- Department of Neurosurgery, Neurological Institute, Cleveland Clinic Abu Dhabi, Al Maryah Island, Abu Dhabi, United Arab Emirates
- Department of Neurosurgery, University of Tübingen, Tübingen, Germany
Correspondence Address:
Florian Roser
Department of Neurosurgery, University of Tübingen, Tübingen, Germany
DOI:10.4103/2152-7806.181986
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Roser F, Ebner FH, Skardelly M. The possibility of seeding vestibular schwannomas through surgery: Limited experience with two cases. Surg Neurol Int 06-May-2016;7:
How to cite this URL: Roser F, Ebner FH, Skardelly M. The possibility of seeding vestibular schwannomas through surgery: Limited experience with two cases. Surg Neurol Int 06-May-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/the-possibility-of-seeding-vestibular-schwannomas-through-surgery-limited-experience-with-two-cases/
Abstract
Background:We present two exceptional cases of possible tumor seeding in benign vestibular schwannoma (VS) patients occurring years after initial microsurgical resection.
Case Description:We retrospectively analyzed the surgical management, histology and documented the growth of new tumor occurrence in close vicinity of the original schwannomas by serial magnetic resonance imaging over a period of 10 years. None of the patients had stigmata of neurofibromatosis, making it a reasonable assumption that the second tumor was due to surgical seeding during the first surgery. Moreover, in the second case, a microsurgical re-exploration showed that the recurrent tumor did not show any adhesion or contact to the caudal cranial nerves as anticipated had this been a new cranial nerve schwannoma.
Conclusions:Surgical seeding of VSs is a rare complication but can occur despite benign histology and generous irrigation during surgery.
Keywords: Iatrogenic implantation, surgical implantation, tumor recurrence, tumor seeding, vestibular schwannoma
INTRODUCTION
Metastatic seeding or iatrogenic implantation of vestibular schwannoma (VS) is a very rare phenomenon. To date, there is only one published case report in the literature.[
CASE PRESENTATION
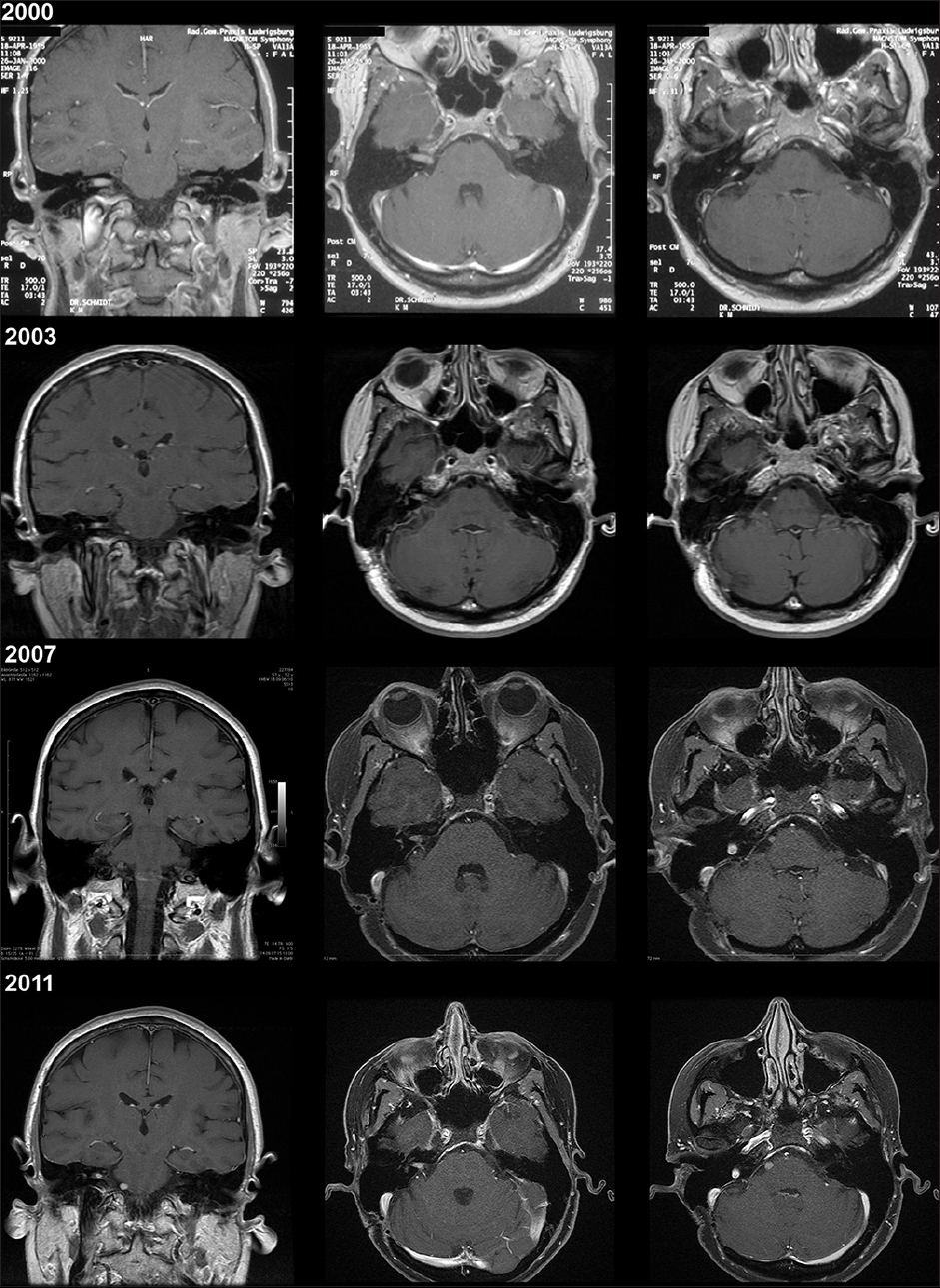
Out of a total number of approximately 1600 surgically treated VSs within one decade at the Department of Neurosurgery (2000–2013), two cases with proposed seeding of VS after initial resection have been identified and followed. The first patient, a 45-year-old female patient was diagnosed with a right-sided VS (T2) in 2000 and had a subsequent incomplete removal of the VS with remnants in the internal auditory canal due to severe adhesions. The histology showed typically benign features without any mitotic activity. In 2003, a tumor nodule was noted at the level of the abducens nerve with subsequent growth over the next years not causing any neurological symptoms. Radiation therapy was applied 7 years after diagnosis in 2011 [
Figure 1
Case 1 with preoperative magnetic resonance imaging of the vestibular schwannoma (upper row, 2000), subsequent follow-up magnetic resonance imaging studies (second row, 2003; third row, 2007; lower row, 2011) with corresponding magnetic resonance imaging sequences and areas of interest: Coronal/axial T1-gadolinium enhanced and axial T1-gadolinium enhanced of the region of later tumor seeding
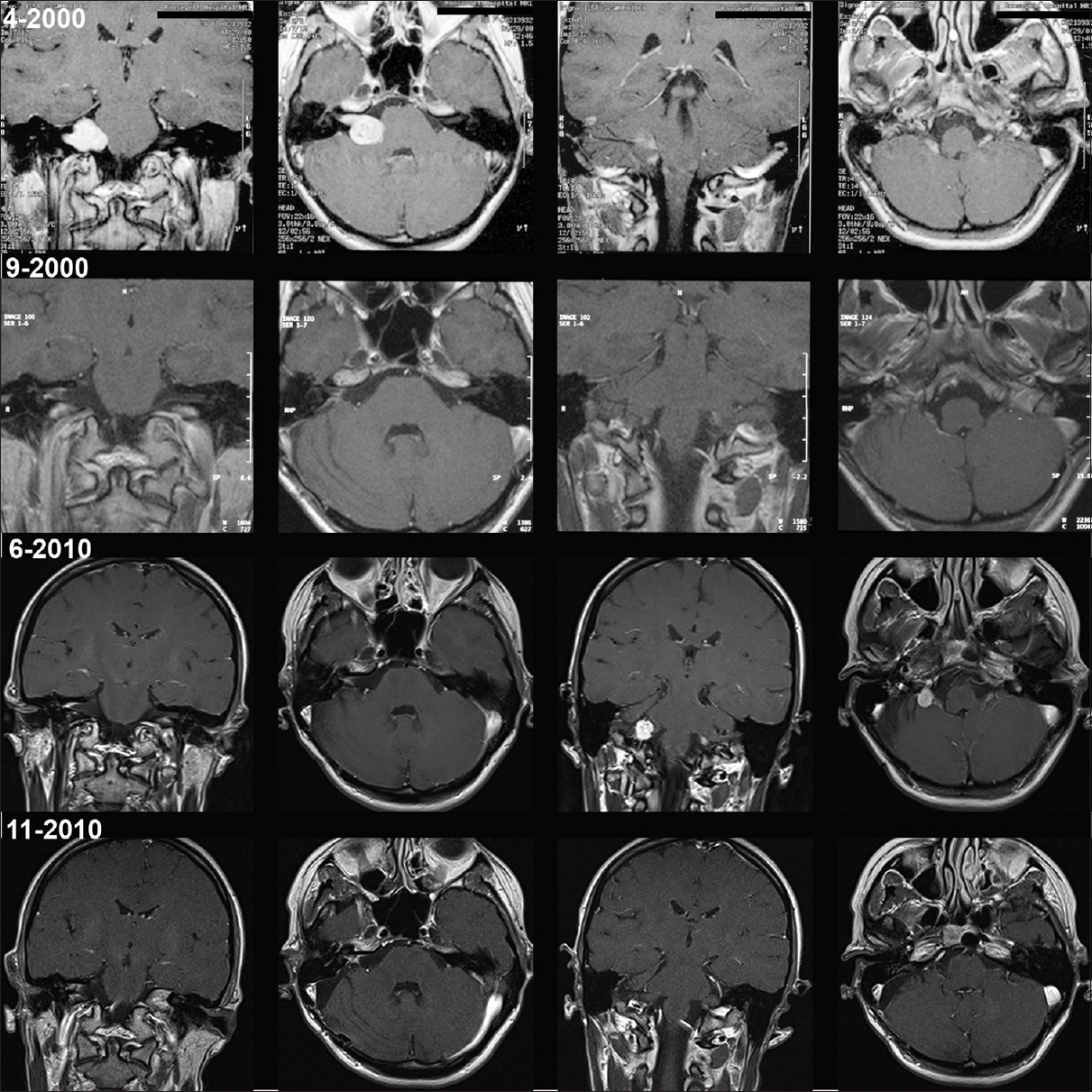
The second patient, a 39-year-old female patient was diagnosed in 2000, with a right-sided VS (T4a) and had total tumor removal performed in the same year via a retrosigmoid approach. No remnants were seen on postoperative magnetic resonance imaging until 2008. At that time, the first occurrence of a tumor nodule was noted at the level of the jugular foramen with remarkable growth over the next years. The tumor was then removed via a re-retrosigmoid craniotomy in 2010. During surgery, no adhesions at the caudal cranial nerves have been seen and a complete resection without any neurological deficits was achieved [
Figure 2
Case 2 with preoperative magnetic resonance imaging of the vestibular schwannoma (upper row, 4–2000). Postoperative magnetic resonance imaging study (second row, 9–2000) and late follow-up magnetic resonance imaging with evidence of seeded tumor at the level of the jugular foramen (third row, 6–2010). Magnetic resonance imaging after re-craniotomy and tumor removal (lower row, 11–2010). Each row with corresponding magnetic resonance imaging sequences and areas of interest: Coronal/axial T1-gadolinium enhanced and coronal/axial T1-gadolinium enhanced of the region of tumor seeding
Histological evaluation of these tumors revealed features typical of VS and similar to those of the tissue obtained from prior resections, no signs of increased mitotic activity, neither at the initial tissue nor in the recurrent tumor. No clinical signs of neurofibromatosis (NF) was evident in either patient and ethylenediaminetetraacetic acid (EDTA) blood screened for NF2 genes was negative. No other tumors have been detected in either of the patients after staging, in the light of secondary tumor manifestation.
DISCUSSION
Metastatic seeding or iatrogenic implantation of numerous types of primary central nervous system tumors, typically along cerebrospinal fluid pathways, is a rare phenomenon and has been reported in association with VS only once.[
Tumor removal concepts in neurosurgery are fundamentally different to other oncological surgical approaches. En bloc or R0 resections are not feasible whether in intra-or extra-axial tumors. In VS surgery, an alternating bimanual dissection with piecemeal removal and cavitron ultrasonic surgical aspiration (CUSA) fragmentation takes place, exposing the patient to the risk of seeding with an opened tumor capsule in every case. Whether the patient gets surgery in the prone or the semi-sitting position, tumor remnants might be flushed away within the cerebello-pontine cistern and tumor particles can settle below the level of surgery. This is evident in both our presented cases in the area of the abducens and the caudal cranial nerves. Here, they might be hardly visible during inspection at the end of the resection or they can become difficult to detect within blood clots or arachnoid membranes. As most of these particles do not have any blood supply they might not flourish and atrophy over time. However, nestled in the right place, it is by all means possible that over time cellular activity may be resuscitated by new nutrition and subsequently develop into a new, secondary tumor. In this scenario, no primary involvement to any nerve bundle is apparent, as noticed in our second case where the recurrent tumor could easily be removed without any attachment to the caudal cranial nerves or its surrounding structures.
Care must be taken during primary resection to minimize risk of secondary tumor implantation in the cerebello-pontine angle. Although rare, reports do exist of seeding through the CUSA. Although reported on for surgery of brain metastasis, significantly higher local recurrences have been reported with the use of the CUSA compared to en bloc resected metastasis.[
One obvious limitation to our study is the very small sample size. In addition, although both patients presented here have been checked for NF2 gene profile, this was obtained by EDTA blood samples only, which gives us a sensitivity of 70%. A definite proof of genetic mosaicism with a sensitivity of 100%, can only be achieved with tumor tissue sampling. In the absence of additional clinical signs of NF and their age far above mean age of initial NF manifestation, we have not considered genetic testing by tumor sampling in our patients. However, changes in the NF genes have been found even in patients who do not fulfill National Institutes of Health criteria of NF. It may be argued that for an appropriate management of patients and family counseling, molecular study of the NF genes should be considered in cases of secondary tumors at the cranial nerves.
CONCLUSIONS
We suggest that strict adherence to oncological principles should be applied in the management of even benign neoplasms such as VS to prevent contamination of surrounding or distal structures with tumor cells and potential recurrence at locations not adjacent to the primary tumor. In cases of schwannomas, secondary tumors should be screened for NF2 gene alterations to rule out genetic mosaicism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahn JH, Lee SH, Kim S, Joo J, Yoo H, Lee SH. Risk for leptomeningeal seeding after resection for brain metastases: Implication of tumor location with mode of resection. J Neurosurg. 2012. 116: 984-93
2. Akai T, Shiraga S, Iizuka H, Kishibe M, Kawakami S, Ueda Y. Recurrent meningioma with metastasis to the skin incision – Case report. Neurol Med Chir (Tokyo). 2004. 44: 600-2
3. Lüdemann WO, Obler R, Tatagiba M, Samii M. Seeding of malignant meningioma along a surgical trajectory on the scalp. Case report and review of the literature. J Neurosurg. 2002. 97: 683-6
4. Patrick TA, Giannini C, Ebersold MJ, Link MJ. Iatrogenic cerebellar implantation of a vestibular schwannoma. Case report. J Neurosurg. 2006. 104: 452-6
5. Schmalisch K, Beschorner R, Psaras T, Honegger J. Postoperative intracranial seeding of craniopharyngiomas – Report of three cases and review of the literature. Acta Neurochir (Wien). 2010. 152: 313-9
6. Singh RV, Yeh JS, Campbell DA. Implantation meningioma in temporalis muscle: Case report. Br J Neurosurg. 1994. 8: 93-5