Published online Apr 15, 2012. doi: 10.4239/wjd.v3.i4.71
Revised: March 21, 2012
Accepted: April 10, 2012
Published online: April 15, 2012
Polycystic ovarian syndrome (PCOS) is the most common endocrine disorder in women. To meet PCOS criteria, women must have a combination of hyperandrogenism, anovulation and ultrasound findings. Almost 10% of all reproductive age women worldwide show signs of PCOS. Although women often seek care for gynecological or body image concerns, many PCOS women are at risk for metabolic syndrome (MS). Many of the metabolic consequences are overlooked and undertreated by physicians because these patients tend to be young, reproductive age women. MS and obesity coexist commonly with PCOS. These young women are predisposed to glucose abnormalities and ultimately diabetes mellitus, dyslipidemia and eventually cardiovascular disease. Bariatric surgery can be an effective means of weight loss in PCOS women. Surgical techniques have become safer and less invasive over time and have been found to be effective in achieving significant weight loss. Surgical options have also increased, giving patients more choices. Bariatric surgery may prevent or reverse metabolic syndrome. Bariatric surgery may also have reproductive benefits in PCOS patients. Although bariatric surgery has historically been performed in older, reproductive aged women, it has recently gained favor in adolescents as well. This is of particular importance due to the prevalence of both PCOS and MS in adolescents. Treatment of PCOS and MS certainly requires a combination of medical therapy, psychological support and lifestyle modifications. These treatments are difficult and often frustrating for patients and physicians. Bariatric surgery can be effective in achieving significant weight loss, restoration of the hypothalamic pituitary axis, reduction of cardiovascular risk and even in improving pregnancy outcomes. Ultimately, bariatric surgery should be considered part of the treatment in PCOS women, especially in those with MS.
- Citation: Malik SM, Traub ML. Defining the role of bariatric surgery in polycystic ovarian syndrome patients. World J Diabetes 2012; 3(4): 71-79
- URL: https://www.wjgnet.com/1948-9358/full/v3/i4/71.htm
- DOI: https://dx.doi.org/10.4239/wjd.v3.i4.71
Polycystic ovarian syndrome (PCOS) is the most common endocrinopathy in women, affecting nearly 10% of women worldwide. Definitions for PCOS vary depending on European, United States or Rotterdam consensus, but diagnostic components include anovulation, hyperandrogenism and targeted ultrasound criteria[1]. Although metabolic abnormalities do not inherently define the syndrome, many PCOS women have hypertension, dyslipidemia, obesity, insulin and glucose abnormalities, and ultimately MS. Treatment for menstrual irregularities, anovulation, infertility and symptoms such as hirsutism is fairly standardized. However, recognition of metabolic abnormalities is commonly delayed and underappreciated. Treatment often lacks aggressiveness. Since these patients are often very young, physicians often misperceive the extent of metabolic disease. Obesity is particularly common in PCOS women; almost 30% of all US women and almost 60% of PCOS women are obese[2].
Here we will review the common metabolic abnormalities seen in women with PCOS. Briefly, different types of bariatric surgery will be discussed as well as the evidence for metabolic and weight improvements from surgery. The role of bariatric surgery in PCOS women will be reviewed with a focus on metabolic improvements. Since many adolescents have PCOS, the evidence, concerns and outcomes in this special patient population will be discussed. Finally, reproductive and pregnancy concerns will be summarized in order to gain a more complete perspective on the potential benefits and concerns of bariatric surgery in these women.
PCOS women have a high prevalence of insulin resistance and impaired glucose tolerance[3,4], both precursors of diabetes mellitus[5,6]. Insulin resistance is difficult to measure but more than 50% of PCOS women, even as adolescents, have insulin resistance and they progress commonly to MS[1,6-8]. MS is defined as a grouping of characteristics predisposing to coronary artery disease and diabetes mellitus. MS requires 3 of the following: central obesity, elevated triglycerides, decreased high-density lipoprotein, hypertension and elevated fasting blood glucose[1]. Its prevalence in the general population is 25%[9,10] and in PCOS women is 40%-50%[11]. The prevalence of MS is young PCOS women < age 40 years (45%-53%) is particularly high relative to age matched counterparts (6%-15%)[10]. Even male relatives of PCOS women have a higher incidence of MS[12,13]. Young women with PCOS have more labile blood pressure regulation[14] and even adolescents show blood pressure regulatory changes[15]. Later in life, menopausal PCOS women have twice the prevalence of hypertension[16]. Clinically, it is an oversimplification to believe that PCOS women just have a higher propensity for MS. However, research[17,18] shows that PCOS itself may be pathophysiologically related to MS. Both gene[19] and protein[20] expression in omental tissue of PCOS women show differences compared to non-hyperandrogenic obese women, suggesting a direct causal connection between PCOS, visceral adiposity and ultimately MS. PCOS women consistently show elevated low-density lipoprotein and triglycerides, lower high-density lipoprotein, increased carotid intimal thickening and an increase in both fatal and non-fatal cardiovascular events. Forty percent of PCOS women have diabetes mellitus before the age of 50 years. These changes are not always dependent on body mass index (BMI)[21]. Carotid intima-media thickening[22] and coronary artery calcification[23] are higher in PCOS women compared to age-matched controls. MS is shockingly prevalent in PCOS adolescents (close to 40%) and to a greater extent in obese PCOS adolescents (> 60%)[24,25]. Obesity, even in very young women, worsens the metabolic features of PCOS.
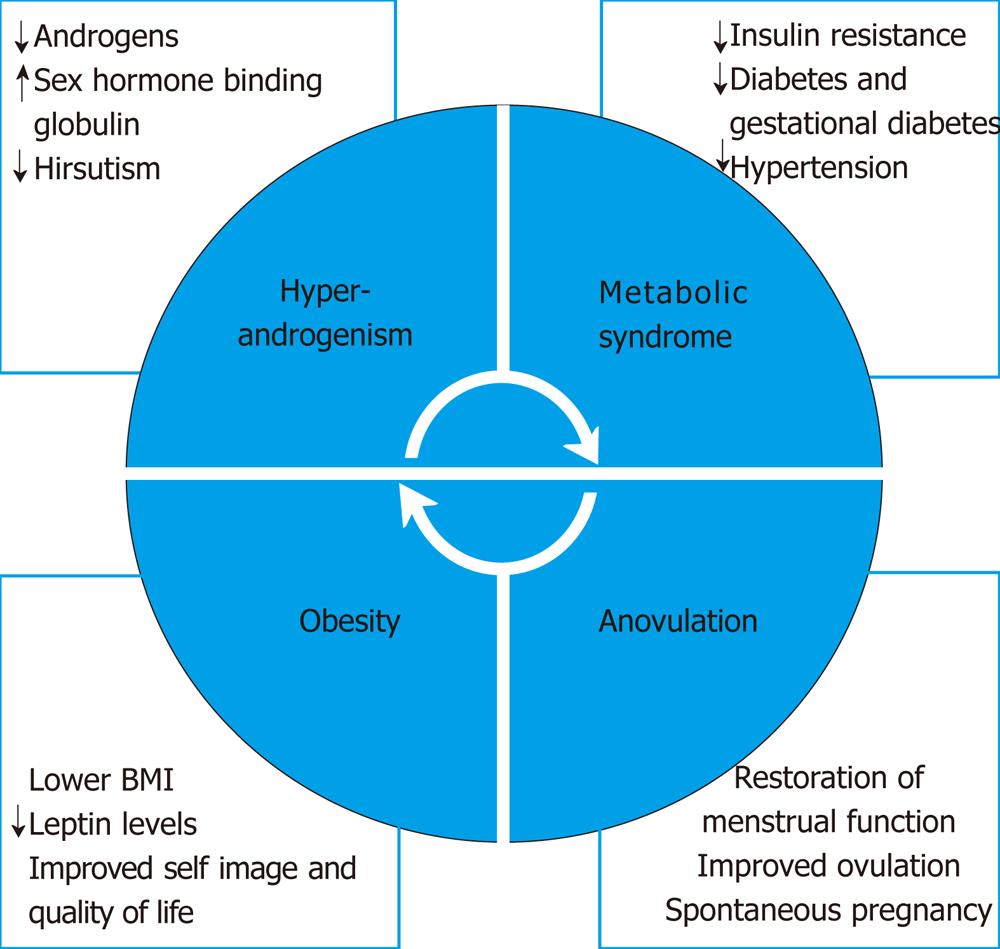
Obesity is an epidemic. Despite universal recognition that weight control is important, it remains perhaps the most difficult morbidity to treat. Obesity is usually defined by BMI and has become the standard research and clinical tool in monitoring weight. Although diet and exercise, “lifestyle modifications”, are first line therapy for overweight women, most studies show limited ability to lose large amounts of excess body weight greater than 10 kg[26-28]. Initial weight loss is a positive predictive factor of long term success[29]. The surrounding social environment, especially family attitudes and support for obese children and adolescents, greatly impacts the success of lifestyle modifications[30]. Pharmacological treatments exist but are limited. Surgical weight loss has been an option for many years but is underutilized, especially in younger patients whose risk factors for coronary artery disease are less glaring. The evidence for lifestyle modifications producing metabolic parameters in PCOS is disappointing[31] (Figure 1).
Bariatric surgery has been available for decades. Most procedures are now performed laparoscopically. Although various procedures have been described and attempted, the 3 most common procedures performed are laparoscopic adjustable gastric banding (LAGB), laparoscopic roux-en-Y gastric bypass (LRYGB) and laparoscopic sleeve gastrectomy (LSG). LAGB involves a band around the proximal stomach which can be progressively inflated and tightened via a subcutaneous port. LRYGB involves surgical diversion of a smaller pouch of stomach to the jejunum. The length of diversion is related to the expected magnitude of effect. In LSG, a portion of the stomach is resected at the fundus, leaving an altered stomach shape and size, thereby affecting ghrelin secretion[32]. A Cochrane review of “surgery for obesity” updated in 2009 showed significantly higher weight loss after surgery for patients with BMI > 30 kg/m2 than after non-surgical intervention. The review also found that metabolic disease improved after surgery[33]. In general, LRYGB leads to a higher degree of excess weight loss than LAGB (68% vs 45% at 4 years); LRYGB also leads to a higher absolute weight loss than LAGB (36 kg vs 22.1 kg at 5 years). More limited data show similar weight loss between LRYGB and LSG and higher weight loss with LSG over LAGB[32]. A meta-analysis of procedures between 2003 and 2007 also shows higher excess weight loss at 1 year for LRYGB over LAGB (62.6% vs 49.4%). Weight loss for each procedure increased subsequently in each of the first 3 years post-operatively[34]. Patient satisfaction was also higher with LRYGB (80% vs 46%) with limited data[35]. Morbidity and mortality in general is inversely proportional to both hospital and surgeon volume. Bariatric surgery is cost effective in comparison to the excessive cost of medical care in these patients for metabolic abnormalities, especially diabetes mellitus[32]. In addition to the overwhelming medical evidence for benefits after surgery, studies also show that patients experience emotional, body image and quality of life improvements after bariatric surgery[36] (Figure 1).
Both short and long term risks of surgery in general have declined over time. A review of 14 studies between 1966 and 2007 showed LAGB had lower short term complications but a higher rate of re-operation. LAGB was associated with a shorter operative time (68 min), reduced hospital stay (2 d) and a lower mortality compared to LRYGB (0.06% vs 0.17%)[35]. However, long term complications were higher after LAGB with re-operation rates higher for band slippage or port problems. A significant number of patients undergoing LAGB have had their bands removed over time after failing to achieve weight loss[37]. Re-operation in LRYGB was mainly for bowel obstruction. Earlier studies showed a higher rate of complications with leak rates as high as 8% and re-operation rates as high as 25% in LAGB patients followed for approximately 10 years[38]. Thirty day readmission rates at a tertiary center for bariatric surgery were overall 6.5% but were approximately twice as high from LRYGB compared to LAGB[39]. LSG is a newer procedure with fewer published results. Complications are purported to be lower because it does not include any surgical bypass and avoids implantation of any device. A recent review of 15 studies including 940 procedures showed a mortality rate of 0.3%, an average hospital stay of 4 d (comparable to LRYGB) and an overall 12.1% complication rate[40].
Almost all bariatric procedures are now performed laparoscopically due to decreased surgical time, shorter hospital stay and quicker recovery. A significant number of procedures are now performed with robotic assistance. A review of 1253 patients undergoing bariatric surgery between 2003 and 2010 included robotic assisted LRYGB (n = 1165) and LAGB (n = 118) procedures. Operative time (213 min), malabsorptive leak rate (2.4%), bleeding (2%) and mortality (no deaths reported) were comparable to traditional laparoscopic procedures[41]. Due to improved visualization, increased accuracy and positioning, it is certainly plausible that robotic assistance will significantly reduce morbidity and surgical complication rates.
Bariatric surgery ameliorates metabolic abnormalities. As noted above, BMI and excess body weight decrease substantially after surgery. More importantly, improvement is noted in glucose abnormalities, dyslipidemia and hypertension. Improvement in diabetes mellitus at 2 year follow up after surgery is proportional to weight loss[42]. Proposed mechanisms include a beneficial alteration in incretin response via earlier and better GLP-1 effects, especially with bypass procedures[43]. Insulin abnormalities may improve very early post-operatively in pre-menopausal women with MS. Fasting glucose and insulin resistance measured by the homeostasis model assessment insulin resistance (HOMA-IR) can decrease > 50% within 1 mo of surgery, whereas insulin sensitivity measured by the euglycemic-hyperinsulinemic clamp does not change as quickly[44]. Studied up to 48 mo post-operatively, young MS patients demonstrated improvements in measurements of HOMA-IR, fasting glucose, area under the curve glucose and area under the curve insulin. Additionally, these measurements parallel reduced leptin levels and increased adiponectin, resistin and ghrelin, indicating biochemical explanation for the success of surgery[45]. Both LRYGB and LGB decrease HOMA-IR, normalize lipid parameters and decrease leptin by 50% immediately post-operatively[46]. A review of 18 studies, including both LRYGB and restrictive procedures, showed reduced insulin values proportional to changes in BMI. HOMA-IR decreased more after LRYGB than restrictive procedures (approximately 70% vs 50%). Adiponectin decreased as well after surgery in similar magnitude[47]. Patients also experienced lowering of cholesterol, low density lipoprotein and triglyceride levels, proportional to weight loss[45,48]. Finally, 75% of patients see improvement in hypertension and more than 50% see complete resolution[49]. Interestingly, the effects on hypertension have been shown to be independent of the magnitude of weight loss. A small study of 20 young (average age 34 years) premenopausal obese women undergoing either LSG or LAGB showed improvements in waist circumference, all lipid parameters, insulin resistance and, most importantly, a reduction in the prevalence of MS from 55% to 0% at 1 year[50]. Recently, a prospective controlled clinical trial compared intensive lifestyle intervention to LRYGB over a one year period. Lifestyle intervention group achieved significant decreases in weight, BMI, glucose and insulin measurements, lipid parameters and hypertension; yet the magnitude of change in the surgical group was generally 2-3 times more beneficial for each parameter[51]. This study included a significant number of women with MS before the age of 40 years (Figure 1).
Although reproductive age women fitting the profile of PCOS are included in many of the studies cited in this review, very few studies have specifically targeted PCOS patients. A limited number (n = 17) of PCOS patients with an average age of 30 years were followed prospectively for up to 26 mo after bariatric surgery (either biliopancreatic diversion or LAGB). Most women (12/17) regained normal menstrual function and most (10/12) had documented spontaneous ovulation. Average weight loss was 41 kg. Patients showed significant improvement in hirsutism, androgen profiles and about a 50% reduction in HOMA-IR[52]. Additionally, a retrospective review of 24 PCOS women with MS undergoing LRYGB between 1997 and 2001 was performed. Follow up for more than 2 years showed that all women resumed normal menstrual cycles, half had resolution of hirsutism and HbA1C decreased from 8.2% to 5.1% within 3 mo. Dyslipidemia, hypertension and diabetes mellitus almost completely resolved. Interestingly, within a short time frame, 5 women became pregnant spontaneously after surgery[53]. In another prospective 6 year study of patients undergoing LAGB (81% of whom were women), 78% of women saw improvement in metabolic syndrome and 48% showed improvement in PCOS specifically with regards to menstrual cycles, fertility and/or hirsutism. No women with IGT had developed diabetes at follow up during the 6 years[54]. Basic science has provided insight into clinical decision making. By chronic exposure to androgens, animal models of PCOS incorporate increased food uptake, weight gain, anovulation, fat mass and glucose intolerance. PCOS rats who underwent vertical sleeve gastrectomy showed significant weight loss, lower food intake, lower fat mass and normalized fasting insulin values[55].
PCOS presents a unique challenge since many obese PCOS women are adolescents. Although patients and physicians may at first be wary of a young patient considering surgical weight loss, these patients have an important opportunity. Bariatric surgery may actually provide primary prevention of coronary artery disease, eliminate MS and cause meaningful, long term reduction in morbidity and mortality. Young women with PCOS show evidence of atherosclerosis by abnormal carotid intimal medical thickness measurements[56,57] and the prevalence of diabetes mellitus before the age of 50 is exceptionally high and estimated at 3-4 times the general population prevalence[8]. So, even some PCOS women aged 25-40 years with MS probably already have early coronary artery disease and thus are no longer candidates for primary prevention. Of course, a comprehensive approach to weight loss in adolescents is very important. Almost 20% of adolescents are overweight by the age of 19 years[58]. It is very important for young women to initiate diet and exercise. Pharmacological treatments are also an option for adolescent obesity, although data are limited. Orlistat and sibutramine were originally FDA approved for this purpose but sibutramine was removed due to cardiovascular concerns and orlistat has considerable GI side effects and liver toxicity[58].
Adolescent bariatric surgery has been studied with limited data. Criteria for adolescent surgery were originally defined in 2004. Eligibility included BMI > 40 kg/m2, comorbidities, physical maturity, failure by lifestyle modifications for at least 6 mo and proper evaluation and understanding[59]. Guidelines were updated in 2009 and are more similar to adult recommendations with BMI > 40 kg/m2 or BMI > 35 kg/m2 with significant comorbidities, including MS[60]. Surgery is an important consideration in adolescents since lifestyle modifications produce modest benefits[61,62] with limited continuation rates[63]. Both LRYGB and LAGB have been studied in adolescents and are FDA approved. LRYGB results in approximately 35% reduction in BMI and resolution of hypertension[64]. LAGB has been more popular with adolescents, possibly due to reduced hospital stay or perceived lower rates of complications. Studies show that LAGB in adolescents decreases BMI by more than 10 units and results in reduction in glucose abnormalities and hypertension in more than 80% of patients[65]. Recent randomized control trials of LAGB in adolescents showed significant excess weight loss (almost 80%), reduction in MS and improved insulin sensitivity[66]. LSG is currently not FDA approved in adolescents. No adequate data exist in adolescents. At this time, although adult data are promising, all data for adolescents must be extrapolated.
Reproductive concerns may also lead PCOS women with MS to consider bariatric surgery. Does surgery optimize both fertility and pregnancy? The relationship between PCOS, obesity and infertility has been documented for many years. Known effects include anovulation, miscarriage, impairment in folliculogenesis and altered endometrial receptivity[67-71]. Modest weight loss often increases the odds of spontaneous ovulation[72]. At the current time and with current surgical risks, bariatric surgery should not be considered a “fertility treatment” for ovulation induction. However, pregnancy risks in woman with MS are high. These women face difficulty in managing diabetes, pre-eclampsia, growth disorders, higher rates of cesarean delivery, higher maternal mortality and increase their children’s risks for metabolic disease in the future[73].
Women already take pregnancy into consideration when electing for bariatric surgery. A recent retrospective study of 1538 women who underwent bariatric surgery showed that 40% of these women reported a history of infertility and that 55% of these women who reported obesity by the age of 18 had infertility. More importantly, 30% of women under the age of 45 cited future pregnancy as a major concern for them. This is especially important given that 54% had hypertension, 33% had diabetes mellitus and 46% had sleep apnea[74]. Clearly, these data show that women undergoing surgery are aware of their own reproductive risks and a significant number of these women are thinking ahead.
Bariatric surgery in reproductive age women has been shown to decrease menstrual irregularities[53,75,76]. PCOS women have less hyperandrogenism post operatively[52,77] and sex hormone binding globulin increases after bariatric surgery[77-79]. LH and FSH levels have been reported to increase after surgery[77,78]. On a more functional level, ovulatory function measured by luteal LH and progesterone secretion improved postoperatively, although levels were still below normal expected values[80]. Additionally, leptin levels decrease after bariatric surgery, reflecting improved reproductive metabolic status[81]. Many of these women (10%-25%) have subclinical hypothyroidism which was significantly reduced following bariatric surgery[82,83]. These changes certainly would suggest improved reproductive function and more studies are certainly needed to gain a better understanding.
The specific impact of bariatric surgery on fertility treatment is very limited but there are enough reported pregnancies to gain important insights. An older study after vertical banded gastroplasty showed a higher conception rate and lower miscarriage rate following surgery[84]. In one of the largest data sets, a retrospective study found that in 110 obese patients with infertility prior to bariatric surgery, 69 became pregnant following bariatric surgery. A BMI drop greater than 5 kg/m2 was a significant predictor of fertility within 2.5 years of follow up after surgery. The type of bariatric procedure did not impact fertility[85]. A small case series describes successful in vitro fertilization outcomes in women who underwent previous surgery. It reported no treatment complications and high pregnancy rates[86]. There was also a trend to a reduced need for fertility treatment in women attempting pregnancy within 3 years of bariatric surgery compared to their need for fertility treatment prior to surgery[87] and reports show previously anovulatory women conceiving post-operatively without ovulation induction agents[30,76,88,89], and with in vitro fertilization[88]. Caution must be taken in these women during in vitro fertilization as ovarian hyperstimulation syndrome may induce ascites and intra-abdominal pressure which can lead to complications of the bariatric procedure[86]. Another group also showed unexpected spontaneous pregnancies soon after surgery in women with previous primary infertility[90], highlighting the need to discuss contraception in the post-operative period in all reproductive age patients. Do PCOS women with MS have safe pregnancy outcomes? Are their outcomes better than if they had not elected to undergo surgery? Most data show that pregnancy after bariatric surgery is safe[87,91-93]. Comparisons ideally need to be made to the general patient population and to PCOS women with MS who do not undergo surgery. Data from pregnant women who previously underwent surgery more than a decade ago show safe fetal outcomes. Compared to all other deliveries at the same institution, women had comparable rates of meconium, apgar scores, congenital malformations, fetal anemia and perinatal mortality. Of 298 deliveries to women post-bariatric surgery compared to the general population, there was a higher incidence of macrosomia, infertility, intra-uterine growth restriction, hypertension, diabetes mellitus and cesarean delivery. However, these women were still on average obese and obesity is known to adversely increase all these outcomes[93]. A subsequent study compared outcomes in similar women who had pregnancies before and after bariatric surgery. The incidence of gestational diabetes (OR 0.23, 95% CI: 0.15 to 0.36) and cesarean delivery (OR 0.53, 95% CI: 0.39 to 0.72) were drastically decreased after bariatric surgery. Additionally, bariatric surgery did not increase rates of post-partum hemorrhage, infection, shoulder dystocia or fetal demise[91]. A similar study pairing pregnancy outcomes in 288 pregnancies to women before and after bariatric surgery showed improvements in pregnancy induced hypertension and diabetes mellitus and a decrease in cesarean delivery rate following bariatric surgery. The length of labor decreased as well as neonatal birth weight. Throughout the study, only 8 women were admitted as a result of complications arising from their previous bariatric surgery - 3 for anemia, 3 for gastrointestinal complaints and 2 for gastric band slippage[87]. Another study comparing pregnancy outcomes in 70 women compared to matched controls found that bariatric surgery eliminated gestational diabetes but did not decrease hypertensive disorders in pregnancy. A secondary analysis demonstrated that women had a lower incidence of postpartum hemorrhage, postpartum endometritis and fetal macrosomia, but also had a slightly higher incidence of small for gestational age neonates[92]. Very little is known about long term outcome in children born to women after bariatric surgery. However, one study showed adult obesity was approximately 50% less among those children conceived after maternal surgery[94].
Even if we accept that pregnancy is safe or even safer after bariatric surgery, less is known about timing. Is there an optimal or even a minimal time after surgery that women need to wait until pregnancy? It has always been hypothesized that women should wait at least 12 mo after bariatric surgery before becoming pregnant in order to allow the rapid weight loss and metabolic changes to subside[19]. The most rapid weight gain occurs within 18 mo post-operatively[95]. Theoretical concerns exist due to nutritional and vitamin deficiencies in these women during that time frame of rapid weight loss[96]. An older study of LAGB showed 7 unexpected pregnancies within about 1 year of surgery and some within several months of surgery. Of those women, 5 had successful term deliveries and 2 had early miscarriage. However, 2 patients required reoperation during pregnancy for band complications[97]. Other studies have challenged these fears. Women with primary infertility who conceived naturally within 1 year of LAGB[90] and gastric bypass[98] also had comparable outcomes. A similar cohort of women followed after LAGB showed 20 pregnancies, which occurred on average 16 mo following surgery, had 2 complications attributed to surgery and otherwise improved pregnancy outcomes[88]. One hundred and four pregnancies were followed in women who became pregnant within 1 year (mean 7.0 mo) of bariatric surgery and they were compared to 385 pregnancies (age, BMI matched) conceived > 1 year (mean 56.7 mo) post-operatively. There were no differences in maternal complications (hypertension, gestational diabetes mellitus, anemia), fetal outcomes (intrauterine growth restriction, oligohydramnios, macrosomia, birthweight, apgars or perinatal mortality) or delivery complications (labor induction/augmentation, post-partum hemorrhage, preterm labor or operative delivery)[99]. Other smaller studies have found similar conclusions after LAGB[100], gastric bypass[101] and LRYGB[102]. While more data is needed, studies so far indicate that even a short surgery to pregnancy interval during the period of more rapid weight loss appears to be safe for mother and infant[99-103].
Overall, PCOS is highly prevalent and strongly associated with obesity and MS. Unfortunately, many women, especially adolescents, are not tested for metabolic abnormalities. These women have undiagnosed and potentially worsening MS for many years. Many PCOS women with obesity and/or MS develop coronary artery disease and glucose abnormalities at a very young age and are therefore at risk for life threatening cardiac events. Although women tend to seek medical care for menstrual irregularities and hirsutism, this encounter offers a chance for testing, education and risk prevention of MS. Lifestyle modification and treatment of risk factors are appropriate and even necessary for long term control. Bariatric surgery is a powerful tool that should not be overlooked simply because a woman is young or presents with PCOS and MS rather than diabetes mellitus, myocardial infarction and severe chronic hypertension. Although surgery has both short and long term risks, the potential benefits may be greater in these PCOS women than in older women who are already more advanced with respect to vascular disease. Every woman with PCOS and MS deserves to at least be offered education and counseling regarding the role of bariatric surgery in reducing their illness. More importantly, young women undergoing bariatric surgery should be specifically included in research to improve knowledge of long term outcomes. Bariatric surgery should be considered along with other medical and lifestyle alterations as first line therapy in PCOS women with obesity and MS.
Peer reviewers: Dr. Hanrui Zhang, Dalton Cardiovascular Research Center, University of Missouri, 134 Research Park Dr. Rm. 102, Columbia, MO 65203, United States; Undurti N Das, MD, FAMS, 13800 Fairhill Road, Shaker Heights, OH 44120, United States
S-Editor Wu X L- Editor Roemmele A E- Editor Wu X
| 1. | Garruti G, Depalo R, Vita MG, Lorusso F, Giampetruzzi F, Damato AB, Giorgino F. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod Biomed Online. 2009;19:552-563. [PubMed] [Cited in This Article: ] |
| 2. | Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4734] [Cited by in F6Publishing: 4424] [Article Influence: 316.0] [Reference Citation Analysis (0)] |
| 3. | Teede HJ, Hutchison S, Zoungas S, Meyer C. Insulin resistance, the metabolic syndrome, diabetes, and cardiovascular disease risk in women with PCOS. Endocrine. 2006;30:45-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 4. | Diamanti-Kandarakis E. Insulin resistance in PCOS. Endocrine. 2006;30:13-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Essah PA, Wickham EP, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50:205-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Cussons AJ, Stuckey BG, Watts GF. Metabolic syndrome and cardiometabolic risk in PCOS. Curr Diab Rep. 2007;7:66-73. [PubMed] [Cited in This Article: ] |
| 7. | Traub ML. Assessing and treating insulin resistance in women with polycystic ovarian syndrome. World J Diabetes. 2011;2:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:347-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 648] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 9. | McCullough AJ. Epidemiology of the metabolic syndrome in the USA. J Dig Dis. 2011;12:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356-359. [PubMed] [Cited in This Article: ] |
| 11. | Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 544] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 12. | Coviello AD, Sam S, Legro RS, Dunaif A. High prevalence of metabolic syndrome in first-degree male relatives of women with polycystic ovary syndrome is related to high rates of obesity. J Clin Endocrinol Metab. 2009;94:4361-4366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburú B, Codner E, Cassorla F, Rojas P, Sir-Petermann T. Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:1820-1826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Holte J, Gennarelli G, Berne C, Bergh T, Lithell H. Elevated ambulatory day-time blood pressure in women with polycystic ovary syndrome: a sign of a pre-hypertensive state? Hum Reprod. 1996;11:23-28. [PubMed] [Cited in This Article: ] |
| 15. | Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86:66-71. [PubMed] [Cited in This Article: ] |
| 16. | Dahlgren E, Johansson S, Lindstedt G, Knutsson F, Odén A, Janson PO, Mattson LA, Crona N, Lundberg PA. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril. 1992;57:505-513. [PubMed] [Cited in This Article: ] |
| 17. | Essah PA, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. J Endocrinol Invest. 2006;29:270-280. [PubMed] [Cited in This Article: ] |
| 18. | Macut D, Simic T, Lissounov A, Pljesa-Ercegovac M, Bozic I, Djukic T, Bjekic-Macut J, Matic M, Petakov M, Suvakov S. Insulin resistance in non-obese women with polycystic ovary syndrome: relation to byproducts of oxidative stress. Exp Clin Endocrinol Diabetes. 2011;119:451-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | American College of Obstetricians and Gynecologists. ACOG Committee Opinion number 315, September 2005. Obesity in pregnancy. Obstet Gynecol. 2005;106:671-675. [PubMed] [Cited in This Article: ] |
| 20. | Cortón M, Botella-Carretero JI, López JA, Camafeita E, San Millán JL, Escobar-Morreale HF, Peral B. Proteomic analysis of human omental adipose tissue in the polycystic ovary syndrome using two-dimensional difference gel electrophoresis and mass spectrometry. Hum Reprod. 2008;23:651-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | McGowan MP. Polycystic ovary syndrome: a common endocrine disorder and risk factor for vascular disease. Curr Treat Options Cardiovasc Med. 2011;13:289-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, Kuller LH. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20:2414-2421. [PubMed] [Cited in This Article: ] |
| 23. | Talbott EO, Zborowski JV, Rager JR, Boudreaux MY, Edmundowicz DA, Guzick DS. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5454-5461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Pfeifer SM, Kives S. Polycystic ovary syndrome in the adolescent. Obstet Gynecol Clin North Am. 2009;36:129-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, Stunkard AJ. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111-2120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 565] [Cited by in F6Publishing: 474] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 27. | Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, Miller ER, Dalcin A, Jerome GJ, Geller S. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959-1968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 579] [Cited by in F6Publishing: 525] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 28. | Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, Kumanyika S, Schmitz KH, Diewald LK, Barg R. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969-1979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 347] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 29. | Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT). J Am Coll Nutr. 2009;28:159-168. [PubMed] [Cited in This Article: ] |
| 30. | Fröhlich G, Pott W, Albayrak Ö, Hebebrand J, Pauli-Pott U. Conditions of long-term success in a lifestyle intervention for overweight and obese youths. Pediatrics. 2011;128:e779-e785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;CD007506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Padwal R, Klarenbach S, Wiebe N, Hazel M, Birch D, Karmali S, Sharma AM, Manns B, Tonelli M. Bariatric surgery: a systematic review of the clinical and economic evidence. J Gen Intern Med. 2011;26:1183-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009;CD003641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 34. | Garb J, Welch G, Zagarins S, Kuhn J, Romanelli J. Bariatric surgery for the treatment of morbid obesity: a meta-analysis of weight loss outcomes for laparoscopic adjustable gastric banding and laparoscopic gastric bypass. Obes Surg. 2009;19:1447-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 35. | Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121:885-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 36. | Dixon JB, O'Brien PE. Changes in comorbidities and improvements in quality of life after LAP-BAND placement. Am J Surg. 2002;184:51S-54S. [PubMed] [Cited in This Article: ] |
| 37. | Spivak H, Abdelmelek MF, Beltran OR, Ng AW, Kitahama S. Long-term outcomes of laparoscopic adjustable gastric banding and laparoscopic Roux-en-Y gastric bypass in the United States. Surg Endosc. 2012;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Tolonen P, Victorzon M, Mäkelä J. 11-year experience with laparoscopic adjustable gastric banding for morbid obesity--what happened to the first 123 patients? Obes Surg. 2008;18:251-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Saunders JK, Ballantyne GH, Belsley S, Stephens D, Trivedi A, Ewing DR, Iannace V, Capella RF, Wasielewski A, Moran S. 30-day readmission rates at a high volume bariatric surgery center: laparoscopic adjustable gastric banding, laparoscopic gastric bypass, and vertical banded gastroplasty-Roux-en-Y gastric bypass. Obes Surg. 2007;17:1171-1177. [PubMed] [Cited in This Article: ] |
| 40. | Shi X, Karmali S, Sharma AM, Birch DW. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20:1171-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 41. | Gill RS, Al-Adra DP, Birch D, Hudson M, Shi X, Sharma AM, Karmali S. Robotic-assisted bariatric surgery: a systematic review. Int J Med Robot. 2011;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248-256.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1816] [Cited by in F6Publishing: 1664] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 43. | Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab. 2009;35:518-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Lima MM, Pareja JC, Alegre SM, Geloneze SR, Kahn SE, Astiarraga BD, Chaim EA, Geloneze B. Acute effect of roux-en-y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3871-3875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Jankiewicz-Wika J, Kołomecki K, Cywiński J, Piestrzeniewicz K, Swiętosławski J, Stępień H, Komorowski J. Impact of vertical banded gastroplasty on body weight, insulin resistance, adipocytokine, inflammation and metabolic syndrome markers in morbidly obese patients. Endokrynol Pol. 2011;62:109-119. [PubMed] [Cited in This Article: ] |
| 46. | Woelnerhanssen B, Peterli R, Steinert RE, Peters T, Borbély Y, Beglinger C. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy--a prospective randomized trial. Surg Obes Relat Dis. 2011;7:561-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | Butner KL, Nickols-Richardson SM, Clark SF, Ramp WK, Herbert WG. A review of weight loss following Roux-en-Y gastric bypass vs restrictive bariatric surgery: impact on adiponectin and insulin. Obes Surg. 2010;20:559-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3301] [Cited by in F6Publishing: 2914] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 49. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5073] [Cited by in F6Publishing: 4544] [Article Influence: 227.2] [Reference Citation Analysis (0)] |
| 50. | Marantos G, Daskalakis M, Karkavitsas N, Matalliotakis I, Papadakis JA, Melissas J. Changes in metabolic profile and adipoinsular axis in morbidly obese premenopausal females treated with restrictive bariatric surgery. World J Surg. 2011;35:2022-2030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Hofsø D, Nordstrand N, Johnson LK, Karlsen TI, Hager H, Jenssen T, Bollerslev J, Godang K, Sandbu R, Røislien J. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. 2010;163:735-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 52. | Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millán JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005;90:6364-6369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 53. | Eid GM, Cottam DR, Velcu LM, Mattar SG, Korytkowski MT, Gosman G, Hindi P, Schauer PR. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:77-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Brancatisano A, Wahlroos S, Brancatisano R. Improvement in comorbid illness after placement of the Swedish Adjustable Gastric Band. Surg Obes Relat Dis. 2008;4:S39-S46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Wilson-Pérez HE, Seeley RJ. The effect of vertical sleeve gastrectomy on a rat model of polycystic ovarian syndrome. Endocrinology. 2011;152:3700-3705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Sundararaman PG, Manomani R, Sridhar GR, Sridhar V, Sundaravalli A, Umachander M. Risk of atherosclerosis in women with polycystic ovary syndrome: a study from South India. Metab Syndr Relat Disord. 2003;1:271-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Mak W, Dokras A. Polycystic ovarian syndrome and the risk of cardiovascular disease and thrombosis. Semin Thromb Hemost. 2009;35:613-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Durant N, Cox J. Current treatment approaches to overweight in adolescents. Curr Opin Pediatr. 2005;17:454-459. [PubMed] [Cited in This Article: ] |
| 59. | Inge TH, Krebs NF, Garcia VF, Skelton JA, Guice KS, Strauss RS, Albanese CT, Brandt ML, Hammer LD, Harmon CM. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217-223. [PubMed] [Cited in This Article: ] |
| 60. | Pratt JS, Lenders CM, Dionne EA, Hoppin AG, Hsu GL, Inge TH, Lawlor DF, Marino MF, Meyers AF, Rosenblum JL. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring). 2009;17:901-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 61. | Nemet D, Barkan S, Epstein Y, Friedland O, Kowen G, Eliakim A. Short- and long-term beneficial effects of a combined dietary-behavioral-physical activity intervention for the treatment of childhood obesity. Pediatrics. 2005;115:e443-e449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 62. | Kamath CC, Vickers KS, Ehrlich A, McGovern L, Johnson J, Singhal V, Paulo R, Hettinger A, Erwin PJ, Montori VM. Clinical review: behavioral interventions to prevent childhood obesity: a systematic review and metaanalyses of randomized trials. J Clin Endocrinol Metab. 2008;93:4606-4615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 63. | Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, Goldberg-Gell R, Burgert TS, Cali AM, Weiss R. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697-2704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 349] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 64. | Bondada S, Jen HC, Deugarte DA. Outcomes of bariatric surgery in adolescents. Curr Opin Pediatr. 2011;23:552-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Franco JV, Ruiz PA, Palermo M, Gagner M. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg. 2011;21:1458-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 66. | O'Brien PE, Sawyer SM, Laurie C, Brown WA, Skinner S, Veit F, Paul E, Burton PR, McGrice M, Anderson M. Laparoscopic adjustable gastric banding in severely obese adolescents: a randomized trial. JAMA. 2010;303:519-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 67. | Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140:347-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 314] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 68. | Patel SS, Carr BR. Oocyte quality in adult polycystic ovary syndrome. Semin Reprod Med. 2008;26:196-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 70. | Hartz AJ, Barboriak PN, Wong A, Katayama KP, Rimm AA. The association of obesity with infertility and related menstural abnormalities in women. Int J Obes. 1979;3:57-73. [PubMed] [Cited in This Article: ] |
| 71. | Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5:247-250. [PubMed] [Cited in This Article: ] |
| 72. | Nelson SM, Fleming R. Obesity and reproduction: impact and interventions. Curr Opin Obstet Gynecol. 2007;19:384-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Thompson JA, Regnault TR. In utero origins of adult insulin resistance and vascular dysfunction. Semin Reprod Med. 2011;29:211-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Gosman GG, King WC, Schrope B, Steffen KJ, Strain GW, Courcoulas AP, Flum DR, Pender JR, Simhan HN. Reproductive health of women electing bariatric surgery. Fertil Steril. 2010;94:1426-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr. 1988;7:147-153. [PubMed] [Cited in This Article: ] |
| 76. | Jamal M, Gunay Y, Capper A, Eid A, Heitshusen D, Samuel I. Roux-en-Y gastric bypass ameliorates polycystic ovary syndrome and dramatically improves conception rates: a 9-year analysis. Surg Obes Relat Dis. 2011;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Gerrits EG, Ceulemans R, van Hee R, Hendrickx L, Totté E. Contraceptive treatment after biliopancreatic diversion needs consensus. Obes Surg. 2003;13:378-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Bastounis EA, Karayiannakis AJ, Syrigos K, Zbar A, Makri GG, Alexiou D. Sex hormone changes in morbidly obese patients after vertical banded gastroplasty. Eur Surg Res. 1998;30:43-47. [PubMed] [Cited in This Article: ] |
| 79. | Victor A, Odlind V, Kral JG. Oral contraceptive absorption and sex hormone binding globulins in obese women: effects of jejunoileal bypass. Gastroenterol Clin North Am. 1987;16:483-491. [PubMed] [Cited in This Article: ] |
| 80. | Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, Zeitlian G, Hickmon C, Feng S, Santoro N. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril. 2009;92:1410-1415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594-1602. [PubMed] [Cited in This Article: ] |
| 82. | Chikunguwo S, Brethauer S, Nirujogi V, Pitt T, Udomsawaengsup S, Chand B, Schauer P. Influence of obesity and surgical weight loss on thyroid hormone levels. Surg Obes Relat Dis. 2007;3:631-635; discussion 631-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 83. | Moulin de Moraes CM, Mancini MC, de Melo ME, Figueiredo DA, Villares SM, Rascovski A, Zilberstein B, Halpern A. Prevalence of subclinical hypothyroidism in a morbidly obese population and improvement after weight loss induced by Roux-en-Y gastric bypass. Obes Surg. 2005;15:1287-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Bilenka B, Ben-Shlomo I, Cozacov C, Gold CH, Zohar S. Fertility, miscarriage and pregnancy after vertical banded gastroplasty operation for morbid obesity. Acta Obstet Gynecol Scand. 1995;74:42-44. [PubMed] [Cited in This Article: ] |
| 85. | Musella M, Milone M, Bellini M, Sosa Fernandez LM, Leongito M, Milone F. Effect of bariatric surgery on obesity-related infertility. Surg Obes Relat Dis. 2011;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 86. | Doblado MA, Lewkowksi BM, Odem RR, Jungheim ES. In vitro fertilization after bariatric surgery. Fertil Steril. 2010;94:2812-2814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Aricha-Tamir B, Weintraub AY, Levi I, Sheiner E. Downsizing pregnancy complications: a study of paired pregnancy outcomes before and after bariatric surgery. Surg Obes Relat Dis. 2011;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Dixon JB, Dixon ME, O'Brien PE. Pregnancy after Lap-Band surgery: management of the band to achieve healthy weight outcomes. Obes Surg. 2001;11:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 89. | Marceau P, Kaufman D, Biron S, Hould FS, Lebel S, Marceau S, Kral JG. Outcome of pregnancies after biliopancreatic diversion. Obes Surg. 2004;14:318-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 90. | Martin LF, Finigan KM, Nolan TE. Pregnancy after adjustable gastric banding. Obstet Gynecol. 2000;95:927-930. [PubMed] [Cited in This Article: ] |
| 91. | Burke AE, Bennett WL, Jamshidi RM, Gilson MM, Clark JM, Segal JB, Shore AD, Magnuson TH, Dominici F, Wu AW. Reduced incidence of gestational diabetes with bariatric surgery. J Am Coll Surg. 2010;211:169-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Lesko J, Peaceman A. Pregnancy outcomes in women after bariatric surgery compared with obese and morbidly obese controls. Obstet Gynecol. 2012;119:547-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 93. | Sheiner E, Levy A, Silverberg D, Menes TS, Levy I, Katz M, Mazor M. Pregnancy after bariatric surgery is not associated with adverse perinatal outcome. Am J Obstet Gynecol. 2004;190:1335-1340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 94. | Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, Marceau P. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118:e1644-e1649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 95. | Wax JR, Cartin A, Wolff R, Lepich S, Pinette MG, Blackstone J. Pregnancy following gastric bypass for morbid obesity: effect of surgery-to-conception interval on maternal and neonatal outcomes. Obes Surg. 2008;18:1517-1521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33:13-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 97. | Weiss HG, Nehoda H, Labeck B, Hourmont K, Marth C, Aigner F. Pregnancies after adjustable gastric banding. Obes Surg. 2001;11:303-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Rand CS, Macgregor AM. Medical care and pregnancy outcome after gastric bypass surgery for obesity. South Med J. 1989;82:1319-1320. [PubMed] [Cited in This Article: ] |
| 99. | Sheiner E, Edri A, Balaban E, Levi I, Aricha-Tamir B. Pregnancy outcome of patients who conceive during or after the first year following bariatric surgery. Am J Obstet Gynecol. 2011;204:50.e1-50.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 100. | Dixon JB, Dixon ME, O'Brien PE. Birth outcomes in obese women after laparoscopic adjustable gastric banding. Obstet Gynecol. 2005;106:965-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 101. | Dao T, Kuhn J, Ehmer D, Fisher T, McCarty T. Pregnancy outcomes after gastric-bypass surgery. Am J Surg. 2006;192:762-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Patel JA, Patel NA, Thomas RL, Nelms JK, Colella JJ. Pregnancy outcomes after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 103. | Sheiner E, Balaban E, Dreiher J, Levi I, Levy A. Pregnancy outcome in patients following different types of bariatric surgeries. Obes Surg. 2009;19:1286-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |