- Department of Neurosurgery, University Hospital Essen, Essen, Germany
- Institute of Diagostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany
- Department of Neurosurgery, Klinikum Nordstadt Hannover, Hannover, Germany
Correspondence Address:
Neriman Özkan
Department of Neurosurgery, University Hospital Essen, Essen, Germany
Department of Neurosurgery, Klinikum Nordstadt Hannover, Hannover, Germany
DOI:10.4103/2152-7806.171236
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Neriman Özkan, Jabbarli R, Wrede KH, Sariaslan Z, Stein KP, Dammann P, Ringelstein A, Sure U, Sandalcioglu EI. Surgical management of intradural spinal cord tumors in children and young adults: A single-center experience with 50 patients. Surg Neurol Int 08-Dec-2015;6:
How to cite this URL: Neriman Özkan, Jabbarli R, Wrede KH, Sariaslan Z, Stein KP, Dammann P, Ringelstein A, Sure U, Sandalcioglu EI. Surgical management of intradural spinal cord tumors in children and young adults: A single-center experience with 50 patients. Surg Neurol Int 08-Dec-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/surgical-management-of-intradural-spinal-cord-tumors-in-children-and-young-adults-a-single%e2%80%91center-experience-with-50-patients/
Abstract
Background:Intradural spinal cord tumors (IDSCTs) in children and young adults are rare diseases. This present study is aimed to demonstrate our experience with a large series of children and young adults with IDSCT.
Methods:A total of 50 patients aged
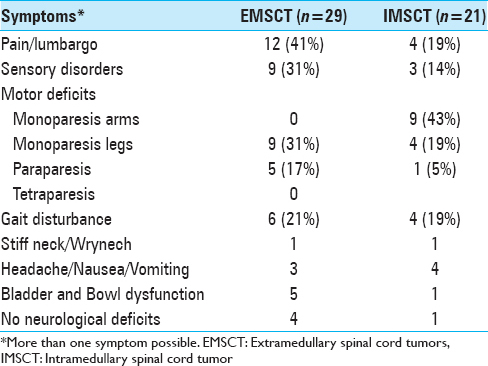
Results:Mean age was 10.3 years (range 6 months–19 years). IDSCT surgery was performed in 44 patients (88%). A common first symptom in patients with EMSCT was neck and back pain (41%), whereas monoparesis of arms (43%) were often seen in patients with IMSCT. The main duration of the symptoms was longer in patients with IMSCT. The postoperative functional outcome was generally comparable to the preoperative functional condition, while better for EMSCT (P P
Conclusion:Due to the mostly mild impact of the surgery on the functional outcome, the surgical treatment of IDSCT in children and young patients can be uniquely advocated.
Keywords: Adolescence, intramedullary tumor in childhood, pediatric neurosurgery, pediatric spine, spinal cord tumor, spinal instrumentation
INTRODUCTION
Spinal tumors are rare and compromise approximately 5–10% of all tumors of the central nervous system in children and young adults.[
Since the first successful removal of an intradural extramedullary fibromyxoma by Gowers and Horsley in 1888,[
The age-related peculiarities of the surgical treatment of spinal cord tumors in children and young adults lie in the body growth and different biological features of neoplastic lesions. However, there are still no specific guidelines or recommendations for the treatment of spinal cord tumors in children and young patients.[
Against this background, we present a large series of children and young adults with intradural spinal cord tumors (IDSCTs) treated at our department during a 20-year period and aimed to derive specific treatment recommendations for pediatric patients upon a single-center experience.
MATERIALS AND METHODS
Patient population
Data of 90 pediatric patients and young adults (younger than 20 years) with the diagnosis of a spinal cord lesion, who were treated at our Neurosurgical Department between January 1990 and December 2010, were retrospectively collected. Children and young adults with extradural spinal cord tumors and spinal abscesses, as well as intradural vascular lesions such as cavernous hemangiomas, arteriovenous fistulas, and arteriovenous malformations, were excluded from this study. Thus, a total of 50 patients fulfilling the inclusion criteria of IDSCTs were identified and enrolled into the study. The study was conducted in accordance with the Declaration of Helsinki and the Guideline for Good Clinical Practice. The study protocol was approved by the local ethic committee of the University of Duisburg-Essen, Germany.
Data management
The data on demographic, clinical, and radiological characteristics of the patients, intraoperative findings, and complications, as well as the parameters of the postoperative course were retrospectively collected. The neurological status was assessed using the Frankel score.[
All patients underwent the clinical examination pre- and post-operatively, as well as 3 months after the surgery. Radiological examination using magnetic resonance imaging (MRI) before and 3 months after surgery was also performed in all cases. Furthermore, depending on the histopathology and clinical status, patients underwent later clinical and MRI-controls (at 6 months and yearly) in certain cases. Therefore, the average follow-up period was 3.5 ± 3.2 years (range 3 months–10 years).
Surgical treatment was performed under standard microsurgical conditions and intraoperative electrophysiological monitoring (with obligatory use of sensory evoked potentials, as well as increasing the use of motor-evoked potentials and D-wave recordings in the recent years). The patients were positioned depending on the location of the tumor: A semi-sitting position was performed for tumors of the upper and middle cervical region and prone position for lesions of the lower cervical and thoracic region including the medullary conus. The extent of tumor resection was judged upon the operative report.
IDSCT were classified into two groups: Intramedullary SCT (IMSCT) and extramedullary SCT (EMSCT). There were 29 (58%) patients suffering from EMSCT and 21 (42%) from IMSCT. The functional outcome was correlated with the histological features, tumor location, and the extension of the tumor. Surgical mortality was referred to death from any cause within 30 days of surgery, whereas the IDSCT mortality was referred to the cases issuing from IDSCT progression.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics 22 (IBM Corporation, USA). Interval-scaled data were expressed as mean and standard deviations and nominal data were expressed as absolute numbers and valid percent.
Correlation analyses were conducted using Spearman's Rho due to not normal distribution of the data. P value of ≤0.05 was considered as statistically significant.
RESULTS
Patient population
The investigated cohort consisted predominantly of males (n = 32, 64%), the mean age was 10.3 years (range 6 months–19 years). There were no differences in age and sex between patients with IMSCT and EMSCT.
Clinical presentation
The mean duration between the first symptoms and diagnosis was 2.8 months (from incidental finding up to 1 year) in patients with IMSCT and 8.1 ± 9.9 months (from incidental finding up to 3 years) in cases with EMSCT. The initial symptoms at the time of admission were also different between both groups. The most common symptom in patients who suffered from EMSCT was back or neck pain. Monoparesis was more often in patients with IMSCT (62%), compared to only nine cases in the EMSCT-group (31%). The rates of gait disturbance were comparable in both groups with 19% and 21% for EMSCT and IMSCT, respectively. A detailed description of symptoms is shown in
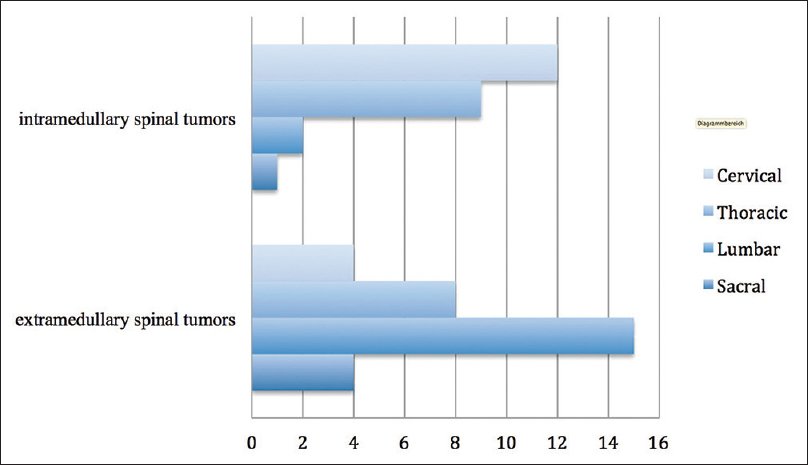
More often IMSCT were located in the cervical part of the spine, whereas EMSCT were more typical for lumbar spine. Tumor locations are presented in
Histopathology
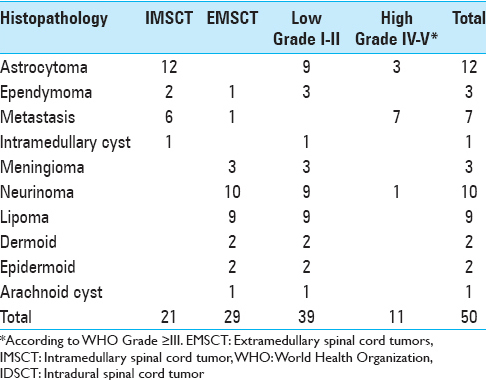
Histological findings of IMSCT and EMSCT are shown in
Neurosurgical treatment
Forty-four patients (88%) underwent surgical treatment of IDSCT. In five cases with intramedullary metastases, intrathecal chemotherapy was administered through an Ommaya reservoir. One patient with intradural extramedullary lipoma underwent conservative management.
Laminoplasty was the standard surgical approach for intraspinal and intradural lesions (27 patients, 61%). Laminectomy was used in the early period of this study for intradural extramedullary tumors. Stabilization of the spine was necessary in only one case of a 14-year-old boy suffering from a spinal metastasis of a colon cancer.
Complete surgical removal was 71% for IMSCT and 59% for EMSCT, respectively.
The surgical morbidity rate was 2%. A postoperative wound infection was observed in one case, and a temporary percutaneous tracheotomy was necessary in 2 patients. Two patients developed pneumonia, which were treated successfully with antibiotic medication.
Illustrative case
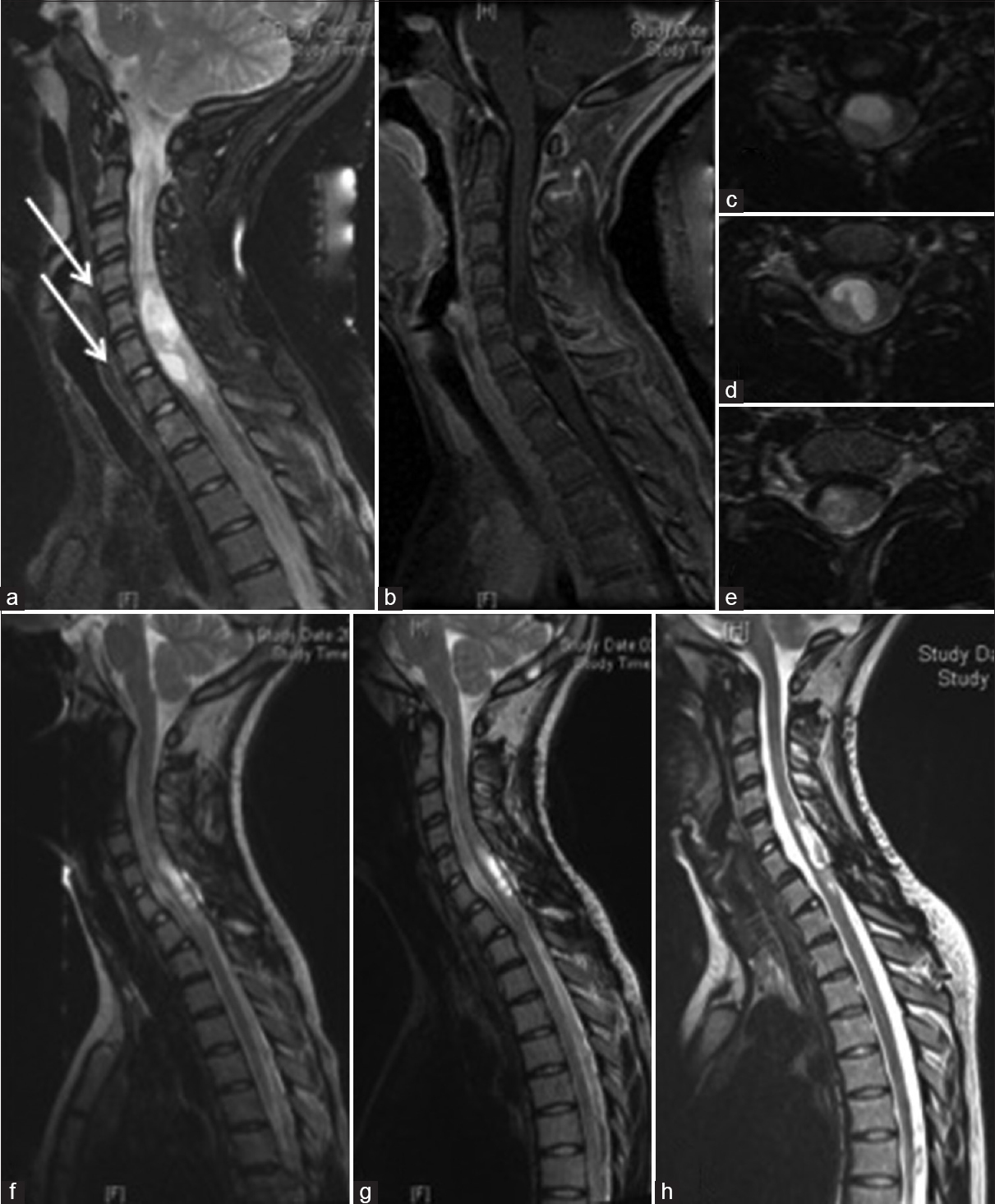
A 14-year-old girl suffered from bilateral arm weakness for 1 year. Gait disturbances or other neurological deficits were not present. An MRI was performed revealing an IMSCT from C5 to C7 with cystic and solid tumor compartments without contrast enhancement. The tumor was removed via a laminotomy of the vertebral arch from C5 to C7 under intraoperative electrophysiological monitoring. The tumor was removed incompletely due to the lack of a clear plane of cleavage. Histopathological examination revealed a fibrillary astrocytoma according to the WHO Grade II. Sensory deficits in arms and legs were observed postoperatively, but improved until discharge and during the postoperative rehabilitation continuously [
Figure 2
A 14-year-old girl with an intramedullary cystic fibrilar astrocytoma World Health Organization II from C5 to C7. (a and b) Preoperative T2 and T1 with contrast enhancement in sagittal view of the tumor and interestingly the vertebral body deformation from C3 to C6 due to the slow tumor growing (arrow), (c-e) T2 in axial view preoperatively, (f) 5 months after surgery with tumor rests in the apical and caudal part of the initial tumors, (g) T2 sagittal view 10 months after surgery and (h) 6 years after surgery, only the postoperative defect of the initial tumor were seen
Early postoperative outcome
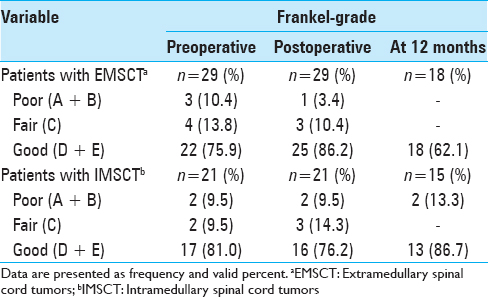
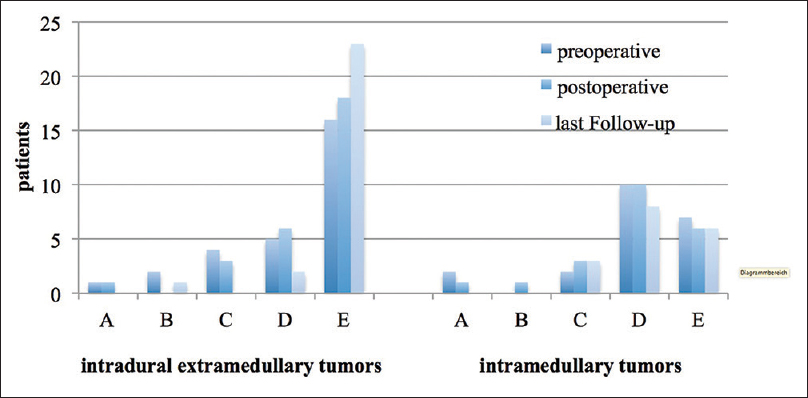
In summary, 17 patients (38%) developed transitory neurological symptoms (new sensory deficits, paresis, or gait disturbances) directly after the surgery. However, these symptoms were mostly reversible, so that the postoperative Frankel score at discharge was comparable with the preoperative score.
Postoperative transitory neurological deterioration was predominantly observed in IMSCT patients (n = 12). Among them, only 1 patient with an IMSCT suffered from a persistent postoperative neurological deterioration from Fankel Grade E to C. In turn, EMSCT patients were more likely to improve in their neurological condition postoperatively [
Follow-up
The mean duration of follow-up was 3.5 years (range 3 months–10 years). Spinal deformity after surgery was not detected except for 1 female patient after laminectomy. This patient had to be re-operated for spine stabilization using dorsal transpedicular screw and rod fixation. In other case with the removal of a spinal lipoma at the age of 1 year and 11 years later repeated surgery with detethering was required because of the development of a tethered cord.
Short-term follow-up at 12 months after discharge was available for 33 (66%) patients (18 with EMSCT, 15 with IMSCT). Nine (50%) patients with EMSCT recovered in this time up to the preoperative Frankel score. Among IMSCT patients, the complete recovery could be achieved in only 3 patients (20%).
At the 12 months follow-up, the Frankel score of the EMSCT-group was higher than the Frankel score of the IMSCT-group [
The WHO grading of the tumor was significantly correlated with the functional outcome at discharge (P = 0.017) and at later follow-ups (P = 0.02). Moreover, there was a statistically significant positive correlation between the preoperative Frankel score and the last follow-up (P = 0.002) [
Recurrence
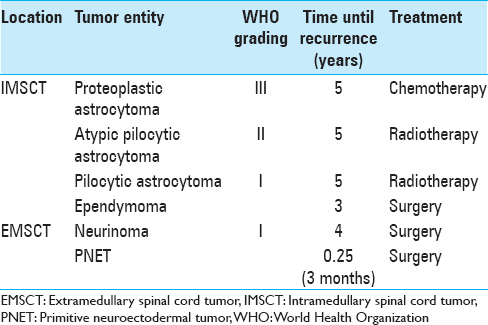
Tumor recurrence was seen in 7 (14%) patients during the whole observational time. In all of these cases, the initial tumor resection was performed subtotally. Four of them (57%) had IMSCT. The detailed description of the cases with recurrent IDSCT is given in
Mortality
Five individuals (10%) died during the follow-up due to the tumor progress. These cases include two 14-year-old patients with intramedullary anaplastic astrocytoma (WHO Grade III) who died 2 and 5 years after the surgery, respectively. Two other patients died 1 and 3 years after surgery, suffering from metastasis from colon carcinoma and medulloblastoma. Finally, a 14-year-old male suffered from malignant peripheral nerve sheath tumor and died 2 months postoperatively.
DISCUSSION
After the implementation of a modern neuroradiological imaging and microsurgical techniques, better functional outcome after IDSCT surgery could be achieved, especially for IMSCT.[
The specific aspects of perioperative care for spinal tumors and spine trauma in the mature spine have been widely described in the modern literature.[
Clinical presentation
The symptoms of IDSCT in children and young adults are attributed to spinal cord compression, and include pain and motor weakness of the extremities. The most common symptoms of patients with EMSCT were pain/back pain (41%). Patients suffering from IMSCT presented more common with the motor weakness of extremities (52%). Constantini et al.[
In our cohort, the mean duration of the symptoms before admission was 8.1 months for EMSCT and 2.8 months for IMSCT. Observation is partially based on spinal cord plasticity allowing considerable tumor progression without the development of meaningful neurological deficits.[
Such long clinical “tolerability” of IDSCT explain the fact that Dincer et al.[
Surgery
Due to the high-risk of secondary spinal deformity in the pediatric patients,[
On the other hand, the use of prophylactic fusion has certain challenges in the pediatric population, such as the risk of neuromuscular scoliosis and “crank-shaft”- deformity due to the continued growth of the anterior spine column, a high rate of pseudarthrosis and the difficulties of screw placement based on the small pedicles.[
In our opinion, the reposition of the posterior arches with micro plates may prevent the development of postoperative scare and, therefore, protects the nervous structures, but does not prevent the development of spinal deformities. Our current surgical strategy consists of the use of laminoplasty without prophylactic fusion and continuous clinical and radiological follow-up that may warrant the timely detection of the secondary spinal deformity in younger individuals.
The extension of the tumor resection depends on tumor histology and location of the tumor.[
In our cohort, the majority of EMSCT were resected completely, except of lipomas and intradural extramedullary metastasis. Intradural meningiomas were completely resected, whereas in one case a Grade I meningioma was recurrent and, therefore, re-surgery was performed.
Astrocytomas are characterized as infiltrative growing tumors, without clear border to the spinal cord tissue and, therefore, often leading to an incomplete tumor removal.[
Functional outcome
Functional outcome after surgery has correlated strongly with the preoperative neurological status, both in EMSCT and in IMSCT patients. Postoperative temporary worsening was seen more likely in patients with IMSCT. We agree with the hypothesis of Epstein,[
Interestingly, at the time of admission the functional grade was good in 75% of EMSCT and 81% of IMSCT patients. Both groups resulted in an improvement or total functional recovery at discharge.
There was no surgery-related mortality in our study. The overall mortality from tumor progression was 10% (n = 8), mainly due to leptomeningeal metastases. Previous reports based upon intramedullary tumors described even higher rates of mortality due to tumor progression (up to 22%).[
In summary, the postoperative functional outcome in the presented cohort is comparable with the previous reports.[
Study limitations
This study presents a retrospective, single-institution analysis and, therefore, faces certain limitations. Retrospective analysis introduces recall bias and difficulty for controlling of confounders. However, randomized prospective studies are difficult for surgical diseases, particularly for pediatric IDSCTs. Then, the histopathological and age heterogeneity of the presented data should also be mentioned as study limitations. Finally, due to the nonuniformly performed postoperative clinical and radiological follow-up, our data do not allow any assumption about the correlations between the surgical radicality and postoperative outcome.
CONCLUSION
IDSCTs are good candidates for radical surgical treatment. As to the surgical technique, we advocate laminotomy to reduce the secondary surgery-related complications. Multicenter prospective database may be helpful in the development of optimal surgical strategies in children and young adults with IDSCTs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahmed R, Menezes AH, Awe OO, Mahaney KB, Torner JC, Weinstein SL. Long-term incidence and risk factors for development of spinal deformity following resection of pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr. 2014. 13: 613-21
2. Ahmed R, Menezes AH, Awe OO, Torner JC. Long-term disease and neurological outcomes in patients with pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr. 2014. 13: 600-12
3. Arseni C, Horvath L, Iliescu D. Intraspinal tumours in children. Psychiatr Neurol Neurochir. 1967. 70: 123-33
4. Babu R, Hazzard MA, Huang KT, Ugiliweneza B, Patil CG, Boakye M. Outcomes of percutaneous and paddle lead implantation for spinal cord stimulation: A comparative analysis of complications, reoperation rates, and health-care costs. Neuromodulation. 2013. 16: 418-26
5. Baysefer A, Akay KM, Izci Y, Kayali H, Timurkaynak E. The clinical and surgical aspects of spinal tumors in children. Pediatr Neurol. 2004. 31: 261-6
6. Constantini S, Houten J, Miller DC, Freed D, Ozek MM, Rorke LB. Intramedullary spinal cord tumors in children under the age of 3 years. J Neurosurg. 1996. 85: 1036-43
7. Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: Surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000. 93: S183-93
8. Cooper PR, Epstein F. Radical resection of intramedullary spinal cord tumors in adults. Recent experience in 29 patients. J Neurosurg. 1985. 63: 492-9
9. Cristante L, Herrmann HD. Surgical management of intramedullary spinal cord tumors: Functional outcome and sources of morbidity. Neurosurgery. 1994. 35: 69-74
10. de Jonge T, Slullitel H, Dubousset J, Miladi L, Wicart P, Illés T. Late-onset spinal deformities in children treated by laminectomy and radiation therapy for malignant tumours. Eur Spine J. 2005. 14: 765-71
11. Dincer F, Dincer C, Baskaya MK. Results of the combined treatment of paediatric intraspinal tumours. Paraplegia. 1992. 30: 718-28
12. Ekelund L, Cronqvist S. Roentgenological changes in spinal malformations and spinal tumours in children. Radiologe. 1973. 13: 541-6
13. el-Mahdy W, Kane PJ, Powell MP, Crockard HA. Spinal intradural tumours: Part I – Extramedullary. Br J Neurosurg. 1999. 13: 550-7
14. Epstein FJ. Spinal cord tumors in children. J Neurosurg. 1995. 82: 516-7
15. Epstein FJ, Farmer JP. Trends in surgery: Laser surgery, use of the cavitron, and debulking surgery. Neurol Clin. 1991. 9: 307-15
16. Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: The result of surgery in 38 patients. J Neurosurg. 1993. 79: 204-9
17. Farwell JR, Dohrmann GJ. Intraspinal neoplasms in children. Paraplegia. 1977. 15: 262-73
18. Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008. 51: 245-50
19. Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969. 7: 179-92
20. Friedman WA, Grundy BL. Monitoring of sensory evoked potentials is highly reliable and helpful in the operating room. J Clin Monit. 1987. 3: 38-44
21. Gowers WR, Horsley V. A case of tumour of the spinal cord. Removal; recovery. Med Chir Trans. 1888. 71: 377-430
22. Gunzburg R, Szpalski M, Aebi M.editors. Vertebral Tumors. Philadelphia, PA, USA: Lippincott William and Wilkins; 2008. p.
23. Huisman TA. Pediatric tumors of the spine. Cancer Imaging. 2009. 9: S45-8
24. Jallo GI, Kothbauer KF, Epstein FJ. Contact laser microsurgery. Childs Nerv Syst. 2002. 18: 333-6
25. Jenkinson MD, Simpson C, Nicholas RS, Miles J, Findlay GF, Pigott TJ. Outcome predictors and complications in the management of intradural spinal tumours. Eur Spine J. 2006. 15: 203-10
26. Kane PJ, el-Mahdy W, Singh A, Powell MP, Crockard HA. Spinal intradural tumours: Part II – Intramedullary. Br J Neurosurg. 1999. 13: 558-63
27. Katsumi Y, Honma T, Nakamura T. Analysis of cervical instability resulting from laminectomies for removal of spinal cord tumor. Spine (Phila Pa 1976). 1989. 14: 1171-6
28. Klekamp J. Treatment of intramedullary tumors: Analysis of surgical morbidity and long-term results. J Neurosurg Spine. 2013. 19: 12-26
29. Lapinksy AS, Richards BS. Preventing the crankshaft phenomenon by combining anterior fusion with posterior instrumentation. Does it work?. Spine (Phila Pa 1976). 1995. 20: 1392-8
30. Li TY, Chu JS, Xu YL, Yang J, Wang J, Huang YH. Surgical strategies and outcomes of spinal ependymomas of different lengths: Analysis of 210 patients: Clinical article. J Neurosurg Spine. 2014. 21: 249-59
31. Lin Y, Jea A, Mekonian SC, Lan S. Treatment of pediatric grad II spinal ependymonas: A population-based study. J Neurosurg Petiatr. 2014. 15: 243-9
32. Rubinstein LJ.editors. Tumors of neuroglial cells. Washington, DC: Armed Forces Institute of Pathology; 1972. p.
33. Loh AH, Aung L, Ha C, Tan AM, Quah TC, Chui CH. Diagnostic delay in pediatric solid tumors: A population based study on determinants and impact on outcomes. Pediatr Blood Cancer. 2012. 58: 561-5
34. Long RR, Wirth FP. Reversible somatosensory evoked potential changes with neodymium: Yttrium-aluminum-garnet laser use. Neurosurgery. 1987. 21: 465-7
35. Manzano G, Green BA, Vanni S, Levi AD. Contemporary management of adult intramedullary spinal tumors-pathology and neurological outcomes related to surgical resection. Spinal Cord. 2008. 46: 540-6
36. Matson DD, Tachdjian MO. Intraspinal tumors in infants and children; review of 115 cases. Postgrad Med. 1963. 34: 279-85
37. McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990. 72: 523-32
38. McGirt MJ, Garcés-Ambrossi GL, Parker SL, Sciubba DM, Bydon A, Wolinksy JP. Short-term progressive spinal deformity following laminoplasty versus laminectomy for resection of intradural spinal tumors: Analysis of 238 patients. Neurosurgery. 2010. 66: 1005-12
39. Safaee M, Oh MC, Mummaneni PV, Weinstein PR, Ames CP, Chou D. Surgical outcomes in spinal cord ependymomas and the importance of extent of resection in children and young adults. J Neurosurg Pediatr. 2014. 13: 393-9
40. Wong AP, Dahdaleh NS, Fessler RG, Melkonian SC, Lin Y, Smith ZA. Risk factors and long-term survival in adult patients with primary malignant spinal cord astrocytomas. J Neurooncol. 2013. 115: 493-503
41. Yasuoka S, Peterson HA, Laws ER Jr, MacCarty CS. Pathogenesis and prophylaxis of postlaminectomy deformity of the spine after multiple level laminectomy: Difference between children and adults. Neurosurgery. 1981. 9: 145-52
42. Yasuoka S, Peterson HA, MacCarty CS. Incidence of spinal column deformity after multilevel laminectomy in children and adults. J Neurosurg. 1982. 57: 441-5
43. Zieger M, Dörr U. Pediatric spinal sonography. Part I: Anatomy and examination technique. Pediatr Radiol. 1988. 18: 9-13
44. Zieger M, Dörr U, Schulz RD. Pediatric spinal sonography. Part II: Malformations and mass lesions. Pediatr Radiol. 1988. 18: 105-11