- Department of Neurosurgery, Barrow Neurological Institute, St. Joseph's Hospital and Medical Center, Phoenix, Arizona, USA
- Department of Neurosurgery, Irkutsk State Medical University, Irkutsk, Russia
Correspondence Address:
Mark C. Preul
Department of Neurosurgery, Barrow Neurological Institute, St. Joseph's Hospital and Medical Center, Phoenix, Arizona, USA
DOI:10.4103/sni.sni_36_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Evgenii Belykh, Laeth George, Xiaochun Zhao, Alessandro Carotenuto, Leandro Borba Moreira, Kaan Yağmurlu, Baran Bozkurt, Vadim A. Byvaltsev, Peter Nakaji, Mark C. Preul. Microvascular anastomosis under 3D exoscope or endoscope magnification: A proof-of-concept study. 04-Jun-2018;9:115
How to cite this URL: Evgenii Belykh, Laeth George, Xiaochun Zhao, Alessandro Carotenuto, Leandro Borba Moreira, Kaan Yağmurlu, Baran Bozkurt, Vadim A. Byvaltsev, Peter Nakaji, Mark C. Preul. Microvascular anastomosis under 3D exoscope or endoscope magnification: A proof-of-concept study. 04-Jun-2018;9:115. Available from: http://surgicalneurologyint.com/surgicalint-articles/microvascular-anastomosis-under-3d-exoscope-or-endoscope-magnification-a-proof%e2%80%91of%e2%80%91concept-study/
Abstract
Background:Extracranial–intracranial bypass is a challenging procedure that requires special microsurgical skills and an operative microscope. The exoscope is a tool for neurosurgical visualization that provides view on a heads-up display similar to an endoscope, but positioned external to the operating field, like a microscope. The authors carried out a proof-of-concept study evaluating the feasibility and effectiveness of performing microvascular bypass using various new exoscopic tools.
Methods:We evaluated microsurgical procedures using a three-dimensional (3D) endoscope, hands-free robotic automated positioning two-dimensional (2D) exoscope, and an ocular-free 3D exoscope, including surgical gauze knot tying, surgical glove cutting, placental vessel anastomoses, and rat vessel anastomoses. Image quality, effectiveness, and feasibility of each technique were compared among different visualization tools and to a standard operative microscope.
Results:3D endoscopy produced relatively unsatisfactory resolution imaging. It was shown to be sufficient for knot tying and anastomosis of a placental artery, but was not suitable for anastomosis in rats. The 2D exoscope provided higher resolution imaging, but was not adequate for all maneuvers because of lack of depth perception. The 3D exoscope was shown to be functional to complete all maneuvers because of its depth perception and higher resolution.
Conclusion:Depth perception and high resolution at highest magnification are required for microvascular bypass procedures. Execution of standard microanastomosis techniques was unsuccessful using 2D imaging modalities because of depth-perception-related constraints. Microvascular anastomosis is feasible under 3D exoscopic visualization; however, at highest magnification, the depth perception is inferior to that provided by a standard operative microscope, which impedes the procedure.
Keywords: Anastomosis, bypass, 3D, endoscope, exoscope, microscope, stereoscopy
INTRODUCTION
Endoscopy has evolved from pure diagnostics to use in ever-expanding therapeutics, requiring the surgeon to be facile in specialized hand–eye coordination and visuospatial appreciation of three-dimensional (3D) space in a two-dimensional (2D) medium. Digital 3D visualization has been widely used in computer gaming, commercial film industry, and in manufacturing and has assumed a substantial place in medicine, including endoscopy and exoscopy. Neurovascular bypass surgery, or cerebral bypass surgery, is a technically challenging specialty which stands to benefit from modern advances in medical technology; namely, the development of 3D endoscopes, exoscopes, and other imaging-centric surgical hardware.
Revascularization using microvascular anastomosis techniques has shown utility in a variety of disease states, particularly those concerning cerebral hemodynamic instability and perfusion defects. In strokes, studies have shown benefit in candidates requiring urgent reperfusion when intra-arterial thrombolysis (ITA) is unavailable or contraindicated, with one study showing significant improvement in NIH stroke scales, neurological outcomes, and “good” outcomes (as defined by Rankin scale).[
Prior studies have compared conventional binocular microscopes versus 3D scope visualization modalities for microvascular anastomosis. Kotsougiani et al.[
The continuing advancement of endoscope and exoscope optical technology is overcoming prior limitations related to visualization during fine surgical procedures. Imaging hardware with higher resolutions can produce sharper, brighter, and higher quality images, thus addressing the limitations posed by previous studies. In this study, we examined these higher resolution 3D imaging modalities with comparison to previous surgical visualization. The goal of this study was to assess feasibility and effectiveness of various new micro-visualization tools used for the creation of a microvascular anastomosis. The rat arterial anastomosis was selected as a final relevant model for assessment simulating a neurovascular bypass procedure. Part-task microsuturing simulation was used for quantitative analysis.
MATERIALS AND METHODS
Visualization tools
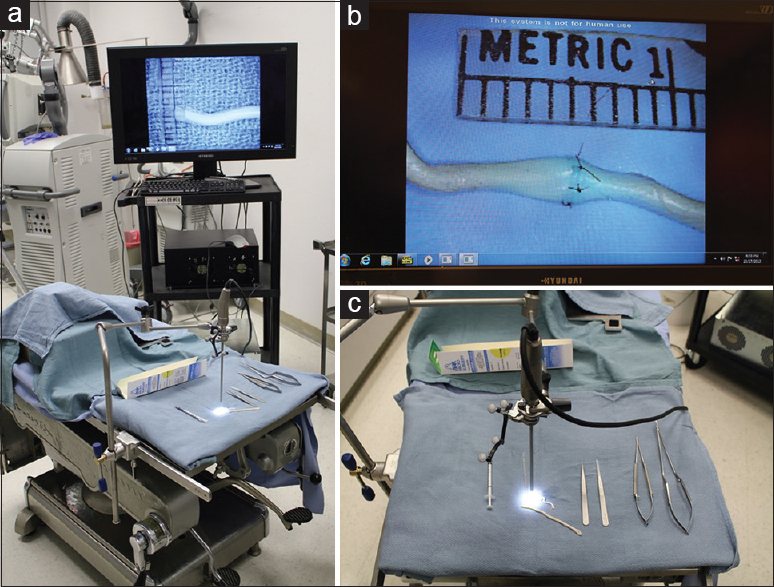
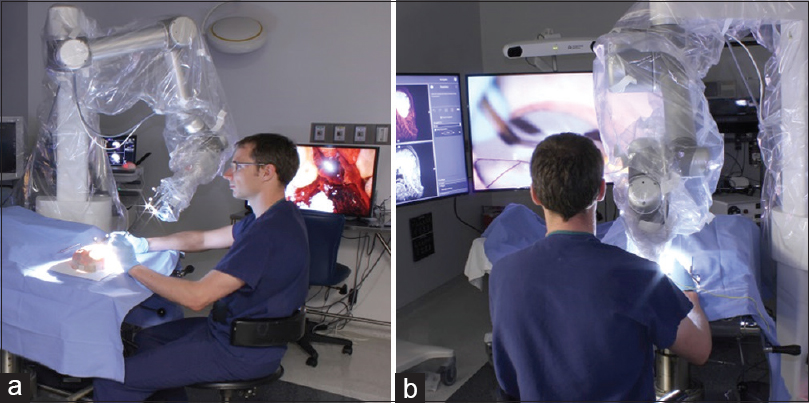
We used four visualization tools in this study. First, we used a straight, 0 degrees stereoscopic endoscope with integrated camera (720-pixel resolution) and a dedicated circular polarization system for visualization (VSii; Visionsense, Philadelphia, PA, USA). Then we assessed a hands-free robotic automated positioning 2D exoscope with two illumination devices and one high-resolution camera attached to the optical component with heads-up display (Synaptive Medical Inc., Toronto, Canada). Finally, we investigated a robotic visualization system with an ocular-free 3D exoscope and 4K resolution heads-up display (KINEVO 900; Carl Zeiss AG, Oberkochen, Germany). The standard neurosurgical operative microscope (PENTERO 900; Carl Zeiss AG) was used as a control. Operators' subjective feedback and ability to perform microsurgical manipulations were assessed on dry and wet simulation models. The assessment was performed in a neurosurgical research laboratory and performed consequentially based on the availability of visualization tools within years 2016–2017.
Dry microsurgery models
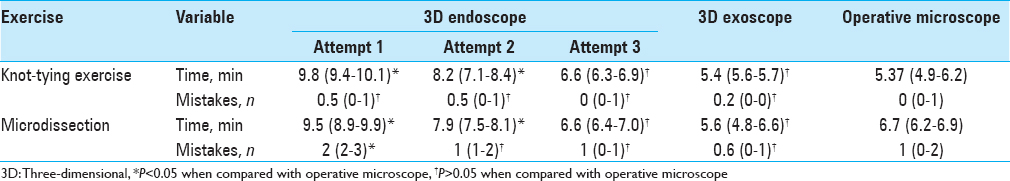
Microsurgical performance under 3D visualization was assessed quantitatively using two exercises: knot tying on surgical gauze and dissection with microscissors in different directions on a surgical glove.[
During the first exercise, a trainee was required to connect two nearby threads of surgical gauze with five knots. Each knot was composed of three throws using a 10-0 monofilament suture. The time required for five knots and the number of mistakes was estimated on a scale from zero (no mistakes) to a maximum of five mistakes, indicating a mistake in each knot. Knot assessment was done under 10× magnification using a standard operative microscope.
During the second exercise (dissection with microscissors), a latex glove was placed under a microsurgical field restriction device with tension applied through tape. A circumference was drawn with a pen and having a line width of 1 mm. The trainee was required to excise the circle by cutting through the 1-mm line and not exceed the borders of the line, as indicated by the ink. We estimated the time required to complete the exercise and number of mistakes, measured as the number of cuts beyond the limit of the line.
All exercises were performed by one neurosurgeon familiar with microvascular bypass techniques. Ten exercises were performed per day within 3 days to assess the acquisition of skills under 3D visualization. An additional 10 exercises were performed under the operative microscope as a control.
Microvascular anastomosis models
The first model used included human and bovine placentas, which have been validated as appropriate, accessible, and anatomically relevant models for surgical practice. In previous studies, human placenta was found to approximate cerebral and superficial temporal arteries, whereas bovine placental arteries were found to approximate the internal carotid and radial arteries, making them an appropriate selection.[
Other models used in the study were rat femoral and carotid arteries used to form end-to-side anastomoses. Similar to the human and bovine training models described above, studies have validated the use of rat femoral and carotid artery grafts in the creation of microvascular end-to-side anastomoses, with significant utility for microvascular training in terms of sustained patency and feasibility.[
Statistical analysis
Data are presented as median and interquartile range. Non-parametric tests were used to assess differences in the groups. P < 0.05 was selected as a lower threshold of significance.
RESULTS
3D endoscopy system functionality, similar to standard endoscopy, requires initial top–bottom and left–right orientation. Coordinating hand movements while observing on the display screen was at first uncomfortable and unfamiliar, compared with the more usual manner of visualization through microscope oculars. For the selected task, we directed the endoscope vertically downward, which was comparable with the usual surgical trajectory of an operative microscope. The size of the 3D endoscopic field of view (FOV) at a distance of 2 cm was 1.5 × 1.0 cm and was similar to the 1.5 × 1.5 cm FOV of the standard operative microscope at the 20-cm working distance and maximum magnification.
Using low-definition 3D endoscopic visualization, microsurgical maneuvers were performed including knot tying and suturing of vessels with a diameter of 2–4 mm. End-to-end and end-to-side anastomosis were completed on bovine placental arteries [
However, we observed a steep increase in performance speed over a period of 10 repetitions of exercises, which were performed to adapt to 3D endoscopic visualization. By the third day of training, the time required to complete the exercises and number of mistakes showed no statistical difference when compared to using the operative microscope [
Although the 2D exoscope provided a high-resolution picture on a large monitor and comfortable position for an operator [
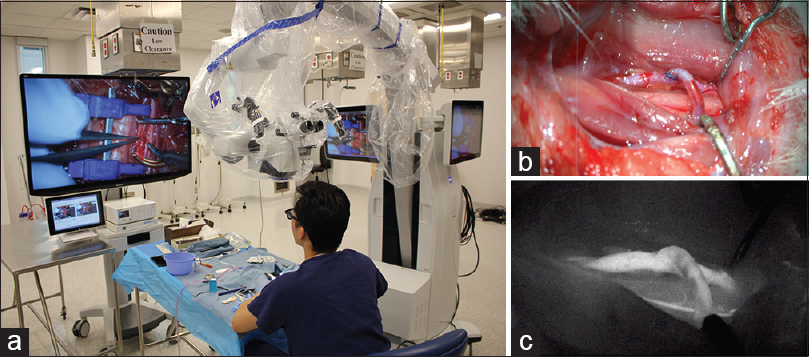
Using 3D exoscopic visualization, six consecutive end-to-side microvascular anastomoses were completed on rat carotid arteries over four consecutive practice sessions. Patency of anastomoses was confirmed by indocyanine green (ICG) injection in 5/6 anastomoses. Depth perception at high magnification (10–15×) was sufficient to perform delicate microsurgical manipulations such as puncturing a vessel wall and knot tying [
Figure 3
Bypass under 3D exoscopic visualization. (a) Position of the operator and operating room setup. (b) Completed end-to-side anastomosis as seen on the heads-up display. (c) ICG confirms patency of the anastomosis. Figure used with permission from the Barrow Neurological Institute, Phoenix, Arizona
DISCUSSION
3D visualization has been shown to have a benefit in surgical education, particularly for observers as an aid to understand surgical anatomy and technical nuances.[
Microsurgical manipulations are possible with 2D visualization under an HD operative microscope, but technically demanding microsurgical techniques require depth perception and high optical resolution to easily and successfully complete tasks and anastomoses. Although there are many factors that can influence microsurgical performance,[
The ability to display a real-time high-quality surgical video has been explored related to robotic-assisted surgery. Feasibility of a robotic-assisted (da Vinci; Intuitive Inc., Surgical, Sunnyvale, CA, USA) superficial temporal artery-to-middle cerebral artery anastomosis has been demonstrated in a cadaveric head, with 3D optics and a decrease in physiologic tremor,[
There are several significantly different technical aspects of 3D exoscopic or 3D endoscopic visualization compared with a standard operative microscope. Change in magnification and FOV size is possible with all systems assessed in this study. Unlike in the microscope, with 3D endoscopy the distance of the endoscope relative to the object mainly defines the degree of magnification. One of the primary benefits of 3D endoscopy is that there is almost no need to adjust the focal length during movements, compared to the standard microscope. However, several of the newest microscopes and exoscopes have focus autocorrection functions to aid in presenting a constantly clear picture. The shortcomings of the assessed stereoscopic endoscope system included lower image quality; however, newer full HD 3D endoscopes were not assessed in our study. There is also some elliptical distortion of the image on the screen from the stereoscopic endoscope. Objects in the center of the screen appear closer to the camera than objects near the edge of the screen. In addition, instruments may block the light from the endoscope or may collide with the endoscope. The need to wear special 3D polarizing glasses might be considered a common limitation for all 3D heads on display systems. The main advantage of 3D endoscopic and exoscopic systems we assessed for microanastomosis was the perception of the volume of objects and depth of structures for planning, targeting, and controlling fine movements, which was not possible with 2D visualization. The smaller size of the endoscope or exoscope system may be viewed as an advantage when compared with operating microscopes.
Overall, this study demonstrates the feasibilty and possibility of performing microvascular anastomosis in vivo under 3D exoscopic visualization. Compared with the standard operating microscope, however, depth perception at high magnification was found to be lacking. At the level of detail required for performing neurovascular bypass on vessels measuring less than 2 mm, this decline in visual clarity and depth perception may represent a compromising factor. Because the 3D exoscope is a relatively novel piece of neurosurgical technology, like any new technological innovation, it must progress through the dogmatic lifecycle of early surgeon adopters before being accepted by the majority. The learning curve with the 3D exoscope is similar to a previous history of the 2D endoscope, which received significant pushback and reluctance for implementation in the operating room. However, unlike the 2D endoscope, the added advantage of increased depth perception with the 3D exoscope should mitigate this learning curve. Even as early adopters of this technology, we noted a steep learning curve such that anastomosis under 3D exoscope visualization is still not as fluid as with surgical microscope. However, with training and improvements in this technology, the efficiency will follow, as we have seen with surgeons using the 2D endoscope. It should be noted, however, that while these tasks act as surrogates for operative tasks (e.g., dissection, microsuturing), they are not exact reproductions of maneuvers in the operative theater. An attempt was made to mitigate this disparity by using a validated animal vessel model; nonetheless, it remains difficult to definitively recommend one modality over the other, in the absence of larger studies with a focus on additional in vivo operative tasks.
With regard to limitations to this study, the newest generation of full HD 3D endoscopes was not assessed, and thus the authors are unable to compare this modality to the others studied here. In addition, all exercises were performed by a single experimenter trained in microsurgical anastomosis techniques.
CONCLUSION
Creation of a microvascular anastomosis is feasible under exoscopic 3D 4K resolution visualization in synthetic, animal, and placental vessel models. Suturing an anastomosis of a larger caliber vessel was possible under lower quality 3D endoscopic magnification. Microanastomosis technique was unsuccessful using 2D imaging modalities because of depth perception related constraints. This pilot study indicates expanded studies to determine utility of 3D endoscopic or 3D exoscopic visualization tools as an alternative to standard microscopic procedures in neurosurgery. Larger, focused studies are needed before definitive recommendations regarding one operative modality over another. As these technologies evolve, it is critical to evaluate visualization platforms that perform best for obtaining optimal surgical outcomes, and for trainees as they begin to acquire fine surgical skills.
Financial support and sponsorship
This research was supported with funds from the Barrow Neurological Foundation, the Women's Board of the Barrow Neurological Institute, and by the Newsome Chair in Neurosurgery Research held by Dr. Preul.
Conflicts of interest
PN received consultant fees from Carl Zeiss AG. Exoscopes were provided by Synaptive Medical Inc. and Carl Zeiss AG, but they did not take part in design of the experiments, examination of data, or writing the article. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as a potential conflict of interest.
References
1. Abdulrauf SI, Urquiaga JF, Patel R, Albers JA, Belkhair S, Dryden K. Awake high-flow extracranial to intracranial bypass for complex cerebral aneurysms: Institutional clinical trial results. World Neurosurg. 2017. 105: 557-67
2. Belykh E, Byvaltsev V. Off-the-job microsurgical training on dry models: Siberian experience. World Neurosurg. 2014. 82: 20-4
3. Belykh E, Lei T, Safavi-Abbasi S, Yagmurlu K, Almefty RO, Sun H. Low-flow and high-flow neurosurgical bypass and anastomosis training models using human and bovine placental vessels: A histological analysis and validation study. J Neurosurg. 2016. 125: 915-28
4. Belykh E, Onaka NR, Abramov IT, Yagmurlu K, Byvaltsev VA, Spetzler RF. Systematic review of factors influencing surgical performance: Practical recommendations for microsurgical procedures in neurosurgery. World Neurosurg. 2018. 112: e182-e207
5. Belykh EG, Byval'tsev VA, Nakadzhi P, Lei T, Oliviero MM, Nikiforov SB. [A model of the arterial aneurysm of the brain for microneurosurgical training]. Zh Vopr Neirokhir Im N N Burdenko. 2014. 78: 40-5
6. Benet A, Tabani H, Griswold D, Yousef S, Rubio RR, Lawton MT. Supracerebellar-infratentorial approach for resection of tectal and thalamic cavernous malformations: 3-dimensional operative video. Oper Neurosurg. 2018. 14: 316-
7. Buess G, van Bergen P, Kunert W, Schurr M. Comparative study of various 2-D and 3-D vision systems in minimally invasive surgery. Chirurg. 1996. 67: 1041-6
8. Burkhardt JK, Winklhofer S, Fierstra J, Wegener S, Esposito G, Luft A. Emergency extracranial-intracranial bypass to revascularize salvageable brain tissue in acute ischemic stroke patients. World Neurosurg. 2018. 109: e476-e85
9. Byval'tsev VA, Suzuki Y. [Combined treatment for Moya-Moya disease, by using direct anastomosis and revascularization: Experience of 225 operations]. Zh Vopr Neirokhir Im N N Burdenko. 2007. 11: 6-
10. Cao C, Indraratna P, Doyle M, Tian DH, Liou K, Munkholm-Larsen S. A systematic review on robotic coronary artery bypass graft surgery. Ann Cardiothorac Surg. 2016. 5: 530-
11. Dash H, Kononov A, Maloney J, Browne E. A simple arteriotomy method for microsurgical end-to-side anastomoses: Technical aspects of use in training and laboratory applications. JReconstrMicrosurg. 1993. 9: 381-4
12. Dodge JT, Brown BG, Bolson EL, Dodge HT. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992. 86: 232-46
13. Dubovoy AV, Ovsyannikov KS, Guzhin VE, Cherepanov AV, Galaktionov DM, Perfil'ev AM. [The use of high-flow extracranial-intracranial artery bypass in pathology of the cerebral and brachiocephalic arteries: Technical features and surgical outcomes]. Zh Vopr Neirokhir Im N N Burdenko. 2017. 81: 5-21
14. Hanna GB, Shimi SM, Cuschieri A. Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet. 1998. 351: 248-51
15. Heath MD, Cohen-Gadol AA. Intraoperative stereoscopic 3D video imaging: Pushing the boundaries of surgical visualisation and applications for neurosurgical education. Br JNeurosurg. 2012. 26: 662-7
16. Hirschl RA, Caragine LP. Robotic-assisted superficial temporal artery-to-middle cerebral artery anastomosis. JRobot Surg. 2008. 2: 165-7
17. Hwang G, Oh CW, Bang JS, Jung CK, Kwon O-K, Kim JE. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery. 2011. 68: 723-30
18. Joo W, Funaki T, Yoshioka F, Rhoton AL. Microsurgical anatomy of the infratemporal fossa. Clin Anat. 2013. 26: 455-69
19. Kalani MY, Hu YC, Spetzler RF. A double-barrel superficial temporal artery-to-superior cerebellar artery (STA-SCA) and STA-to-posterior cerebral artery (STA-PCA) bypass for revascularization of the basilar apex. J Clin Neurosci. 2013. 20: 887-9
20. Kamath S. Observations on the length and diameter of vessels forming the circle of Willis. J Anat. 1981. 133: 419-
21. Kari E, Oyesiku NM, Dadashev V, Wise SK. Comparison of traditional 2-dimensional endoscopic pituitary surgery with new 3-dimensional endoscopic technology: Intraoperative and early postoperative factors. International forum of allergy & rhinology. 2012. p.
22. Kikkawa Y, Ikeda T, Takeda R, Nakajima H, Ogura T, Ooigawa H. Results of early high-flow bypass and trapping for ruptured blood blister-like aneurysms of the internal carotid artery. World Neurosurg. 2017. 105: 470-7
23. Kim T, Oh CW, Bang JS, Kim JE, Cho WS. Moyamoya disease: Treatment and outcomes. J Stroke. 2016. 18: 21-
24. Kotsougiani D, Hundepool CA, Bulstra LF, Shin DM, Shin AY, Bishop AT. The learning rate in three dimensional high definition video assisted microvascular anastomosis in a rat model. J Plast ReconstrAesthetic Surg. 2016. 69: 1528-36
25. Lee SH, Jung Y, Ryu JW, Choi SK, Kwun BD. Surgical revascularization for the treatment of complex anterior cerebral artery aneurysms: Experience and illustrative review. World Neurosurg. 2017. p.
26. Marcus HJ, Hughes-Hallett A, Cundy TP, Di Marco A, Pratt P, Nandi D. Comparative effectiveness of 3-dimensional vs 2-dimensional and high-definition vs standard-definition neuroendoscopy: A preclinical randomized crossover study. Neurosurgery. 2013. 74: 375-81
27. Martins C. Rhoton's Lab. World Neurosurg. 2016. 92: 623-36
28. Mokhtari P, Tayebi Meybodi A, Lawton MT, Payman A, Benet A. Transfer of learning from practicing microvascular anastomosis on silastic tubes to rat abdominal aorta. World Neurosurg. 2017. 108: 230-5
29. Russin JJ. The arborization bypass: Sequential intracranial-intracranial bypasses for an unruptured fusiform MCA aneurysm. J Clin Neurosci. 2017. 39: 209-11
30. Taffinder N, Smith S, Huber J, Russell R, Darzi A. The effect of a second-generation 3D endoscope on the laparoscopic precision of novices and experienced surgeons. Surg Endosc. 1999. 13: 1087-92
31. Tayebi Meybodi A, Lawton MT, Mokhtari P, Yousef S, Gandhi S, Benet A. Microsurgical bypass training rat model, Part 1: Technical nuances of exposure of the aorta and iliac arteries. World Neurosurg. 2017. 107: 925-34
32. Tayebi Meybodi A, Lawton MT, Yousef S, Mokhtari P, Gandhi S, Benet A. Microsurgical bypass training rat model: Part 2-anastomosis configurations. World Neurosurg. 2017. 107: 935-43
33. Usachev DY, Lukshin VA, Shmigel'skiy AV, Akhmedov AD, Sosnin AD, Kozlova KA. [Creation of extracranial-intracranial microvascular anastomosis under regional anesthesia (a case report and a review of the literature)]. Zh Vopr Neirokhir Im N N Burdenko. 2017. 81: 88-95
34. White TG, O'Donnell D, Rosenthal J, Cohen M, Aygok G, Nossek E. Trends in cerebral revascularization in the era of pipeline and carotid occlusion surgery study. World Neurosurg. 2016. 91: 285-96
35. Williamson RW, Abla AA, Zabramski JM, Nakaji P, Spetzler RF, Wanebo JE. Revascularization of Moyamoya angiopathy in older adults. World Neurosurg. 2017. 99: 37-40
36. Wong AK, Davis GB, Nguyen TJ, Hui KJ, Hwang BH, Chan LS. Assessment of three-dimensional high-definition visualization technology to perform microvascular anastomosis. J Plast Reconstr Aesthetic Surg. 2014. 67: 967-72
37. Yaşargil MG. Personal considerations on the history of microneurosurgery. JNeurosurg. 2010. 112: 1347-