Published online May 15, 2019. doi: 10.4239/wjd.v10.i5.311
Peer-review started: March 4, 2019
First decision: April 13, 2019
Revised: April 17, 2019
Accepted: May 1, 2019
Article in press: May 1, 2019
Published online: May 15, 2019
The coexistence of sarcopenia and obesity is referred to as sarcopenic obesity (SO) and it has been hypothesized that the two components of SO may synergistically increase their negative effects. However, many uncertainties still surround this condition especially with regard to its potential negative effects on health outcomes.
To conduct a systematic review to determine the prevalence of sarcopenia among adults with overweight and obesity and to investigate whether SO was associated with a higher risk of type 2 diabetes (T2D).
This study was conducted in adherence with the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines. Literature searches, study selection, methodology development and quality appraisal were performed independently by two authors and the data were collated by means of meta-analysis and narrative synthesis.
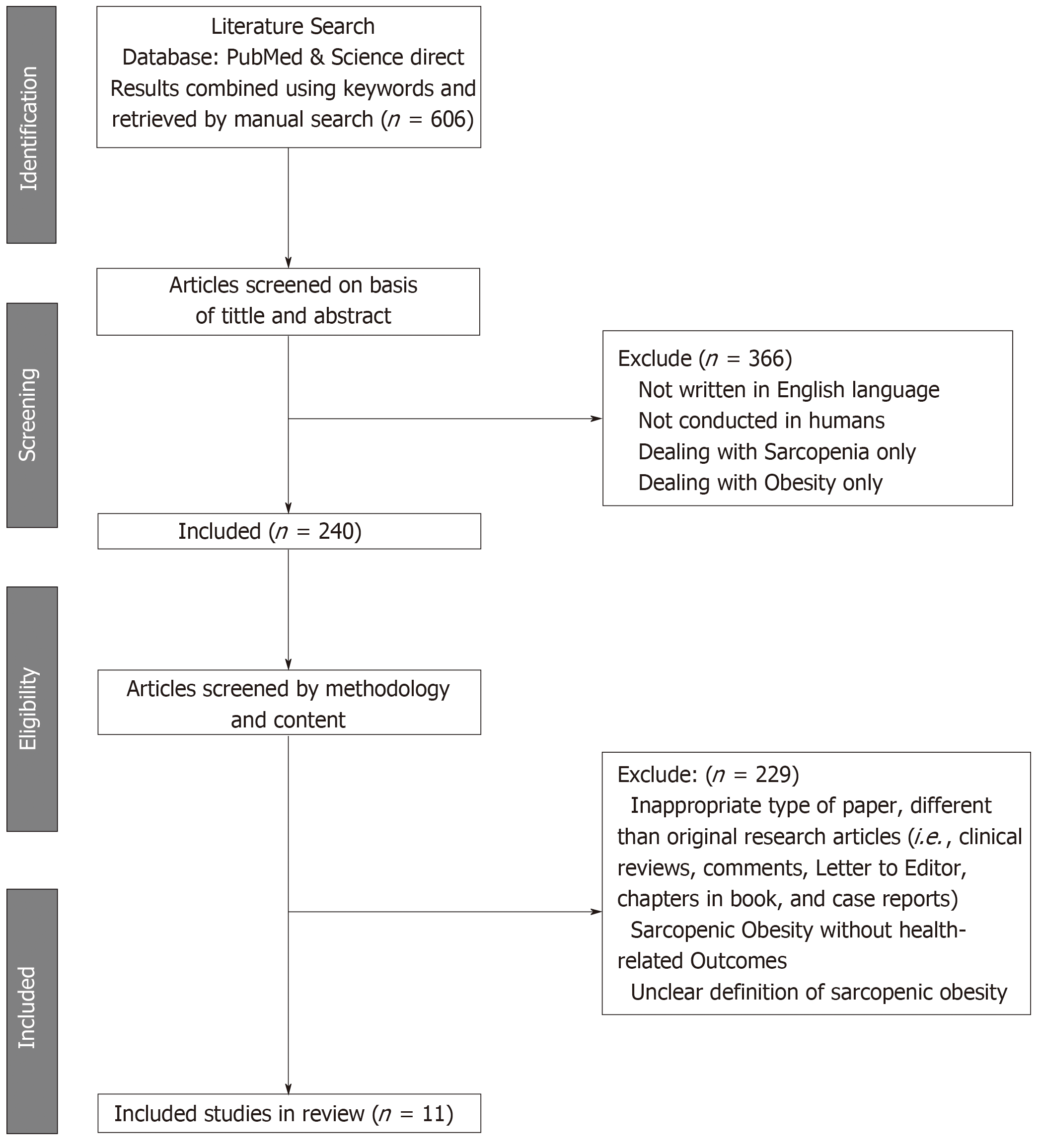
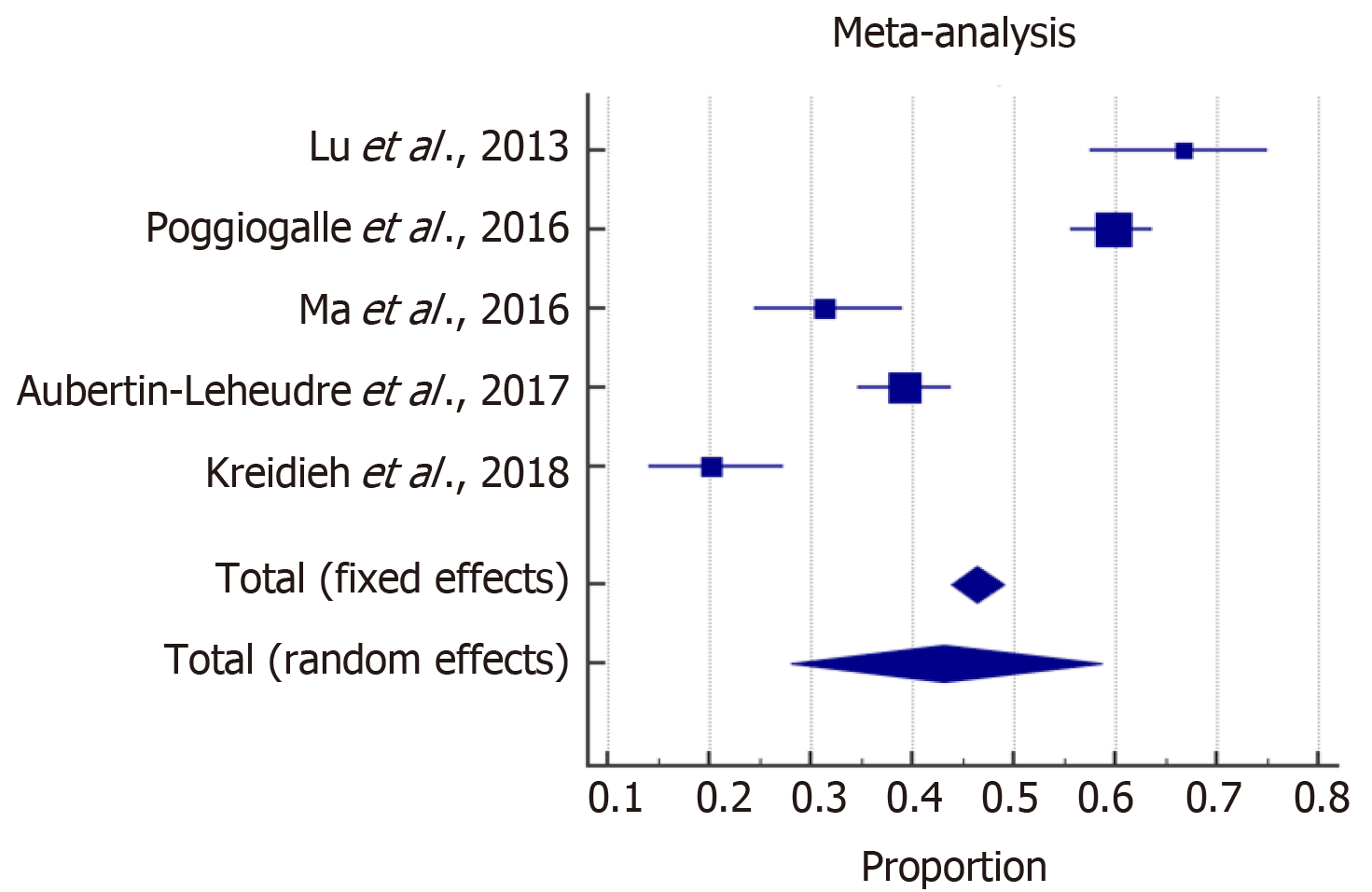
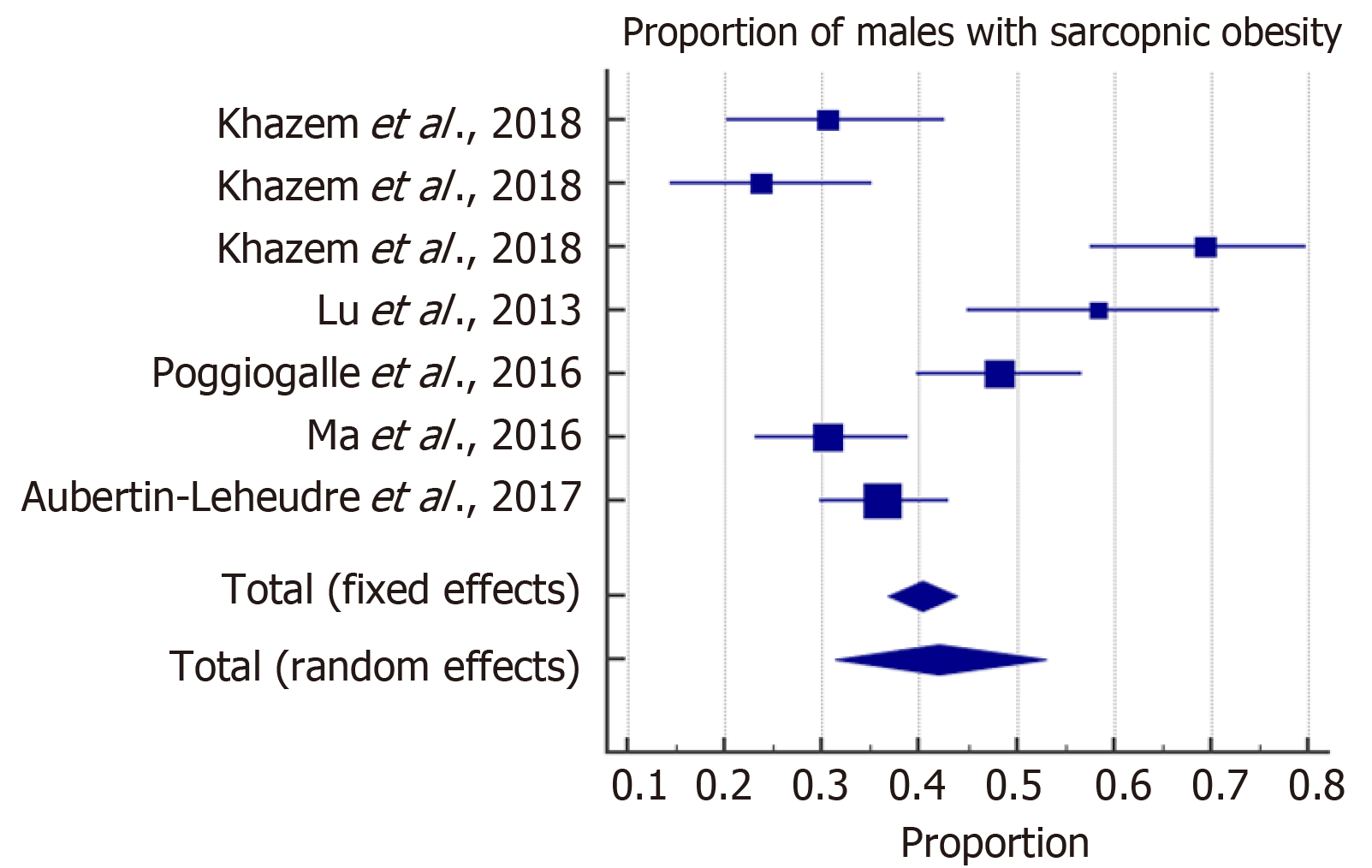
Of the 606 articles retrieved, 11 studies that comprised a total of 60118 adults with overweight and obesity of both genders met the inclusion criteria and were reviewed, revealing two main findings. First, the overall prevalence of sarcopenia is 43% in females and 42% in males who are with overweight and obesity. Secondly, the presence of SO increases the risk of T2D by 38% with respect to those without SO (OR = 1.38, 95%CI: 1.27-1.50).
A high prevalence of sarcopenia has been found among adults with overweight and obesity regardless of their gender and this condition seems to be associated with a higher risk of T2D. Clinician should be aware of this scenario in their clinical practice for the better management of both obesity and T2D.
Core tip: The coexistence of sarcopenia and obesity is referred to a phenotype termed sarcopenic obesity, defined as the increase in body fat deposition, and the reduction in lean mass and muscle strength. Since many uncertainties still surround this condition, especially with regard to its potential negative effects on health outcomes, we conducted this systematic review and found a high prevalence of sarcopenia among adults with obesity. Moreover, this condition seems to be associated with a higher risk of type 2 diabetes (T2D). Clinicians should be aware of this scenario in their clinical practice for better management of obesity and T2D.
- Citation: Khadra D, Itani L, Tannir H, Kreidieh D, El Masri D, El Ghoch M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: A systematic review and meta-analysis. World J Diabetes 2019; 10(5): 311-323
- URL: https://www.wjgnet.com/1948-9358/full/v10/i5/311.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i5.311
A condition that occurs because of the coexistence of sarcopenia and obesity has been termed sarcopenic obesity (SO)[1-7]. Many uncertainties still surround this phe-nomenon with regard to its definition and its potential negative effects on health outcomes, especially those related to obesity, namely the so-called cardio-metabolic diseases[8,9] such as type 2 diabetes (T2D), cardiovascular diseases, dyslipidaemia and metabolic syndrome[5,6,10-13]. In fact, it has been hypothesized that the two components of SO may synergistically increase their negative effects on health, however this is still a matter of debate[14-16].
Several studies have been conducted with a specific focus on determining the association between SO and T2D, however data regarding the contention that individuals with SO are likely to have poorer glycaemic profiles (i.e., hyperglycaemia, high HbA1c, insulin resistance, etc.,) are still contradictory and require further clarification[11,17-23]. Moreover, to the best of our knowledge no systematic review posing this issue as a primary outcome has yet been conducted in order to provide an unbiased interpretation of the evidence published to date. In light of these considerations, we set out to systematically review the published literature with the aim of determining the prevalence of sarcopenia among adults with overweight and obesity and to investigate whether SO was associated with higher risk of T2D, in accordance with the PICO process[24-26] as detailed below: P – population: Individuals in the overweight or obese categories, however they were defined [i.e., body mass index (BMI), body fat percentage, waist circumference, etc.,][27]; I – seeking treatment (i.e., weight-loss or any other treatment if recruited from a clinical setting), otherwise non-treated if subjects were recruited from the general population; C – comparison: Comparison between individuals with sarcopenia and those without SO and with the healthy control group (when available); O – outcome: (i) Prevalence of SO however it was defined in the studies’ Methods section (i.e., low muscle mass, low muscle strength, low physical performance, increased visceral adiposity, increased waist circumference etc.,) and assessed [i.e., bioelectric impedance analysis (BIA), dual-energy X-ray absorptiometry (DXA), handgrip, etc.,] among the entire obesity groups in the two genders; (ii) The prevalence of T2D however it was defined in the studies’ Methods section (i.e., fasting plasma glucose and glycated hemoglobin A1c, oral glucose tolerance test etc.,) in the SO and non-SO groups.
The review conformed to the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines[28-30] and was registered in the PROSPERO Registry, No. CRD42018111931[31].
All studies that evaluated SO and T2D in adults were included, provided that they met the following criteria: (i) Studies written in English; (ii) Original research with a cross sectional or longitudinal design; and (iii) Prospective or retrospective observational (analytical or descriptive), experimental or quasi-experimental controlled or non-controlled studies, documenting clearly the prevalence of SO, as well as the association or relationship between SO and T2D. No reviews or non-original articles (i.e., case reports, editorials, “Letters to the Editor” and book chapters) were included.
The literature search was designed and performed independently in duplicate by two authors, namely the principal (DK) and the senior investigator (ME). The PubMed and Science direct databases[32] were systematically screened using the MeSH terms and a manual search was carried out to retrieve other articles that had not been identified via the initial search strategy. Publication date was not considered an exclusion criterion for the purposes of this review.
Two independent authors (DK and ME) screened the articles for their methodology and suitability for inclusion. The quality appraisal was conducted according to the Newcastle-Ottawa Scale (NOS), which relies on a 9-star system whereby scores of 0-3, 4-6 and 7-9 are considered poor, moderate and good quality, respectively[33]. Consensus discussion was used to resolve disagreements between reviewers.
The title and abstract of each paper were initially assessed by two independent authors (DK and ME) for language suitability and subject matter relevance, the selected studies were then assessed in terms of their suitability for inclusion and the quality of the methodology. The studies that passed both rounds of screening are presented in Table 1.
| Study | Design | Definition of SO | Body composition | Gender | Sample | Mean age | Mean BMI | Prevalence Sarcopenic Obesity | Prevalence of Diabetes |
| Sénéchal et al[35], 2012 | Cross sectional | Dynapenic obesity, defined as low leg muscle strength, combined with abdominal obesity | Kin- Com dynamometer | M-F | T = 1963 | Non DO: 65.5 ± 9.6; DO: 65.4 ± 9.9 | Non DO: 30.8 ± 4.5; DO: 29.9 ± 4.6 | DO: n = 566/1963 (Did not distinguish in gender) | T2D: Non DO: n = 196; DO: n = 130 |
| Lu et al[18], 2013 | Cross sectional | Defined by combination of total skeletal muscle mass/wt. (100) and BMI ≥ 25 kg/m2 | BIA | M-F | T = 180; M = 60; F = 120 | Non SO: 69.9 ± 7.3; SO: 61.1 ± 9.9 | Non SO: 26.8 ± 1.6; SO: 27.8 ± 2.6 | n = 35/60 in males; n = 80/120 in females | T2D: Non SO: n = 12/65; SO: n = 17/115 |
| Poggiogalle et al[36], 2015 | Cross sectional | Defined by ASMM/h2 or ASMM/wt. < 2SD of sex specific mean combined with assessment of FM and FFM | DXA | M-F | T = 727; M = 141; F = 586 | 46.49 ± 13.73; 46.99 ± 13.76 | 38.85 ± 5.88; 38.84 ± 5.79 | SO: n = 68/141 in males; n = 350/586 in females | Pre-diabetes or T2D: Non-SO: n = 69; SO: n = 155 |
| Ma et al[37], 2016 | Retrospective; Cross sectional | SO: BMI > 30 kg/m2 and 24 h- UC < median | Sex-specific 24-h urinary creatinine excretion | M-F | T = 310; M = 144; F = 166 | 71.8 ± 7.6 | 34.1 ± 4.0 | SO: n = 44/144 in males; n = 52/166 in females | T2D: Non SO: n = 51; SO: n = 40 |
| Xiao et al[38], 2017 | Retrospective | FMI/FFMI ratio > 95 percentile of sex, BMI and ethnicity specific population-representative references | BIA | M-F | T = 144; M = 45; F = 99 | Non SO: 56.6 ± 12.7; SO: 54.6 ± 10.1 | Non SO: 44.0 ± 7.6; SO: 49.1 ± 8.3 | SO: 73/144 in total; (Did not distinguish in gender) | T2D: Non SO: n = 36/71; SO: n = 34/71 |
| Kang et al[39], 2017 | Cross sectional | ASM/Wt < 1 SD the mean of the reference group, and BMI ≥ 25 kg/m2 | DXA | F | T = 1555 | Non SO: 61.05 ± 0.44; SO: 62.91 ± 0.44 | Non SO: 26.80 ± 0.07; SO: 27.93 ± 0.11 | SO: n = 855/1555 (All females) | T2D: Non SO: n = 105/700; SO: n = 165/855 |
| Aubertin-Leheudre et al[40], 2017 | Cross sectional | Dynapenic obesity, defined as low handgrip strength (≤ 19.9 in females; ≤ 31.9 in males), combined with BMI ≥ 30 kg/m2 | Jamar Handheld Dynamometer | M-F | T = 670; M = 213; F = 457 | Non SO: 76.3 ± 4.7; SO: 78.0 ± 4.6 | Non SO: 35.6 ± 4.8; SO: 34.9 ± 4.8 | SO: n = 77/213 in males; n = 179/457 in females | T2D: Non SO: n = 133/414; SO: n = 81/256 |
| Park et al[41], 2018 | Cross sectional | SO defined by combination of SMI < 2 SD and WC ≥ 90 cm for men and ≥ 85 cm women | BIA | M-F | T = 53818; M = 38820; F = 14998 | Non SO: 40.5 ± 9.2; SO: 40.0 ± 11.3 | Non SO: 26.9 ± 2.2; SO: 30.7 ± 3.4 | n = 6513; M = 3341; F = 3172 | T2D; Non-SO: n = 2176; SO: n = 391 |
| Kreidieh et al[42], 2018 | Cross sectional | ALM/BMI < 0.512 | BIA | F | T = 154 | 33.26 ± 14.65 | 31.42 ± 4.94 | n = 31 | T2D: Non SO: n = 3/123; SO: n = 4/31 |
| Khazem et al[43], 2018 | Cross sectional | ALM/BMI < 0.789, (ALM/Wt.) × 100% < 25.72, and (ALM/Wt.) × 100% < 29.60 | BIA | M | T = 72 | 32.79 ± 13.65 | 33.69 ± 5.85 | 23.9%-69.4% | T2D: Non SO: n = 1/22; SO: n = 3/50 |
| Scott et al[46], 2018 | Cross sectional (includes a longitudinal part) | ALM/height < 7.26 kg/m2 combined with handgrip strength < 30 kg and/or low gait speed ≤ 0.8 m/s. Obesity was defined as body fat percentage ≥ 30% | DXA Handgrip strength Gait speed | M | T = 525 | Non SO: 75.9 ± 4.7; SO: 80.3 ± 6.5 | Non SO: 30.7 ± 3.4; SO: 27.2 ± 2.3 | n = 80 | High fasting glucose or diabetes medications: Non SO: n = 177/445; SO: n = 29/80 |
The 11 studies that met the inclusion criteria have been presented as a narrative synthesis. In addition, a meta-analysis was conducted on the included studies using Med Calc. software[34]. The Mantel Haenszel fixed and random effect models were used to estimate the overall effect size and 95%CI. The pooled estimate and 95%CI of the prevalence of SO among males and females in the included studies was estimated similarly.
The initial search retrieved 606 papers. After the first round of screening, 366 papers were excluded for: (i) Languages other than English; (ii) Non-human studies; and (iii) Dealing with obesity without sarcopenia, or the latter without the former. The second round of screening excluded 229 articles due to: (i) Inappropriate paper type, not original research articles (i.e., clinical reviews, Letters to the Editor, chapters in a book and case reports); (ii) Descriptions of SO, but not health-related outcomes; and (iii) An unclear definition of SO or identification of individuals with this condition. Accordingly, following the screening process, 11 articles were included in the systematic review and underwent narrative synthesis and meta-analysis (Figure 1). The NOS checklist proved that the studies were of a high quality (n = 11) (mean score = 7.36 points) (Table 2).
| Author | Sénéchal et al[35], 2012 | Lu et al[18], 2013 | Poggiogalle et al[36], 2016 | Ma et al[37], 2016 | Xiao et al[38], 2017 | Kang et al[39], 2017 | Aubertin- Leheudre et al[40], 2017 | Park et al[41], 2018 | Scott et al[46], 2018 | Kreidieh et al[42], 2018 | Khazem et al[43], 2018 |
| Selection | |||||||||||
| Represents cases with independent validation | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cases are consecutive or obviously representative | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Controls from the community | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Controls have no history of sarcopenic obesity | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Comparability | |||||||||||
| Controls are comparable for the most important factors | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Control for any additional factor | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Ascertainment of exposure | |||||||||||
| Secured record or structured interview where blind to/control status | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Same method of ascertainment for cases and controls | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cases and controls have completed follow up | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Total score | 7 | 7 | 8 | 8 | 6 | 8 | 7 | 8 | 8 | 7 | 7 |
In 2012, Sénéchal et al[35] conducted a cross-sectional evaluation in which the authors assessed dynapenic obesity, defined as low leg muscle strength combined with abdominal obesity, in 1963 individuals with abdominal obesity. Of these patients, 566 had dynapenic obesity (data per gender is not available). Regardless of gender the mean age and mean BMI in the dynapenic obesity and non-dynapenic obesity groups were 65.4 ± 9.9 years and 29.9 ± 4.6 kg/m2 and 65.5 ± 9.6 years and 30.8 ± 4.5 kg/m2 respectively. Furthermore, 130 of the 566 individuals with dynapenic obesity had T2D compared to 196 of the 1397 individuals in the non-dynapenic obesity group.
One year later, Lu et al[18] completed a cross sectional study in which they assessed SO defined as the coexistence of obesity (BMI ≥ 25 kg/m2) and sarcopenia based on the skeletal muscle index estimated by BIA. A sample of 180 individuals with obesity (60 males and 120 females) was recruited. Of the 60 males included in the sample 35 had SO compared to 80 of the 120 females. Regardless of gender the mean age and BMI in the SO group were 61.1 ± 9.9 years and 27.8 ± 2.6 kg/m2, and 69.9 ± 7.3 years and 26.8 ± 1.6 kg/m2 in the non-SO group. Moreover, 12 of the 65 patients in the SO group had T2D compared to 17 of the 115 patients in the non-SO group.
In early 2016, Poggiogalle et al[36] conducted a cross sectional study in which the authors assessed SO using DXA, with SO defined as the coexistence of obesity (BMI ≥ 30 kg/m2) and sarcopenia (ASMM: height2 < 6.54 and < 4.82 kg/m2 for males and females respectively) or (ASMM: weight < 0.2827 and < 0.2347 for males and females respectively). This study enrolled a sample of 727 individuals with obesity (141 males and 586 females), with mean ages of 45.63 ± 13.53 and 45.76 ± 13.58 years, and mean BMIs of 37.56 ± 5.99 and 37.80 ± 5.77 kg/m2 respectively for each gender. Of the 141 male patients 68 had SO, while 350 of the 586 females had the condition. In addition, 155 of the 418 patients had pre-diabetes or T2D in the SO group compared to 70 of the 309 patients in the non-SO group.
In the same year, Ma et al[37] performed a cross-sectional evaluation on SO defined by BMI and sex-specific 24-h urinary creatinine excretion, in 310 patients (166 females and 144 males) with obesity (BMI ≥ 30 kg/m2). Fifty-four of the 144 males and 52 of the 166 females had SO. The mean BMI and age of the SO group were 34.1 ± 4.0 kg/m2 and 71.8 ± 7.6 years, while they were 34.9 ± 4.4 kg/m2 and 67.8 ± 6.8 years in the non-SO group, respectively. Furthermore, 40 of the 106 patients had T2D in the SO group in comparison to 51 of the 204 patients in the non-SO group.
In 2017, Xiao et al[38] performed a retrospective study on the prevalence of SO and its association with health outcomes in patients seeking weight loss treatment in a bariatric surgery setting. Body composition analysis was conducted by means of BIA and SO was defined by a fat mass:fat-free mass index (FMI: FFMI) ratio greater than the 95th percentile of sex, BMI and ethnicity-specific population-representative references. A sample of 144 adults with obesity (99 females and 45 males) were enrolled, with a mean age of 55.6 ± 11.5 years and a mean BMI of 46.6 ± 8.4 kg/m2. Of the 144 patients included in the sample 73 had SO (data per gender is not available). The mean age and BMI of the individuals with obesity only were 56.6 ± 12.7 years and 44.0 ± 7.6 kg/m2, compared to 54.6 ± 10.1 years and 49.1 ± 8.3 kg/m2 in those with SO. Furthermore, 34 of the 73 patients had T2D in the SO group in comparison to 36 of the 71 patients in the non-SO group.
In 2017, Kang et al[39] conducted a large cross-sectional study to assess the association between SO and metabolic syndrome in postmenopausal women. SO was defined by the co-existence of sarcopenia (ASM/weight < 1 standard deviation below the mean of the reference group) and a BMI cut-off point for obesity which referred to a score of of 25 kg/m2 on the basis of the Asia-Pacific obesity criterion. The study included 1555 females with obesity, of whom 855 had SO, with a mean age of 62.91 ± 0.44 years and a mean BMI of 27.93 ± 0.11 kg/m2. On the other hand, 700 did not have SO and had a mean age of 61.05 ± 0.44 years and a mean BMI of 26.80 ± 0.07 kg/m2. In addition, 165 of the 855 patients had T2D in the SO group while 105 of the 700 patients in the non-SO group had T2D.
In the same year, a cross-sectional study by Aubertin-Leheudre et al[40] aimed to examine the association between dynapenic obesity and metabolic risk factors in older adults (age ≥ 70 years). Dynapenic obesity was defined as low handgrip strength (u 19.9 in females; ≤ 31.9 in males) combined with a BMI of ≥ 30 kg/m2. The study included 670 participants with obesity (213 males and 457 females), of whom 256 had dynapenic obesity, with a mean age of 78.0 ± 4.6 years and a mean BMI of 34.9 ± 4.8 kg/m2, and 414 did not have dynapenic obesity, with a mean age of 76.3 ± 4.7 years and a mean BMI of 35.6 ± 4.8 kg/m2. Furthermore, 81 of the 256 individuals in the dynapenic obesity group had T2D while 133 of 414 individuals in the non-dynapenic obesity group had T2D.
In 2018, Park et al[41] conducted a large cross sectional study in two sites, which included a total of 53818 adults with overweight and obesity of both genders (38820 males and 14998 females), of whom 6513 had SO defined as below two standard deviations of the mean of the skeletal muscle mass index for young adults assessed by BIA and a waist circumference of ≥ 90 cm for men and ≥ 85 cm for women. The mean age and BMI of the individuals with obesity only were 40.5 ± 9.2 years and 26.9 ± 2.2 kg/m2 compared to those with SO who had a mean age of 40.0 ± 11.3 years and a mean BMI of 30.7 ± 3.4 kg/m2. Moreover, 391 of the 6513 patients had T2D in the SO group compared to 2176 of the 47305 patients in the non-SO group.
In 2018, Kreidieh et al[42] conducted a cross sectional controlled study in which body composition measurements were conducted by BIA using a definition that in addition to appendicular lean mass (ALM) also involved BMI, and patients were considered affected by SO if ALM:BMI < 0.512. The study included 154 females with overweight and obesity with a mean age of 33.26 ± 14.65 years and a mean BMI of 31.42 ± 4.94 kg/m2. Of the 154 female patients 31 had SO. Moreover, four of the 31 patients had T2D in the SO group compared to three of the 123 patients in the non-SO group.
In 2018, Khazem el al[43] performed a cross-sectional controlled study on 72 adult males with overweight and obesity with a mean age of 32.79 ± 13.65 years and a mean BMI of 33.69 ± 5.84 kg/m2. In this study the authors used three different definitions proposed by Batsis et al[44], Levine and Crimmins[21], and Oh et al[45] based on ALM:BMI and (ALM: weight) × 100% to define SO. Body composition was assessed by BIA. Based on each formula the prevalence of SO varied between 23.9% and 69.4%. However, based on the definition that was revealed to be more useful from the clinical perspective, 50 of the 72 patients had a reduced lean body mass with a prevalence of 69.4%. Moreover, three of the 50 patients had T2D in the SO group in comparison to one of the 22 patients in the non-SO group.
Finally, in 2018 Scott et al[46] conducted a large sampled study that aimed to investigate the cross-sectional association between SO and components of metabolic syndrome in community-dwelling older men. SO was defined by the co-existence of sarcopenia as ALM/height < 7.26 kg/m2 combined with handgrip strength < 30 kg and/or low gait speed ≤ 0.8 m/s, while obesity was defined as a body fat percentage of o 30%. The study included 525 males with obesity, of whom 80 had SO, with a mean age of 80.3 ± 6.5 years and mean BMI of 27.2 ± 2.3 kg/m2 and 445 did not have SO, with a mean age of 75.9 ± 4.7 years and mean BMI of 30.7 ± 3.4 kg/m2. Furthermore, 29 of the 80 individuals in the SO group had T2D in comparison to 177 of the 445 individuals in the non-SO group.
The meta-analysis estimated the overall prevalence of SO among males and females. With high heterogeneity among the included studies, a random effect model was considered for the estimation of the overall prevalence of SO. The forest plots in Figures 2 and 3 show that SO affected 43% (95%CI: 28-59) of females and 42% (95%CI: 31-53) of males. In addition, the overall odds ratios of T2D in patients with SO as compared to those without SO are presented in Figure 4. The fixed effect weighted pooled odds for T2D in patients with SO indicated an increased risk of T2D of approximately 38% compared to those without SO (OR: 1.38, 95%CI: 1.27-1.50). The heterogeneity analysis revealed moderate variability (I2 = 60%).
This systematic review aimed to provide benchmark data on the prevalence of sarcopenia in individuals with overweight and obesity and to assess any potential association between SO and T2D in this population. The major finding is that sarcopenia seems to affect approximately 40%-45% of individuals with overweight and obesity of both genders, and the co-existence of both conditions, namely sarcopenia and excess weight/obesity increases the risk of T2D by nearly 38% when compared with those who had excess weight or obesity alone. The underlying mechanism behind this association is still unclear, however it seems that there is a bi-directional interaction between obesity, chronic inflammation, insulin resistance and sarcopenia[19]. In fact, the chronic inflammation plays an important role in the pathogenesis of T2D. For this reason we speculate that coexistence of both obesity and sarcopenia under the so-called phenotype “SO”, may have a synergistic effect with chronic inflammation being a common “denominator” seen in both conditions, which seems to exacerbate further glucose metabolism impairment (i.e., insulin resistance, pre-diabetes and T2D)[19].
The clinical implication of this review’s findings is the awareness of the high prevalence of sarcopenia in the overweight/obese population that should be raised among clinicians and patients. Secondly, these results reveal the importance of screening for SO in individuals affected by excess weight and obesity, since this condition also seems to be strongly associated with T2D.
This systematic review has certain strengths. To the best of our knowledge this is the first systematic review to assess the overall prevalence of SO in males and females with overweight and obesity. In fact, the studies that have been conducted on this topic reported varying levels of prevalence that ranged between 0 and 100%, depending on the applied definition of SO[47,48]. Higher prevalence tends to be reported in studies that accounted for body mass (i.e., BMI), whereas a lower prevalence is reported in those that did not[43,49]. A low prevalence may also be explained by the use of definitions that have primarily been developed from studies in older cohorts and these may not be applicable to younger adults[47].
However, this systematic review also has certain limitations. Foremost, our results need to be interpreted with caution with regard to the association between SO and the prevalence of T2D, since the cross-sectional design of the studies (i.e., non cohort), included in our systematic review indicates only simple associations between SO and T2D at best and does not provide solid information regarding any causal relationships between the two conditions[50,51]. In other words, these studies lack evidence to determine if SO may lead to the onset or deterioration of T2D, since very few studies have longitudinally investigated the “real” effects of SO on health[52]. These shortcomings in the current research indicate the need to design longitudinal studies to clarify the real effect of SO on the onset and progression of T2D.
In conclusion, a high prevalence of sarcopenia has been found among adults with overweight and obesity regardless of their gender, and this condition seems to be associated with a higher risk of T2D. Clinicians should be aware of this scenario in their clinical practice for better management of both obesity and T2D.
The coexistence of sarcopenia and obesity has been termed as sarcopenic obesity (SO). Several studies have been conducted in order to determine any potential association between SO phenotype and type 2 diabetes (T2D). However, the available data are still contradictory and require further clarification.
To our knowledge no systematic review on the primary outcome related to the association between SO and T2D has been conducted yet to provide an unbiased interpretation of the evidence published to date.
We set out to systematically review the published literature with the aim of determining the prevalence of sarcopenia among adults with overweight and obesity and to investigate whether SO was associated with higher risk of T2D.
The review conformed to the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines, and data were collated by means of narrative synthesis and meta-analysis.
The prevalence of SO in adult with overweight and obesity is 43% in females and 42% in males, and the presence of this condition increases the risk of T2D by 38% with respect to those without SO.
A high prevalence of sarcopenia has been found among adults with overweight and obesity regardless of their gender, and this condition seems to be associated with a higher risk of T2D. The clinical implication of our findings is to raise awareness of the high prevalence of this phenotype in the overweight/obese population, and the importance of screening for SO in individuals affected by excess weight, since this condition seems to be strongly associated with T2D. However, our results need to be interpreted with caution with regard to the association between SO and the prevalence of T2D, since the cross-sectional design of the studies included in our systematic review indicates only associations between the two conditions and that does not provide information regard the causal relationships.
The current research indicates the need to design longitudinal studies to clarify the real effect of SO on the onset and progression of T2D. In other words, the available studies lack in evidence to determine if SO may lead to the onset or deterioration of T2D, since very few studies have longitudinally investigated the “real” effects of SO on health.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Lebanon
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chien CW, Dinc M, Chen GX S-Editor: Dou Y L-Editor: A E-Editor: Xing YX
| 1. | Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med. 2016;31:1054-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 2. | Polyzos SA, Margioris AN. Sarcopenic obesity. Hormones (Athens). 2018;17:321-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes. 2013;20:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Choi KM. Sarcopenia and sarcopenic obesity. Endocrinol Metab (Seoul). 2013;28:86-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | dos Santos EP, Gadelha AB, Safons MP, Nóbrega OT, Oliveira RJ, Lima RM. Sarcopenia and sarcopenic obesity classifications and cardiometabolic risks in older women. Arch Gerontol Geriatr. 2014;59:56-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care. 2010;33:1652-1654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 399] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 7. | Hwang B, Lim JY, Lee J, Choi NK, Ahn YO, Park BJ. Prevalence rate and associated factors of sarcopenic obesity in korean elderly population. J Korean Med Sci. 2012;27:748-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Visser M, van Venrooij LM, Vulperhorst L, de Vos R, Wisselink W, van Leeuwen PA, de Mol BA. Sarcopenic obesity is associated with adverse clinical outcome after cardiac surgery. Nutr Metab Cardiovasc Dis. 2013;23:511-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Park SH, Park JH, Song PS, Kim DK, Kim KH, Seol SH, Kim HK, Jang HJ, Lee JG, Park HY, Park J, Shin KJ, Kim Di, Moon YS. Sarcopenic obesity as an independent risk factor of hypertension. J Am Soc Hypertens. 2013;7:420-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 807] [Cited by in F6Publishing: 729] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 11. | El Ghoch M, Calugi S, Dalle Grave R. Sarcopenic Obesity: Definition, Health Consequences and Clinical Management. The Open Nutrition Journal. 2018;12:70-73. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Baek SJ, Nam GE, Han KD, Choi SW, Jung SW, Bok AR, Kim YH, Lee KS, Han BD, Kim DH. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008-2010 Korea National Health and Nutrition Examination Survey. J Endocrinol Invest. 2014;37:247-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Messier V, Karelis AD, Lavoie ME, Brochu M, Faraj M, Strychar I, Rabasa-Lhoret R. Metabolic profile and quality of life in class I sarcopenic overweight and obese postmenopausal women: a MONET study. Appl Physiol Nutr Metab. 2009;34:18-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Gusmao-Sena MH, Curvello-Silva K, Barreto-Medeiros JM, Da-Cunha-Daltro CH. Association between sarcopenic obesity and cardiovascular risk: where are we? Nutr Hosp. 2016;33:592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Barazzoni R, Bischoff SC, Boirie Y, Busetto L, Cederholm T, Dicker D, Toplak H, Van Gossum A, Yumuk V, Vettor R. Sarcopenic obesity: Time to meet the challenge. Clin Nutr. 2018;37:1787-1793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 16. | Barazzoni R, Bischoff S, Boirie Y, Busetto L, Cederholm T, Dicker D, Toplak H, Van Gossum A, Yumuk V, Vettor R. Sarcopenic Obesity: Time to Meet the Challenge. Obes Facts. 2018;11:294-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 17. | Kim TN, Park MS, Lim KI, Choi HY, Yang SJ, Yoo HJ, Kang HJ, Song W, Choi H, Baik SH, Choi DS, Choi KM. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean Sarcopenic Obesity Study. Clin Endocrinol (Oxf). 2013;78:525-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Lu CW, Yang KC, Chang HH, Lee LT, Chen CY, Huang KC. Sarcopenic obesity is closely associated with metabolic syndrome. Obes Res Clin Pract. 2013;7:e301-e307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010;5:e10805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 406] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 20. | Murai J, Nishizawa H, Otsuka A, Fukuda S, Tanaka Y, Nagao H, Sakai Y, Suzuki M, Yokota S, Tada H, Doi M, Fujishima Y, Kita S, Funahashi T, Maeda N, Nakamura T, Shimomura I. Low muscle quality in Japanese type 2 diabetic patients with visceral fat accumulation. Cardiovasc Diabetol. 2018;17:112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Levine ME, Crimmins EM. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring). 2012;20:2101-2106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Kim K, Park SM. Association of muscle mass and fat mass with insulin resistance and the prevalence of metabolic syndrome in Korean adults: a cross-sectional study. Sci Rep. 2018;8:2703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Kim JA, Hwang SY, Chung HS, Kim NH, Seo JA, Kim SG, Kim NH, Choi KM, Baik SH, Yoo HJ. Proportion and Characteristics of the Subjects with Low Muscle Mass and Abdominal Obesity among the Newly Diagnosed and Drug-Naïve Type 2 Diabetes Mellitus Patients. Diabetes Metab J. 2019;43:105-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12-A13. [PubMed] [Cited in This Article: ] |
| 25. | Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1070] [Cited by in F6Publishing: 1333] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 26. | Aslam S, Emmanuel P. Formulating a researchable question: A critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 2010;31:47-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Purnell JQ, Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP. Definitions, Classification, and Epidemiology of Obesity 2018. . [PubMed] [Cited in This Article: ] |
| 28. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16777] [Cited by in F6Publishing: 16380] [Article Influence: 1092.0] [Reference Citation Analysis (1)] |
| 29. | McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1683] [Cited by in F6Publishing: 1635] [Article Influence: 272.5] [Reference Citation Analysis (0)] |
| 30. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11206] [Cited by in F6Publishing: 10347] [Article Influence: 689.8] [Reference Citation Analysis (0)] |
| 31. | Andrade R, Pereira R, Weir A, Ardern CL, Espregueira-Mendes J. Zombie reviews taking over the PROSPERO systematic review registry. It's time to fight back! Br J Sports Med. 2017;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | PubMed. Available from: http://www.njrcentre.org.uk/. [Cited in This Article: ] |
| 33. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8858] [Cited by in F6Publishing: 10821] [Article Influence: 772.9] [Reference Citation Analysis (0)] |
| 34. | Med Calc. Available from: http://www.njrcentre.org.uk/. [Cited in This Article: ] |
| 35. | Sénéchal M, Dionne IJ, Brochu M. Dynapenic abdominal obesity and metabolic risk factors in adults 50 years of age and older. J Aging Health. 2012;24:812-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Poggiogalle E, Lubrano C, Sergi G, Coin A, Gnessi L, Mariani S, Lenzi A, Donini LM. Sarcopenic Obesity and Metabolic Syndrome in Adult Caucasian Subjects. J Nutr Health Aging. 2016;20:958-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Ma J, Hwang SJ, McMahon GM, Curhan GC, Mclean RR, Murabito JM, Fox CS. Mid-adulthood cardiometabolic risk factor profiles of sarcopenic obesity. Obesity (Silver Spring). 2016;24:526-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Xiao J, Cain A, Purcell SA, Ormsbee MJ, Contreras RJ, Kim JS, Thornberry R, Springs D, Gonzalez MC, Prado CM. Sarcopenic obesity and health outcomes in patients seeking weight loss treatment. Clin Nutr ESPEN. 2018;23:79-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Kang SY, Lim GE, Kim YK, Kim HW, Lee K, Park TJ, Kim J. Association between Sarcopenic Obesity and Metabolic Syndrome in Postmenopausal Women: A Cross-sectional Study Based on the Korean National Health and Nutritional Examination Surveys from 2008 to 2011. J Bone Metab. 2017;24:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Aubertin-Leheudre M, Anton S, Beavers DP, Manini TM, Fielding R, Newman A, Church T, Kritchevsky SB, Conroy D, McDermott MM, Botoseneanu A, Hauser ME, Pahor M; LIFE Research Group. Dynapenia and Metabolic Health in Obese and Nonobese Adults Aged 70 Years and Older: The LIFE Study. J Am Med Dir Assoc. 2017;18:312-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Park CH, Do JG, Lee YT, Yoon KJ. Sarcopenic obesity associated with high-sensitivity C-reactive protein in age and sex comparison: a two-center study in South Korea. BMJ Open. 2018;8:e021232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Kreidieh D, Itani L, El Masri D, Tannir H, Citarella R, El Ghoch M. Association between Sarcopenic Obesity, Type 2 Diabetes, and Hypertension in Overweight and Obese Treatment-Seeking Adult Women. J Cardiovasc Dev Dis. 2018;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Khazem S, Itani L, Kreidieh D, El Masri D, Tannir H, Citarella R, El Ghoch M. Reduced Lean Body Mass and Cardiometabolic Diseases in Adult Males with Overweight and Obesity: A Pilot Study. Int J Environ Res Public Health. 2018;15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999-2004. Nutr Res. 2015;35:1031-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 45. | Oh C, Jho S, No JK, Kim HS. Body composition changes were related to nutrient intakes in elderly men but elderly women had a higher prevalence of sarcopenic obesity in a population of Korean adults. Nutr Res. 2015;35:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Scott D, Cumming R, Naganathan V, Blyth F, Le Couteur DG, Handelsman DJ, Seibel M, Waite LM, Hirani V. Associations of sarcopenic obesity with the metabolic syndrome and insulin resistance over five years in older men: The Concord Health and Ageing in Men Project. Exp Gerontol. 2018;108:99-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Johnson Stoklossa CA, Sharma AM, Forhan M, Siervo M, Padwal RS, Prado CM. Prevalence of Sarcopenic Obesity in Adults with Class II/III Obesity Using Different Diagnostic Criteria. J Nutr Metab. 2017;2017:7307618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 48. | Lee DC, Shook RP, Drenowatz C, Blair SN. Physical activity and sarcopenic obesity: definition, assessment, prevalence and mechanism. Future Sci OA. 2016;2:FSO127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 49. | El Ghoch M, Rossi AP, Calugi S, Rubele S, Soave F, Zamboni M, Chignola E, Mazzali G, Bazzani PV, Dalle Grave R. Physical performance measures in screening for reduced lean body mass in adult females with obesity. Nutr Metab Cardiovasc Dis. 2018;28:917-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Mahajan A. Limitations of cross-sectional studies. Neurol India. 2015;63:1006-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Setia MS. Methodology Series Module 3: Cross-sectional Studies. Indian J Dermatol. 2016;61:261-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 52. | Cuthbertson DJ, Bell JA, Ng SY, Kemp GJ, Kivimaki M, Hamer M. Dynapenic obesity and the risk of incident Type 2 diabetes: the English Longitudinal Study of Ageing. Diabet Med. 2016;33:1052-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |