, especially with comminution), exceedingly poor bone quality, and cemented femoral stems, as these factors have been associated with worse outcomes (10,23,28,89-91). In these instances, patient factors such as medical comorbidities and anesthesia risk could guide treatment, as these patients would be more likely to do poorly with prolonged immobilization and less likely to tolerate revision surgery (38,65-67). In fact, medical fragility itself may be an indication for revision arthroplasty in particularly worrisome patients, although better alternatives may exist. Finally, surgeons should always be prepared in the event that intraoperative assessment indicates the need for a change in operative plan.
Osteosynthesis of type B1 fractures
Once the decision has been made to proceed with osteosynthesis, there is controversy with regard to the optimal method for reducing and fixing the fracture. The available literature does not offer a consensus, and it is mostly comprised of small to medium sized case series providing support for or against the use of specific techniques. Low institutional case volumes make high quality, well-controlled studies quite difficult. While many of the challenges with fixation of type B1 fractures remain the same, considerable variability exists with regard to fracture configuration and bone quality; thus, operative treatment must be tailored to meet the needs of each case.
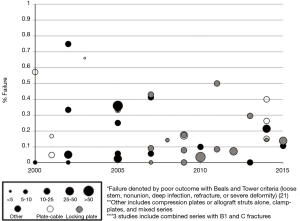
While there are reports of good outcomes (3-17), many studies point to a high failure rate for osteosynthesis of type B1 fractures (Figure 2) (18-36). Several authors have accepted that unrecognized femoral stem loosening may in part be to blame for high failure rates (23,38-40,92,93). Its contribution to failure is not clear, but may be the result of increased motion and strain at the fracture site or another underlying pathology. However, it is clear that despite the stability of the implant, both the biomechanical and biologic conditions for fracture healing are poor in the presence of intramedullary prostheses. Not only does the physical presence of the implant and possibly the cement mantle obstruct adequate proximal fixation, prior reaming and occlusion of the canal contribute to endosteal ischemia. Ensuing periosteal devascularization during fracture reduction and fixation with plates and cables further impairs the biologic response (18,64,94). These suboptimal conditions play a large role in the reported failures, which commonly include early construct failure (plate or screw pullout or fracture), non-union or malunion, refracture at the end of the plate or implant, or stem loosening.
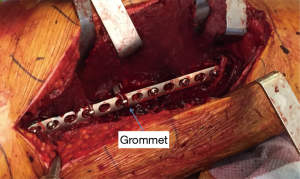
Gaining stability of the fracture site is critical for healthy fracture healing. Too-rigid fixation can also be a problem, as some degree of movement is necessary for callus formation, and increasing stiffness of a construct can lead to mechanical fatigue (95,96). Local stress at the fracture site can be avoided by leaving 3 or 4 screw holes empty, effectively increasing working length (2,97). Also, screw head inserts in empty holes can reduce the risk for plate fracture at these points of weakness (95). Engaging at least 4 cortices proximally and 8 distally has been recommended, although only 4 cortices distally may be necessary for more proximal fractures (2). Screw pullout occurs due to dynamic loading of daily activity, and patients with osteoporotic bone have a higher risk of screw pull out. Bicortical screws are less likely to pull out and provide better rotational stability, but could jeopardize the stability of the stem or the integrity of the cement mantle when aimed proximally (95). Most reports suggest that violation of the cement mantle with proximal screws does not lead to premature femoral stem loosening (8,98,99). Cables can be placed around the plate through specialized grommets (Figure 3), effectively reducing the risk for plate pullout. These cables also can help bring the plate into contact with the bony contour, which is an effective method for reducing soft tissue impingement.
The fracture site, or the femoral stem in more proximal fractures, should be bypassed by at least two cortical diameters, although one study reported a lower risk for refracture with femoral-spanning plates (4,26,41,94). While biplanar fixation and allograft struts have been advocated, most authors in recent years have stressed the importance of minimally disruptive techniques that avoid significant soft tissue dissection and tissue stripping to maximize biologic healing (4,41,95). Allograft struts provide additional support and eventually incorporate to improve femoral bone stock, although they require complete stripping of the anterior or medial femoral cortex (4,40,41). Outcomes have been mixed, and one study by Moore found a longer time to union and increased risk for deep infection with allograft struts (90,91,100,101). Unstable fracture patterns such as transverse or short oblique patterns with significant comminution or patients with poor bone may still benefit from placement of a cortical strut allograft. An accurate, direct fracture reduction, despite its blood supply disruption, is also still a priority to avoid early failure (2). Finally, early mechanical loading is a likely culprit in plate failure, even when bone quality and fixation are optimal (22,94,95,102). Some degree of protected weight bearing is recommended for 6-8 weeks, with advance to full weight bearing with clinical and radiologic signs of fracture healing (66,95).
A wide range of constructs have been used to meet the fixation requirements around an implanted femoral stem. Historically, cerclage techniques with wires, cables, or bands and clamp (Mennen) plates have been used alone, but these do not provide adequate fixation (15,30,91,94,103). The first specialized plate designed to avoid the pitfalls of proximal fixation, including violation of the stem or cement mantle and poor screw purchase, was the Ogden plate. This modified plate used heavy-duty bands for proximal fixation and screws distal to the prosthesis (104,105). Currently, there is evidence to support the use of three common constructs: plate-cable systems, locking plates, and compression plates. Each method has particular advantages and drawbacks with no one method demonstrating superiority in all cases. In fact, many newer plate designs allow for flexibility in using multiple methods for fixation as they are needed.
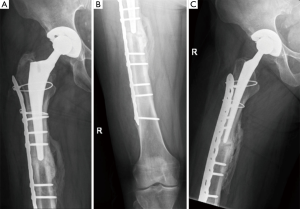
More modern applications of the “Ogden method”, or plate-cable systems, are an improvement on the original design and continue to be utilized (Figure 4) (105,106). In 10 studies on plate-cable systems used since 2000, the rate of failure defined by poor outcome by the Beals and Tower criteria, which includes loose stem, nonunion, deep infection, refracture, or severe deformity, ranged from 0-66% although the range was more reasonable (15-33%) in studies with greater than 15 patients (3,14,21,23,26-30,36,94). These improved results may be a result of outcomes from large-volume centers that have greater experience treating periprosthetic femur fractures. Early plate-cable constructs seem to have relied more on cables alone for proximal fixation; however, proximal fixation can now be achieved with a combination of cables and unicortical or bicortical screws. Biomechanical studies suggest plate-cable constructs with proximal unicortical screws alone or unicortical screws with cables provide added stability in compression, lateral bending, and torsion compared to proximal cables alone (106). Some series have also reported good results with proximal bicortical screws; these constructs should have superior rotational stability (66). Several authors have postulated that failure with plate-cable systems is the result of varus malpositioning of the femoral stem, which results from poor torsional stability (18,27,29).
As indicated in Figure 2, the use of locking plate technology for type B1 fracture fixation has increased in recent years. No studies on the use of locking plates published prior to 2007 are shown, but 8 studies performed between 2007 and 2010 can be seen. According to a recent systematic review by Dehghan et al., 36% of type B1 fractures were treated with locking plates (61). Locking plates are particularly suitable for fixation in patients with poor bone quality, which is quite common with periprosthetic fractures. Locking plates do not depend on friction between the plate-screw and bone interface for stability, and the fixed-angle between screw and bone mitigates the burden on adequate screw purchase. As the plate does not need to be pressed directly against the bone, there is the added benefit of less tissue disruption and less risk for refracture at the end of the plate (95). Newer locking plate designs allow for screw placement at oblique angles for easier insertion anterior or posterior to the implant (11). In 14 studies performed since 2007, the rate of failure of locking plates ranged from 0-50%, and this range was 3.5-17.4% in studies with greater than 20 patients (4-6,9,16,22,26,35,39,102,107,108). Dehghan et al. reported a higher rate of nonunion and hardware complications with locking plates, although they attributed this to an overreliance on the locking plate to gain stability, inadequate reduction, and selection bias for use of locking plates in more difficult cases, such as those with severe osteoporosis (61). In addition to locking plates, some periprosthetic fractures with simple fracture patterns (transverse or short oblique) and no comminution are best treated by absolute stability via a dynamic compression plate (41,109). While a few studies reported on the use of dynamic compression plating alone, many of the commonly used locking plates now offer both dynamic compression and locking options.
Revision arthroplasty
Revision THA with stem exchange is the treatment of choice for periprosthetic fractures with obvious or subtle clinical signs of femoral implant failure or difficult to treat fracture patterns. Revision can also be considered in fragile, elderly patients to avoid further reoperation and minimize mortality. Some authors have suggested that revision THA be combined with osteosynthesis, although this requires a prolonged operation and 2-stage revisions are not optimal in elderly patients (38,52). Simple long-stem revision can be time-consuming, difficult, and expensive, especially if the stem is well-fixed, but experienced surgeons have reported comparatively low complication and reoperation rates (106). In a large study on exchange with a long-stem implant, the overall complication rate was 10%, including 2.5% dislocations, 3.3% implant loosening, 1.7% device fracture, 1.7% deep infection, and 0.8% refracture (38). Laurer reported no implant failures and one case of peroneal palsy in 16 patients undergoing revision THA for periprosthetic fractures (type B1 and C), whereas 8 implant failures occurred in 16 similar patients undergoing osteosynthesis (39). Generally, the level of the fracture as well as the presence and location of pathologic bone are considered when deciding the best type of implant to use. Similar to a fracture plate, the revision stem should span the fracture site for a minimum of two cortical diameters (about 4 cm) (59,72,110). In more proximal fractures with intact proximal femoral bone stock, a standard-length stem may be considered. With poor proximal bone, a long-stem revision stem may be sufficient if the isthmus and distal femur are largely free of pathology, whereas more complex bone grafting may be necessary if the distal femur is affected by osteolysis (40).
For patients with stable prostheses that are considered to be high risk for decompensation with prolonged immobilization, in situ lengthening of an indwelling prosthesis may be a viable option. This method involves placement of a slotted retrograde intramedullary nail over the conical tip of the retained prosthesis. Similar to intramedullary nailing of typical femoral fractures, this technique could allow for early mobilization and is less demanding than long-stem revision arthroplasty. Meyer et al. reported the successful use of this technique in 18 patients; the only complication was loosening of a single femoral stem. It appears that there is some risk for loosening of the femoral prosthesis while coupling the stem and nail. While this method currently requires a custom-made nail based on preoperative computed tomography scans, special standardized implants could be manufactured if this were to become a common treatment method (111). However, this technique is not commonly used in the United States.
Conclusions
Management of periprosthetic fractures around the femoral stem can be challenging because these fractures require extensive surgical expertise. Poor biologic healing often plagues such fractures, adequate proximal fixation can be difficult to achieve, and failing implants within pathologic bone may be difficult to recognize. However, careful clinical evaluation and proper operative technique can improve outcomes for patients suffering from these difficult to treat injuries. Firstly, femoral stem stability should be adequately assessed intraoperatively in all cases. If the fracture site cannot be accessed directly, dynamic testing under fluoroscopy is indicated. When femoral stem stability remains in question, surgical dislocation should be performed for definitive evaluation. For fractures around a well-fixed stem (Vancouver B1 fractures) in normal bone, the mainstay of operative treatment is ORIF. This can be effectively accomplished with a variety of constructs. Cables and non-locking screws can be used for fixation in good quality bone, whereas locking screws should be used in patients with poor bone stock. Compression plating can be advantageous for simple fracture patterns without comminution. The use of allograft struts is controversial; they may be beneficial for augmentation of unstable fracture configurations with comminution or poor bone. Unlike fractures around uncompromised implants, revision arthroplasty should be considered in patients with loose femoral stems (Vancouver B2 fractures), in patients with a history of worsening hip pain or dysfunction, or femur fractures with minimal trauma. Improved outcomes may be achieved by performing revision arthroplasty in patients with difficult to treat fracture patterns (transverse or short oblique with comminution), exceedingly poor bone quality, or cemented stems. Additionally, osteosynthesis of periprosthetic fractures is associated with a high 1-year mortality rate. Elderly, fragile patients may benefit from treatment methods that allow for early mobilization, such as revision arthroplasty or even effective stem lengthening with a retrograde nail.
Acknowledgements
We would like to thank Katherine Huff for her assistance with editing and formatting the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Moreta J, Aguirre U, de Ugarte OS, et al. Functional and radiological outcome of periprosthetic femoral fractures after hip arthroplasty. Injury 2015;46:292-8. [PubMed]
- Marsland D, Mears SC. A review of periprosthetic femoral fractures associated with total hip arthroplasty. Geriatr Orthop Surg Rehabil 2012;3:107-20. [PubMed]
- Lever JP, Zdero R, Nousiainen MT, et al. The biomechanical analysis of three plating fixation systems for periprosthetic femoral fracture near the tip of a total hip arthroplasty. J Orthop Surg Res 2010;5:45. [PubMed]
- Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury 2009;40:1180-6. [PubMed]
- Chakravarthy J, Bansal R, Cooper J. Locking plate osteosynthesis for Vancouver Type B1 and Type C periprosthetic fractures of femur: a report on 12 patients. Injury 2007;38:725-33. [PubMed]
- Ebraheim NA, Gomez C, Ramineni SK, et al. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma 2009;66:1152-7. [PubMed]
- Haddad FS, Duncan CP, Berry DJ, et al. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am 2002;84-A:945-50. [PubMed]
- Haidar SG, Goodwin MI. Dynamic compression plate fixation for post-operative fractures around the tip of a hip prosthesis. Injury 2005;36:417-23. [PubMed]
- Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop 2012;83:648-52. [PubMed]
- Kim Y, Tanaka C, Tada H, et al. Treatment of periprosthetic femoral fractures after femoral revision using a long stem. BMC Musculoskelet Disord 2015;16:113. [PubMed]
- Meiners J, Faschingbauer M, Voigt C, et al. Polyaxial locked implants in the treatment of type vancouver B1 periprosthetic fractures of the femur: Retrospective clinical examination in 58 cases with review of the literature. Eur J Trauma Emerg Surg 2010;36:53-59.
- Ricci WM, Bolhofner BR, Loftus T, et al. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. J Bone Joint Surg Am 2005;87:2240-5. [PubMed]
- Sen R, Prasad P, Kumar S, et al. Periprosthetic femoral fractures around well fixed implants: a simple method of fixation using LC-DCP with trochanteric purchase. Acta Orthop Belg 2007;73:200-6. [PubMed]
- Shah N, McCabe J. Dall-Miles cable and plate system for periprosthetic femoral fracture. Eur J Orthop Surg Traumatol 2002;12:137-9. [PubMed]
- Wang JW, Wang CJ. Periprosthetic fracture of the femur after hip arthroplasty: The clinical outcome using cortical strut allografts. J Orthop Surg (Hong Kong) 2000;8:27-31. [PubMed]
- Xue H, Tu Y, Cai M, et al. Locking compression plate and cerclage band for type B1 periprosthetic femoral fractures preliminary results at average 30-month follow-up. J Arthroplasty 2011;26:467-71.e1.
- Zhang Y, Fan X, Liu Y, et al. Limited open reduction and double plates internal fixation for treatment of Vancouver type B1 periprosthetic femoral fracture after hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2013;27:1428-31. [PubMed]
- Agarwal S, Andrews CM, Bakeer GM. Outcome following stabilization of type B1 periprosthetic femoral fractures. J Arthroplasty 2005;20:118-21. [PubMed]
- Ahuja S, Chatterji S. The Mennen femoral plate for fixation of periprosthetic femoral fractures following hip arthroplasty. Injury 2002;33:47-50. [PubMed]
- Aigner C, Marschall C, Reischl N, et al. Cortical strut grafts, an alternative to conventional plating in periprosthetic fractures of the femur. Z Orthop Ihre Grenzgeb 2002;140:328-33. [PubMed]
- Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res 1996.238-46. [PubMed]
- Buttaro MA, Farfalli G, Paredes Núñez M, et al. Locking compression plate fixation of Vancouver type-B1 periprosthetic femoral fractures. J Bone Joint Surg Am 2007;89:1964-9. [PubMed]
- Corten K, Vanrykel F, Bellemans J, et al. An algorithm for the surgical treatment of periprosthetic fractures of the femur around a well-fixed femoral component. J Bone Joint Surg Br 2009;91:1424-30. [PubMed]
- Holder N, Papp S, Gofton W, et al. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg 2014;57:209-13. [PubMed]
- Holley K, Zelken J, Padgett D, et al. Periprosthetic fractures of the femur after hip arthroplasty: an analysis of 99 patients. HSS J 2007;3:190-7. [PubMed]
- Moloney GB, Westrick ER, Siska PA, et al. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg 2014;134:9-14. [PubMed]
- Sandhu R, Avramidis K, Johnson-Nurse C. Dall-Miles cable and plate fixation system in the treatment of periprosthetic femoral fractures: a review of 20 cases. J Orthop Surg (Hong Kong) 2005;13:259-66. [PubMed]
- Spina M, Rocca G, Canella A, et al. Causes of failure in periprosthetic fractures of the hip at 1- to 14-year follow-up. Injury 2014;45 Suppl 6:S85-92. [PubMed]
- Tadross TS, Nanu AM, Buchanan MJ, et al. Dall-Miles plating for periprosthetic B1 fractures of the femur. J Arthroplasty 2000;15:47-51. [PubMed]
- Tsiridis E, Haddad FS, Gie GA. Dall-Miles plates for periprosthetic femoral fractures. A critical review of 16 cases. Injury 2003;34:107-10. [PubMed]
- Tsiridis E, Narvani AA, Timperley JA, et al. Dynamic compression plates for Vancouver type B periprosthetic femoral fractures: a 3-year follow-up of 18 cases. Acta Orthop 2005;76:531-7. [PubMed]
- Young SW, Pandit S, Munro JT, et al. Periprosthetic femoral fractures after total hip arthroplasty. ANZ J Surg 2007;77:424-8. [PubMed]
- Khashan M, Amar E, Drexler M, et al. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury 2013;44:1556-60. [PubMed]
- Lindahl H, Malchau H, Herberts P, et al. Periprosthetic femoral fractures classification and demographics of 1049 periprosthetic femoral fractures from the Swedish National Hip Arthroplasty Register. J Arthroplasty 2005;20:857-65. [PubMed]
- Müller M, Kääb M, Tohtz S, et al. Periprosthetic femoral fractures: outcome after treatment with LISS internal fixation or stem replacement in 36 patients. Acta Orthop Belg 2009;75:776-83. [PubMed]
- Venu KM, Koka R, Garikipati R, et al. Dall-Miles cable and plate fixation for the treatment of peri-prosthetic femoral fractures-analysis of results in 13 cases. Injury 2001;32:395-400. [PubMed]
- Eingartner C, Ochs U, Egetemeyer D, et al. Treatment of periprosthetic femoral fractures with the Bicontact revision stem. Z Orthop Unfall 2007;145 Suppl 1:S29-33. [PubMed]
- Katzer A, Ince A, Wodtke J, et al. Component exchange in treatment of periprosthetic femoral fractures. J Arthroplasty 2006;21:572-9. [PubMed]
- Laurer HL, Wutzler S, Possner S, et al. Outcome after operative treatment of Vancouver type B1 and C periprosthetic femoral fractures: open reduction and internal fixation versus revision arthroplasty. Arch Orthop Trauma Surg 2011;131:983-9. [PubMed]
- Ninan TM, Costa ML, Krikler SJ. Classification of femoral periprosthetic fractures. Injury 2007;38:661-8. [PubMed]
- Pike J, Davidson D, Garbuz D, et al. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg 2009;17:677-88. [PubMed]
- Chevillotte CJ, Ali MH, Trousdale RT, et al. Inflammatory laboratory markers in periprosthetic hip fractures. J Arthroplasty 2009;24:722-7. [PubMed]
- Weissman BN. Imaging of total hip replacement. Radiology 1997;202:611-23. [PubMed]
- Manaster BJ. From the RSNA refresher courses. Total hip arthroplasty: radiographic evaluation. Radiographics 1996;16:645-60. [PubMed]
- Ostlere S, Soin S. Imaging of prosthetic joints. Imaging 2003;15:270-285.
- Lindahl H, Garellick G, Regnér H, et al. Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg Am 2006;88:1215-22. [PubMed]
- Brady OH, Garbuz DS, Masri BA, et al. The reliability and validity of the Vancouver classification of femoral fractures after hip replacement. J Arthroplasty 2000;15:59-62. [PubMed]
- Naqvi GA, Baig SA, Awan N. Interobserver and intraobserver reliability and validity of the Vancouver classification system of periprosthetic femoral fractures after hip arthroplasty. J Arthroplasty 2012;27:1047-50. [PubMed]
- Rayan F, Dodd M, Haddad FS. European validation of the Vancouver classification of periprosthetic proximal femoral fractures. J Bone Joint Surg Br 2008;90:1576-9. [PubMed]
- Duncan CP, Masri BA. Fractures of the femur after hip replacement. Instr Course Lect 1995;44:293-304. [PubMed]
- Bethea JS 3rd, DeAndrade JR, Fleming LL, et al. Proximal femoral fractures following total hip arthroplasty. Clin Orthop Relat Res 1982.95-106. [PubMed]
- Johansson JE, McBroom R, Barrington TW, et al. Fracture of the ipsilateral femur in patients wih total hip replacement. J Bone Joint Surg Am 1981;63:1435-42. [PubMed]
- Mont MA, Maar DC. Fractures of the ipsilateral femur after hip arthroplasty. A statistical analysis of outcome based on 487 patients. J Arthroplasty 1994;9:511-9. [PubMed]
- Grammatopoulos G, Pandit H, Kambouroglou G, et al. A unique peri-prosthetic fracture pattern in well fixed femoral stems with polished, tapered, collarless design of total hip replacement. Injury 2011;42:1271-6. [PubMed]
- Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am 1999;30:183-90. [PubMed]
- Fredin HO, Lindberg H, Carlsson AS. Femoral fracture following hip arthroplasty. Acta Orthop Scand 1987;58:20-2. [PubMed]
- Garcia-Cimbrelo E, Munuera L, Gil-Garay E. Femoral shaft fractures after cemented total hip arthroplasty. Int Orthop 1992;16:97-100. [PubMed]
- Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am 1992;23:249-57. [PubMed]
- Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect 1998;47:243-9. [PubMed]
- Meek RM, Norwood T, Smith R, et al. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br 2011;93:96-101. [PubMed]
- Dehghan N, McKee MD, Nauth A, et al. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma 2014;28:721-7. [PubMed]
- Franklin J, Malchau H. Risk factors for periprosthetic femoral fracture. Injury 2007;38:655-60. [PubMed]
- Singh JA, Jensen MR, Harmsen SW, et al. Are gender, comorbidity, and obesity risk factors for postoperative periprosthetic fractures after primary total hip arthroplasty? J Arthroplasty 2013;28:126-31.e1-2.
- Zuurmond RG, van Wijhe W, van Raay JJ, et al. High incidence of complications and poor clinical outcome in the operative treatment of periprosthetic femoral fractures: An analysis of 71 cases. Injury 2010;41:629-33. [PubMed]
- Langenhan R, Trobisch P, Ricart P, et al. Aggressive surgical treatment of periprosthetic femur fractures can reduce mortality: comparison of open reduction and internal fixation versus a modular prosthesis nail. J Orthop Trauma 2012;26:80-5. [PubMed]
- Ricci WM. Periprosthetic femur fractures. J Orthop Trauma 2015;29:130-7. [PubMed]
- Bhattacharyya T, Chang D, Meigs JB, et al. Mortality after periprosthetic fracture of the femur. J Bone Joint Surg Am 2007;89:2658-62. [PubMed]
- Pavlou G, Panteliadis P, Macdonald D, et al. A review of 202 periprosthetic fractures--stem revision and allograft improves outcome for type B fractures. Hip Int 2011;21:21-9. [PubMed]
- Iesaka K, Kummer FJ, Di Cesare PE. Stress risers between two ipsilateral intramedullary stems: a finite-element and biomechanical analysis. J Arthroplasty 2005;20:386-91. [PubMed]
- Lehmann W, Rupprecht M, Nuechtern J, et al. What is the risk of stress risers for interprosthetic fractures of the femur? A biomechanical analysis. Int Orthop 2012;36:2441-6. [PubMed]
- Jando V, Duffy P, Masri B, Duncan C, et al. Management of periprosthetic fractures. In: Callaghan JJ, Rosenberg AG, Rubash HE, editors. The adult hip. Volume 2 ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:1211.
- Larson JE, Chao EY, Fitzgerald RH. Bypassing femoral cortical defects with cemented intramedullary stems. J Orthop Res 1991;9:414-21. [PubMed]
- Rupprecht M, Sellenschloh K, Grossterlinden L, et al. Biomechanical evaluation for mechanisms of periprosthetic femoral fractures. J Trauma 2011;70:E62-6. [PubMed]
- Wroblewski BM. The mechanism of fracture of the femoral prosthesis in total hip replacement. Int Orthop 1979;3:137-9. [PubMed]
- Harris B, Owen JR, Wayne JS, et al. Does femoral component loosening predispose to femoral fracture?: an in vitro comparison of cemented hips. Clin Orthop Relat Res 2010;468:497-503. [PubMed]
- Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J 2007;83:312-6. [PubMed]
- Schwarzkopf R, Oni JK, Marwin SE. Total hip arthroplasty periprosthetic femoral fractures: a review of classification and current treatment. Bull Hosp Jt Dis (2013) 2013;71:68-78. [PubMed]
- Nixon M, Taylor G, Sheldon P, et al. Does bone quality predict loosening of cemented total hip replacements? J Bone Joint Surg Br 2007;89:1303-8. [PubMed]
- Loudon JR, Older MW. Subsidence of the femoral component related to long-term outcome of hip replacement. J Bone Joint Surg Br 1989;71:624-8. [PubMed]
- Søballe K, Hansen ES, Brockstedt-Rasmussen H, et al. Fixation of titanium and hydroxyapatite-coated implants in arthritic osteopenic bone. J Arthroplasty 1991;6:307-16. [PubMed]
- Garbuz DS, Masri BA, Duncan CP. Periprosthetic fractures of the femur: principles of prevention and management. Instr Course Lect 1998;47:237-42. [PubMed]
- Schmidt AH, Kyle RF. Periprosthetic fractures of the femur. Orthop Clin North Am 2002;33:143-52. ix. [PubMed]
- Haddad FS, Masri BA, Garbuz DS, et al. The prevention of periprosthetic fractures in total hip and knee arthroplasty. Orthop Clin North Am 1999;30:191-207. [PubMed]
- Paterson M, Fulford P, Denham R. Loosening of the femoral component after total hip replacement. The thin black line and the sinking hip. J Bone Joint Surg Br 1986;68:392-7. [PubMed]
- Lavernia CJ. Cost-effectiveness of early surgical intervention in silent osteolysis. J Arthroplasty 1998;13:277-9. [PubMed]
- Harrington IJ, Tountas AA, Cameron HU. Femoral fractures associated with Moore's prosthesis. Injury 1979;11:23-32. [PubMed]
- Somers JF, Suy R, Stuyck J, et al. Conservative treatment of femoral shaft fractures in patients with total hip arthroplasty. J Arthroplasty 1998;13:162-71. [PubMed]
- McElfresh EC, Coventry MB. Femoral and pelvic fractures after total hip arthroplasty. J Bone Joint Surg Am 1974;56:483-92. [PubMed]
- Park MS, Lee YK, Yang KH, et al. Management of periprosthetic femoral fractures. J Arthroplasty 2003;18:903-6. [PubMed]
- Haddad FS, Duncan CP, Berry DJ, et al. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am 2002;84-A:945-50. [PubMed]
- Wu ES, Cherian JJ, Kapadia BH, et al. Outcomes of post-operative periprosthetic femur fracture around total hip arthroplasty: a review. Expert Rev Med Devices 2015;12:61-72. [PubMed]
- Lee SR, Bostrom MP. Periprosthetic fractures of the femur after total hip arthroplasty. Instr Course Lect 2004;53:111-8. [PubMed]
- Lindahl H, Malchau H, Odén A, et al. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br 2006;88:26-30. [PubMed]
- Dargan D, Jenkinson MJ, Acton JD. A retrospective review of the Dall-Miles plate for periprosthetic femoral fractures: twenty-seven cases and a review of the literature. Injury 2014;45:1958-63. [PubMed]
- Graham SM, Moazen M, Leonidou A, et al. Locking plate fixation for Vancouver B1 periprosthetic femoral fractures: a critical analysis of 135 cases. J Orthop Sci 2013;18:426-36. [PubMed]
- Moazen M, Jones AC, Jin Z, et al. Periprosthetic fracture fixation of the femur following total hip arthroplasty: a review of biomechanical testing. Clin Biomech (Bristol, Avon) 2011;26:13-22. [PubMed]
- Charnley J. The healing of human fractures in contact with self-curing acrylic cement. Clin Orthop Relat Res 1966.157-63. [PubMed]
- Cooke PH, Newman JH. Fractures of the femur in relation to cemented hip prostheses. J Bone Joint Surg Br 1988;70:386-9. [PubMed]
- Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty 1992;7:129-35. [PubMed]
- Moore RE, Baldwin K, Austin MS, et al. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty 2014;29:872-6. [PubMed]
- Tsiridis E, Spence G, Gamie Z, et al. Grafting for periprosthetic femoral fractures: strut, impaction or femoral replacement. Injury 2007;38:688-97. [PubMed]
- Kobbe P, Klemm R, Reilmann H, et al. Less invasive stabilisation system (LISS) for the treatment of periprosthetic femoral fractures: a 3-year follow-up. Injury 2008;39:472-9. [PubMed]
- Noorda RJ, Wuisman PI. Mennen plate fixation for the treatment of periprosthetic femoral fractures: a multicenter study of thirty-six fractures. J Bone Joint Surg Am 2002;84-A:2211-5. [PubMed]
- Zenni EJ Jr, Pomeroy DL, Caudle RJ. Ogden plate and other fixations for fractures complicating femoral endoprostheses. Clin Orthop Relat Res 1988.83-90. [PubMed]
- Dennis MG, Simon JA, Kummer FJ, et al. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma 2001;15:177-80. [PubMed]
- Dennis MG, Simon JA, Kummer FJ, et al. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty 2000;15:523-8. [PubMed]
- Füchtmeier B, Galler M, Müller F. Mid-Term Results of 121 Periprosthetic Femoral Fractures: Increased Failure and Mortality Within but not After One Postoperative Year. J Arthroplasty 2015;30:669-74. [PubMed]
- White RR. Fixation of periprosthetic femur fractures around total hip implants without the use of cables or struts. Techniques in Orthopaedics 2013;28:218-224.
- Mukundan C, Rayan F, Kheir E, et al. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop 2010;34:485-9. [PubMed]
- Panjabi MM, Trumble T, Hult JE, et al. Effect of femoral stem length on stress raisers associated with revision hip arthroplasty. J Orthop Res 1985;3:447-55. [PubMed]
- Meyer C, Alt V, Schroeder L, et al. Treatment of periprosthetic femoral fractures by effective lengthening of the prosthesis. Clin Orthop Relat Res 2007.120-7. [PubMed]