Published online Apr 15, 2018. doi: 10.4239/wjd.v9.i4.66
Peer-review started: March 20, 2018
First decision: April 2, 2018
Revised: April 9, 2018
Accepted: April 12, 2018
Article in press: April 12, 2018
Published online: April 15, 2018
The so-called “metabolic syndrome” (MS), constitutes a cluster of metabolic and cardiovascular abnormalities, including fasting glucose, blood pressure, triglycerides, high density lipoprotein cholesterol (HDL-C), and waist circumference that arise from insulin resistance. Obstructive sleep apnea (OSA) syndrome is characterized by recurrent episodes of partial or complete obstruction of the upper airway, involving cessation or significant decreased airflow, with intermittent hypoxemia, frequent arousals from sleep and recurrent oxyhemoglobin desaturations that interfere with normal sleep patterns generating difficulty falling asleep, unrefreshing sleep and loud snoring. The relation between these two entities is known as “Syndrome Z”, and there is no question about the impact of these risk factors on health and disease. This clinical condition presents a growing epidemic Worldwide, affecting approximately 60% of the general population with both MS and OSA due to the constant increase of body mass index in humans. This article presents evidence-based data that focuses on the direct relationship between MS and OSA.
Core tip: Obstructive sleep apnea (OSA), has been tightly-related to several components of metabolic syndrome (MS). However, most of the evidence documented has only evaluated individual components of the MS, or patients with a diagnosis of OSA.
- Citation: Castaneda A, Jauregui-Maldonado E, Ratnani I, Varon J, Surani S. Correlation between metabolic syndrome and sleep apnea. World J Diabetes 2018; 9(4): 66-71
- URL: https://www.wjgnet.com/1948-9358/full/v9/i4/66.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i4.66
The term “metabolic syndrome” (MS) dates back to 1977, when Haller used it to describe the association between hypertension, dyslipidemia, obesity, and disturbed glucose metabolism[1]. He demonstrated how the presence of multiple of these factors increased the risk of developing cardiovascular disease[1]. Phillips, in 1978 suggested that the combination of risk factors not only predisposed to heart disease, but were also related with an increased risk for obesity[2]. These constellation of abnormalities included glucose intolerance, hyperinsulinemia and a high level of triglycerides, glucose, cholesterol and insulin[2]. In the late 1980’s, Gerald Reaven hypothesized that insulin resistance could be the underlying factor, linking these constellations of abnormalities, which he named “Syndrome X” or also known as Metabolic X-syndrome, which is currently known as the MS[3].
The MS remains a global epidemiological challenge[4,5]. This is due to the exponential increase in body mass index (BMI) in humans as the result of an increase in caloric intake, increase in obesity percentage, and increased sedentary life habits[6]. This clinical entity has a cluster of risk factors such as hypertension, central obesity, increased triglycerides, decreased high density lipoprotein cholesterol (HDL-C), increased blood glucose and insulin resistance[7].
Taking in consideration the established guidelines by the World Health Organization (WHO) as a reference to determine the best criteria for diagnosis, specificity and sensitivity between International Diabetes Federation (IDF), revised National Cholesterol Education Program (NCEP-R), NCEP Adult Treatment Panel (ATP)-III, and American Association of Clinical Endocrinologists (AACE) were evaluated, IDF criteria obtained the best overall specificity of 81.3% and sensitivity of 71.2% for proper diagnosis[8].
The parameters set for diagnosis include fasting glucose ≥ 100 mg/dL (or receiving drug therapy for hyperglycemia), blood pressure ≥ 130/85 mmHg (or receiving drug therapy for hypertension), triglycerides ≥ 150 mg/dL (or receiving drug therapy for hypertriglyceridemia), HDL-C < 40 mg/dL in men or < 50 mg/dL in women (or receiving drug therapy for reduced HDL-C) and waist circumference ≥ 94 cm in men or ≥ 80 cm in women (see Table 1)[8].
| Factor | IDF |
| Hypertension | Current antihypertensive therapy or BP ≥ 130/85 mmHg |
| Dyslipidemia - elevated triglycerides | Plasma triglycerides ≥ 150 mg/dL or specific treatment for high triglycerides |
| Dyslipidemia - depressed HDL | HDL < 40 mg/dL in men or < 50 mg/dL in women or specific treatment for low HDL |
| Obesity | Waist circumference > 37 inches in men or > 31.5 inches in women |
| Glucose | Fasting glucose ≥ 100 mg/dL or previously diagnosed type 2 diabetes |
| Requirements for diagnosis | Waist circumference criteria plus any 2 of other criteria. |
| No OSA | AHI < 5/h |
| Mild | AHI 5-14/h |
| Moderate | AHI 15-30/h |
| Severe | AHI > 30/h |
In the vast majority of cases with MS, development of clinical manifestations such as cardiovascular disease, diabetes mellitus type 2 and obstructive sleep apnea (OSA) are known to manifest[7,9,10].
The disorder known as OSA is not new by any means, and it is becoming more prominent due to the increase in obesity over the past few decades[11]. Of historical interest, sleep apnea and snoring have been documented since 4000 BC[12]. Charles Dickens in the 19th century wrote the “Pickwickian Papers”, where one of the characters was “Joe, the fat boy” stating he was always asleep and snoring even on the simplest task such as errands and waiting for a table, leading way to the term “Pickwickian syndrome” in the late 19th century as apneic symptoms[13,14]. Finally, by mid-1960’s, Gastaut, and associates, formally introduced the term “Sleep apnea”, when a closure of the upper airways in obese patients was found, providing the first documented relation between obesity, sleep-induced airway obstruction, sleep fragmentation, and daytime sleepiness, leading way to what is now known as “OSA”[15,16].
These repetitive partial or, in certain advanced cases complete obstructive episodes of the upper airway during periods of sleep, are characterized by cessation of breathing for at least 10 s per minute resulting in hypopneic scenarios leading to apnea[7,9,10]. Grading of OSA is based on the apnea-hypopnea index (AHI), which is defined as number of apnea and hypopnea episode per hour of sleep[17]. Mild OSA has an AHI of 5-14/h, moderate OSA 15-30/h AHI, and severe OSA has an AHI of > 30/h[18,19] (Table 1).
Some have suggested that the MS should include OSA, as part of its clinical manifestations, and should be called “Z syndrome”[7,18]. A study by Xu et al[19] established an independent relationship between these two clinical entities.
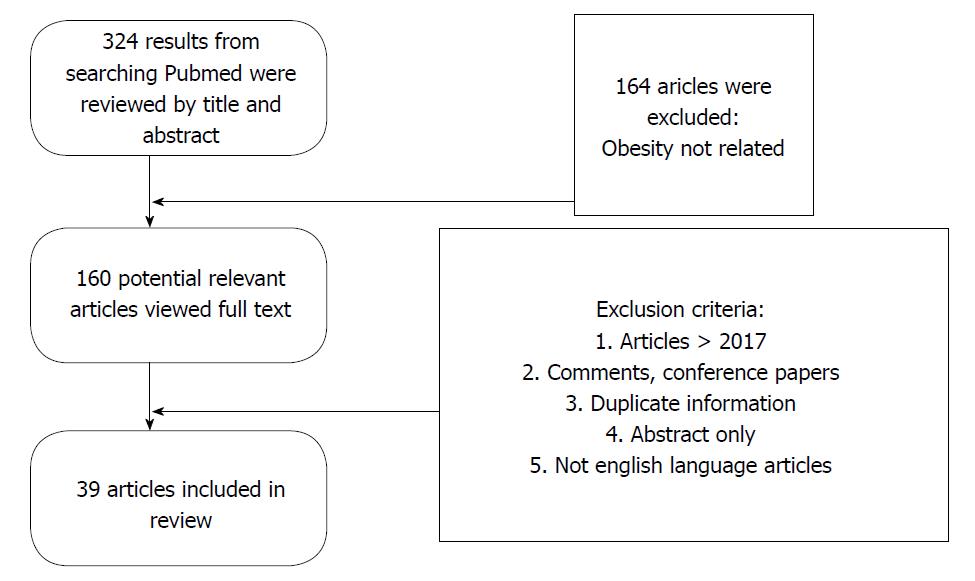
Guidelines for the review were followed[19,20]. The authors independently searched an electronic database (PubMed) using MeSH with the following terms “sleep apnea, obstructive sleep apnea” and “MS” to pinpoint articles up to the year 2017 excluding 2018, which were relevant to determine the correlation between MS and OSA, using the following medical subject headings and keywords: Metabolic syndrome, OSA. Overall, 321 articles were identified, and 39 studies were finally included in this review (Figure 1). All the included article reviewed were full text. Articles not related with obesity, MS, abstract only, duplicate information, comments and conference papers were excluded. Only studies in English language were included with a purpose of universal understanding. The acquired data from all qualified articles were later discussed between authors and any disagreements were resolved.
The prevalence of the MS Worldwide is estimated to be between < 10% to as much as 84%, finding a certain correlation with developed countries, yet depends on various factors such as socioeconomic status, lifestyle, BMI, and region studied[6,21]. Study by Khosravi-Boroujeni et al[22] showed the prevalence of MS have changed from 2001-2013. They also mentioned that prevalence of diabetes has also been increasing over the years. This has been attributed to aging, life style changes, population growth, obesity and decline in physical activity. Central obesity was labelled as a critical component of the MS. The prevalence of the hypertriglyceridemia also declined, due to use of the statins, healthy eating with cutting back on trans-fat and aggressive screenings[22].
Prevalence estimates for OSA range between 4%-24% for men and 2%-16% for women making it slightly higher amongst men, as well as most common after the age of 40 and during the postmenopausal period for women[23]. This prevalence increases for all who have risk factors, such as obesity and diabetes ranking it a significant health care challenge for population worldwide, with the odds as high as nearly double that of normal-weight adults[24]. While examining data, the relationship between MS and OSA was found to coexist up to 60% of cases[25].
There is significant evidence of the correlation between MS and OSA. It is clear that OSA independently leads to insulin resistance, a component of MS[26]. There is evidence that there is lower insulin sensitivity, and a higher fasting insulin level in these patients[27]. In these patients, there is an increase in epinephrine, norepinephrine and/or cortisol leading to an increase in gluconeogenesis and a decrease in skeletal muscle uptake of glucose[28]. In addition, there is a concomitant elevation in systemic inflammatory markers[29-31]. Examples of these are tumor necrosis factor alfa (TNF-α) and interleukin six (IL-6), both of which are independent of obesity and are dependent on OSA[20]. C-reactive protein (CRP) levels are also elevated, and these directly correlates with the elevated TNF-α and IL-6, leading to insulin resistance[26,30,31]. Furthermore, it is thought obesity and OSA have a synergistic, negative effect over glucose metabolism[27,32]. Intermittent hypoxemia has been shown to produce beta cell dysfunction as well as insulin resistance[32].
As noted above, OSA intermittent hypoxemia causes sympathetic excitation, decreasing insulin sensitivity, glucose uptake and stimulates hepatic gluconeogenesis. Moreover, there is activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), with subsequent production of pro-inflammatory mediators such as TNF-α, IL-6, and interleukin eight (IL-8)[32]. Therefore, OSA is linked to inflammation-mediated reaction with sympathetic signaling and oxidative stress, all led by NF-κB[33]. Obesity, on the other hand, leads to hypertrophied, dysfunctional adipocytes[34]. These adipocytes attract cytotoxic T cells (CD8+ T) as well as macrophages with the latter producing pro-inflammatory cytokines like IL-6, TNF-α and inducible nitric oxide synthase (iNOS)[33,34]. There is a release of free fatty acids leading impairment of insulin-signaling pathway, insulin resistance and metabolic consequences. Obesity has adipose tissue mediating pro-inflammatory reactions with compromised vascularity via hypoxemia-derived pathway[34]. Here, the hypoxia-inducible factor 1 alfa is the moderator by increasing expression of pro-inflammatory adipocyte genes mediated by NF-κB gene promoters[32]. What appears to be interesting is that OSA may lead to MS signs and symptoms with the intermittent hypoxemia causing visceral adipose tissue inflammation in rodents or reductionist models without presence of obesity[32].
OSA has shown to lead to early atherosclerosis by causing endothelial failure. In a study by Ciconne, 40 healthy patients were compared against 80 patients with OSA. Of the affected, 26 had mild OSA and 54 had severe OSA. The patients’ demographics such as age, BMI, and neck circumference were comparable among the control group and both OSA groups. The authors hypothesized various episodes of hypoxia and re-oxygenation along with sleep deprivation would lead to systemic inflammation. They measured inflammation markers in venous blood. Patient with OSA had increased carotid intimal thickening, C-reactive protein (CRP), interleukin (IL-6), tumor necrosis factor (TNF-α) and pentraxin (PTX-3). They found CRP which promotes adhesion molecule expression and opsonizes LDL for reuptake by the macrophages within the plaque. IL-6 stimulated the production of CRP and serum amyloid A; the latter reduces HDL-C content. TNF-α stimulates monocytic adhesion to the endothelial surface with infiltration through the vascular wall, and finally their conversion to macrophages; it is also the cause of multiple atherogenic cytokine production. PTX-3 was seen to be elevated in both OSA groups. While PTX-3’s role in the pathophysiology of OSA is not clear yet, it is considered as a specific marker for endothelial inflammation[35].
In a study by Zychowski et al[36], human coronary endothelial cells were incubated with 5% serum, of control and OSA patients for 4 h. They then, performed qPCR to evaluate for endothelial inflammatory markers. OSA serum induced much more endothelial cell expression of VCAM1, ICAM1, IL8, SELP and CCL5 mRNA. This suggested another molecular role of endothelial activation and dysfunction in patients with OSA[36].
As the estimated prevalence of OSA is 2%-3% in healthy children and between 13% and 66% among obese adolescents, it is justified to screen for it[26]. Children with OSA had reportedly 6.49 times increased odds of developing MS when compared with children without OSA[34]. The principal predictor is hypoxemia. Consideration should be given to screening of obese children with MS for OSA since their outcomes are modifiable by lifestyle changes[34].
An excess in weight is more crucial for OSA than either age or gender[37,38]. For every percent in weight reduction, there is a 3% reduction in the AHI[27,39]. Increased waist diameter correlates with an increased incidence of OSA[40]. Obesity is an important factor, more importantly, central obesity. Central obesity is linked to higher leptin production with resistance to said hormone and leading to increase probability of developing OSA. Metabolic abnormalities increase the chance of upper airway collapsibility. Neck circumference is a better predictor of OSA than general obesity. Central obesity impact is greater on the upper airway function when compared to peripheral obesity, as stated earlier[40,41]. Patients with obesity and OSA have approximately a 67% more total neck fat compared to the normal person. This leads to a smaller upper airway area and greater compression on said airway while sleeping. Central obesity is more closely related to fat depositions in the neck, unlike peripheral obesity. This leads to a more notable narrowing of the upper airway while asleep[38,42].
The presence of MS may be the trigger to the development of OSA. This is important, since it is now known that the coexistence of both pathologies within the same patient raises biomarkers, which directly cause or at least raise potential for complications. Detecting MS and OSA is very important. Similarly, known diabetic patients should be screened for OSA and complications could be avoided. Adequate treatment of OSA can help decrease insulin resistance. It is imperative for the clinicians to keep themselves updated with the recent changes in science which can translate in combating and preventing the progression of disease.
There are more pathophysiological theories of how OSA may have a synergistic negative effect with MS that is yet to be made clear. From a direct effect of OSA over the hypothalamic-pituitary-adrenal axis, adrenal medulectomy trials as a possible therapeutic procedure, or a theory of OSA affecting the microbiota of the gastrointestinal tract leading to the development or worsening of MS. The current studies are being performed in mice, but in a near future may lead to a better understanding of the relationship between OSA and MS and possibly to improved patient management.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Ciccone MM, Micheu MM S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Haller H. Epidermiology and associated risk factors of hyperlipoproteinemia. Z Gesamte Inn Med. 1977;32:124-128. [PubMed] [Cited in This Article: ] |
| 2. | Phillips GB. Sex hormones, risk factors and cardiovascular disease. Am J Med. 1978;65:7-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2634] [Cited by in F6Publishing: 2268] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 4. | Alam MF, Nasreen S, Ullah E, Hussain A. The awareness and prevalence of metabolic syndrome in medical community of bahawalpur. Oman Med J. 2011;26:26-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Moreira GC, Cipullo JP, Ciorlia LA, Cesarino CB, Vilela-Martin JF. Prevalence of metabolic syndrome: association with risk factors and cardiovascular complications in an urban population. PLoS One. 2014;9:e105056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Thounaojam MC, Nammi S, Jadeja R. Natural Products for the Treatment of Obesity, Metabolic Syndrome, and Type 2 Diabetes 2016. Evid Based Complement Alternat Med. 2016;2016:9072345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kostoglou-Athanassiou I, Athanassiou P. Metabolic syndrome and sleep apnea. Hippokratia. 2008;12:81-86. [PubMed] [Cited in This Article: ] |
| 8. | Onesi SO, Ignatius UE. Metabolic syndrome: Performance of five different diagnostic criterias. Indian J Endocrinol Metab. 2014;18:496-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Drager LF, Lopes HF, Maki-Nunes C, Trombetta IC, Toschi-Dias E, Alves MJ, Fraga RF, Jun JC, Negrão CE, Krieger EM. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5:e12065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Calvin AD, Albuquerque FN, Lopez-Jimenez F, Somers VK. Obstructive sleep apnea, inflammation, and the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Gibson GJ. Obstructive sleep apnoea syndrome: underestimated and undertreated. Br Med Bull. 2005;72:49-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Asaad T. Sleep in Ancient Egypt. Cairo Egypt: Springer Science 2015; 13-19. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Littleton SW, Mokhlesi B. The pickwickian syndrome-obesity hypoventilation syndrome. Clin Chest Med. 2009;30:467-478, vii-viii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Bickelmann AG, Burwell CS, Robin ED, Whaley RD. Extreme obesity associated with alveolar hypoventilation; a Pickwickian syndrome. Am J Med. 1956;21:811-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 578] [Cited by in F6Publishing: 599] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1427] [Cited by in F6Publishing: 1272] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 16. | Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res. 1966;1:167-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 263] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Wang Z, Zhou X, Zhao W, Liu F, Ni H, Yu Z. Assessing the severity of sleep apnea syndrome based on ballistocardiogram. PLoS One. 2017;12:e0175351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Hudgel DW. Sleep Apnea Severity Classification - Revisited. Sleep. 2016;39:1165-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Xu S, Wan Y, Xu M, Ming J, Xing Y, An F, Ji Q. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulm Med. 2015;15:105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 1053] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 22. | Khosravi-Boroujeni H, Sarrafzadegan N, Sadeghi M, Roohafza H, Talaei M, Ng SK, Phung H, Pourmogaddas A, Ahmed F. Secular Trend of Metabolic Syndrome and Its Components in a Cohort of Iranian Adults from 2001 to 2013. Metab Syndr Relat Disord. 2017;15:137-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Cizza G, de Jonge L, Piaggi P, Mattingly M, Zhao X, Lucassen E, Rother KI, Sumner AE, Csako G; NIDDK Sleep Extension Study. Neck circumference is a predictor of metabolic syndrome and obstructive sleep apnea in short-sleeping obese men and women. Metab Syndr Relat Disord. 2014;12:231-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 441] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 25. | Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472. [PubMed] [Cited in This Article: ] |
| 26. | Nannapaneni S, Ramar K, Surani S. Effect of obstructive sleep apnea on type 2 diabetes mellitus: A comprehensive literature review. World J Diabetes. 2013;4:238-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 59] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 822] [Cited by in F6Publishing: 776] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 28. | Trombetta IC, Maki-Nunes C, Toschi-Dias E, Alves MJ, Rondon MU, Cepeda FX, Drager LF, Braga AM, Lorenzi-Filho G, Negrao CE. Obstructive sleep apnea is associated with increased chemoreflex sensitivity in patients with metabolic syndrome. Sleep. 2013;36:41-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Khoo MC, Oliveira FM, Cheng L. Understanding the metabolic syndrome: a modeling perspective. IEEE Rev Biomed Eng. 2013;6:143-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1662] [Cited by in F6Publishing: 1613] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 32. | Ryan S. Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnoea and metabolic dysfunction. J Physiol. 2017;595:2423-2430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 33. | Gileles-Hillel A, Almendros I, Khalyfa A, Nigdelioglu R, Qiao Z, Hamanaka RB, Mutlu GM, Akbarpour M, Gozal D. Prolonged Exposures to Intermittent Hypoxia Promote Visceral White Adipose Tissue Inflammation in a Murine Model of Severe Sleep Apnea: Effect of Normoxic Recovery. Sleep. 2017;40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Gami AS, Caples SM, Somers VK. Obesity and obstructive sleep apnea. Endocrinol Metab Clin North Am. 2003;32:869-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Ciccone MM, Scicchitano P, Zito A, Cortese F, Boninfante B, Falcone VA, Quaranta VN, Ventura VA, Zucano A, Di Serio F. Correlation between inflammatory markers of atherosclerosis and carotid intima-media thickness in Obstructive Sleep Apnea. Molecules. 2014;19:1651-1662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Zychowski KE, Sanchez B, Pedrosa RP, Lorenzi-Filho G, Drager LF, Polotsky VY, Campen MJ. Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis. 2016;254:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Jalilolghadr S, Yazdi Z, Mahram M, Babaei F, Esmailzadehha N, Nozari H, Saffari F. Sleep architecture and obstructive sleep apnea in obese children with and without metabolic syndrome: a case control study. Sleep Breath. 2016;20:845-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Mortimore IL, Marshall I, Wraith PK, Sellar RJ, Douglas NJ. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 192] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax. 1992;47:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 261] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol (1985). 2005;99:1592-1599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 463] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 41. | Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 563] [Cited by in F6Publishing: 520] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 42. | Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol (1985). 2005;99:2440-2450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |