Published online Mar 27, 2018. doi: 10.4240/wjgs.v10.i3.28
Peer-review started: November 22, 2017
First decision: December 21, 2017
Revised: January 30, 2018
Accepted: February 9, 2018
Article in press: February 9, 2018
Published online: March 27, 2018
The gold standard for curative treatment of locally advanced rectal cancer involves radical resection with a total mesorectal excision (TME). TME is the most effective treatment strategy to reduce local recurrence and improve survival outcomes regardless of the surgical platform used. However, there are associated morbidities, functional consequences, and quality of life (QoL) issues associated with TME; these risks must be considered during the modern-day multidisciplinary treatment for rectal cancer. This has led to the development of new surgical techniques to improve patient, oncologic, and QoL outcomes. In this work, we review the evolution of TME to the transanal total mesorectal excision (TaTME) through more traditional minimally invasive platforms. The review the development, safety and feasibility, proposed benefits and risks of the procedure, implementation and education models, and future direction for research and implementation of the TaTME in colorectal surgery. While satisfactory short-term results have been reported, the procedure is in its infancy, and long term outcomes and definitive results from controlled trials are pending. As evidence for safety and feasibility accumulates, structured training programs to standardize teaching, training, and safe expansion will aid the safe spread of the TaTME.
Core tip: The evaluation and management of rectal cancer have evolved remarkably over the last few decades. Total mesorectal excision (TME) has been recognized as the standard surgical management for curative radical treatment of rectal cancer. While abdominal procedures, whether by the open or minimally invasive approaches, apply the classical concept of “top-to-bottom” dissection, the transanal TME (TaTME) uses the opposite approach of “bottom-to-top” dissection. In this review we discuss the evolution of TME for rectal cancer to the TaTME, its technical aspects, advantages, shortcomings, and current needs. The research and education initiatives as well as future directions of TaTME were also highlighted.
- Citation: Emile SH, de Lacy FB, Keller DS, Martin-Perez B, Alrawi S, Lacy AM, Chand M. Evolution of transanal total mesorectal excision for rectal cancer: From top to bottom. World J Gastrointest Surg 2018; 10(3): 28-39
- URL: https://www.wjgnet.com/1948-9366/full/v10/i3/28.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v10.i3.28
Despite the current multidisciplinary modern management, rectal cancer remains a formidable challenge for the colorectal surgeon. Surgical therapy for rectal cancer has evolved since Dr. Ernest Miles described the abdominoperineal resection in 1908. With this radical resection and the realization that rectal cancer must be tackled from the both abdomen and perineum, Miles reduced the local recurrence rate from nearly 100% to 30%[1]. Defining the “zone of upward spread” he introduced the concept of surgical oncology whereby the tumor, blood supply and nodal tissue needed to be excised.
With better surgical tools enabling a low anastomosis, a shift toward sphincter-saving approaches began, with the anterior resection replacing abdominoperineal resection as the standard curative resection, when possible. These approaches resulted in poor oncologic outcomes for recurrence and overall survival. Technical advancement came to light in 1982, when Heald et al[2] published the total mesorectal excision (TME) technique. The TME entails sharp, nerve-sparing dissection in the avascular plane between the mesorectum and surrounding structures circumferentially. A complete TME with intact fascia and no invasion into the muscular coat or mucosa is an important, positive prognosticator against locoregional tumor recurrence[3].
TME became the gold standard for curative resection from proven better local control and survival[4]. Neoadjuvant and adjuvant chemotherapy and radiotherapy serve as adjuvants to improve the outcome after surgery; the dose and timing of these adjuncts are variable based on the disease stage and patient-related factors[5-14]. However, these adjuncts are not a substitute for a proper TME, with poor surgery yielding an inadequate surgical specimen invariably leading to local recurrence[15]. Additional evidence from the Medical Research Council of United Kingdom CR07 and National Cancer Institute of Canada-CTG CO16 (CR07) trial highlighted the importance of good quality surgery, and how inadequate surgery can be only minimally compensated for by chemoradiotherapy[3,16]. In the early 1990’s, laparoscopic surgery was introduced, and gradually become applied to colon and rectal cancer. While there were initial concerns about the oncological safety of laparoscopy, the Clinical Outcomes of Surgical Therapy (COST) Trial demonstrated the safety, oncologic equivalency, and clinical benefits over open surgery[17]. Abundant support has reported comparable oncologic outcomes and improves short-term benefits of laparoscopic over open surgery for rectal cancer[3,18-21]. The safety of laparoscopy for rectal cancer was less clearly defined initially, as early controlled trials concentrated on the oncologic safety of colon cancer[17,20]. While skepticism remained, the improved outcomes with TME were shown to be generalizable in both open and minimally invasive approaches[3,6,22-28]. Then recent studies further questioned the oncologic equivalence of the laparoscopic approach for rectal cancer. The ALaCaRT and ACOSOG Z6051 trials failed to establish the non-inferiority of laparoscopy compared to open rectal cancer surgery[29,30]. The authors of ALaCaRT recommended using a different platform in low rectal cancers than pure abdominal laparoscopy, as working in the deep pelvis with rigid, straight laparoscopic instruments from difficult angles was challenging and required complex maneuvers[29]. Technical limitations exist with the laparoscopic approach, especially during the distal transection of the rectum, due to limited visualization and restriction working in the confined, bony pelvis[31]. These limitations highlighted the need for other approaches to rectal cancer. Robotic assisted surgery was introduced to address the limitations of laparoscopy, and gained acceptance from the improved visualization, lower conversion rates, better TME quality lower positive CRM rate, and earlier recovery of genitourinary functions[32-34]. Studies reported equivalent oncologic and functional outcomes of both approaches, which raise the issue about the cost-effectiveness of the robotic platform, and the need for more effective and cost-efficient platforms[35-37].
For this review, three of the authors reviewed published data regarding rectal cancer surgery, with attention to surgical techniques over the last several decades leading to the transanal TME. With the defined focus, PubMed and MEDLINE databases and the #colorectal research hashtag on Twitter were searched from database inception through September 15, 2017 for articles and data published with relevant evidence regarding the evolution of surgery for rectal cancer. The following search terms were used: “total mesorectal excision”, “transanal excision”, “local excision”, “laparoscopic colorectal surgery”, and “transanal total mesorectal excision”, “TaTME”, “rectal carcinoma” and “rectal cancer”. Reference lists were manually searched and relevant articles were added if pertinent to the scope of the study. Articles were included if in English and the full content was available. Conference proceedings and videos were not included.
Despite significant advances in technology and use of minimally invasive approaches in many other surgical disciplines, open surgery remains the gold standard for rectal cancer. Technical challenges and subsequent low uptake of laparoscopy in low rectal cancer surgery and contention on the value of robotic platforms have left the door open for a new approach. An ideal approach would involve a short learning curve, low relative cost, reproducibility and clear evidence of patient safety.
To leverage the benefits of a minimally invasive approach, intraluminal, endoscopic, transanal, and hybrid techniques have been expanded in recent years. Additional desire to improve not only oncological outcomes but also function and quality of life outcomes led to investigation of local excision techniques[38,39]. While local excision has improved functional outcomes compared with radical resection, the lack of lymphadenectomy and higher rates of positive resection margins, locoregional recurrence, and lower overall survival means that it may not be directly comparable to TME in terms of oncological outcomes[38-45]. Therefore, it is currently recommended for benign and early (T1) rectal lesions, unless on clinical trial[46,47]. With these outcomes, it was necessary to develop more precise methods for local excision[48].
Advanced endoscopic platforms, combining the transanal and minimally invasive approaches addressed the limitations of conventional transanal resections, and allowed precise dissection of low and mid rectal tumors, a limitation of other platforms to date.
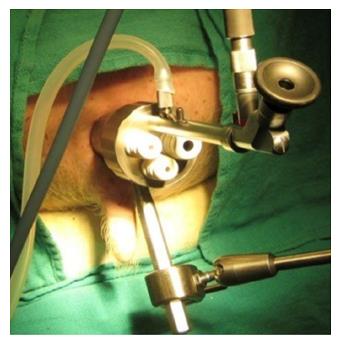
In 1983, Dr. Gerhard Buess developed Transanal Endoscopic Microsurgery (TEM), (Figure 1), offering improved visualization from a stereoscopic magnified view in the gas-dilated rectum for precise excision in an operative space that would be otherwise difficult to reach, as well as significantly lower morbidity, lower local recurrence rates, with a higher rate of negative resection margins than traditional TAE[49-52]. Widespread adoption was limited due to the cost of the specialized instrumentation, additional learning curve, and limited indications for the technique[53-56].
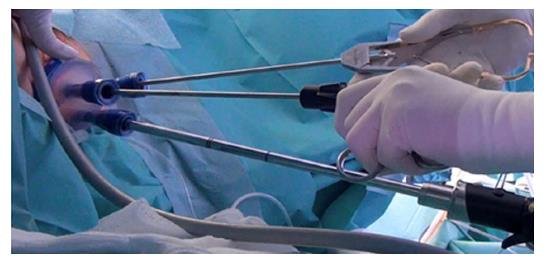
Dr. Sam Atallah introduced Transanal Minimally Invasive Surgery (TAMIS), (Figure 2) as an alternate advanced videoscopic transanal platform that also combines minimally invasive benefits with transanal resection, but addresses some limitations of TEM[55]. The same superior visualization and reach of TEM is offered but using standard laparoscopic equipment reduces the cost and learning curve[55,57,58]. The TAMIS platform may also be less traumatic to the anal sphincter than TEM[57]. A recent systematic review described low conversion rate of 2.3%, and low rates of positive margins, tumor fragmentation, and overall complications of 4.36%, 4.1% and 7.4%, respectively[54]. Both TEM and TAMIS have limitations in patient selection, lack of adequate lymphadenectomy inability to adequately stage the pelvis, and prohibitively high recurrence rates with T2 and more advanced rectal tumors[55,59-62]. TEM and TAMIS remain important in the evolution of the TaTME platform.
Natural orifice transluminal endoscopic surgery (NOTES) further pushed the boundaries of minimally invasive surgery, eliminating the extraction wound, associated pain, risk of wound infection and incisional hernia. The per-oral transgastric approach was first developed in animal models, and then intensely explored across transoral, transanal, transurethral, and transvaginal routes, before being cautiously tested in clinical practice[63,64]. Dr. Mark Whiteford reported a successful NOTES transanal sigmoid colectomy cadaver series in 2007[65], while Dr. Patricia Sylla combined the transgastric endoscopic and TEM platform in a swine rectosigmoid resection series[66].
Dr. Antonio Lacy was instrumental in moving the concept of NOTES out of the “lab” and into potential practise, reporting a sigmoid resection using transvaginal mini-laparoscopic-assisted natural orifice surgery for sigmoid adenocarcinoma[67]. Using the TEM platform in a human rectal cancer series, there seemed to now be a safe alternative to open and laparoscopic TME[68]. Several colorectal series followed, affirming the feasibility of NOTES[69-76]. However for the majority NOTES remains experimental, with concerns over the operative platform, accidental organ injury and viscerotomy closure[64,73]. The potential of performing complex colorectal dissection using existing transanal endoscopic platforms fueled the movement towards the TaTME.
Hybrid approaches to rectal cancer were occurring long before NOTES. For sphincter preservation and conservation of adequate function in very distal lesions, Dr. Gerald Marks developed the TransAnal Abdominal TransAnal Proctosigmoidectomy with colo-anal anastomosis (TATA) technique in 1984, a transanally initiated TME dissection that offers a direct, precise distal dissection, assuring adequate distal margins[77,78]. Dr. John Marks routinely integrated laparoscopic and robotic approaches with TATA, adding the advantages of minimally invasive surgery to this groundbreaking procedure. The TATA introduced the concept of “bottom-up” technique, in contrast to the “top-down” traditional technique followed in the abdominal procedures.
TaTME extends the TATA’s principle of initiating the TME dissection transanally (bottom-up) and accomplishes the most difficult part of the dissection from the caudal side[77]. Sylla and Lacy first described the TaTME in 2010[79] followed by an early case series of 20 patients[68], and a further validated series in 140 patients[80]. Since these early reports, numerous series have described the safety and feasibility of taTME even in challenging patients. The theoretical advantages of access and visualization have established this technique as not only a credible alternative to more traditional approaches which has the potential to provide optimal outcomes for oncologic resection of low rectal cancers[81-91].
Indications for TaTME: TaTME is mainly indicated for treatment of malignant tumors affecting the middle and lower third of the rectum. Moreover, it can be applied in benign conditions affecting the rectum such as Crohn’s disease and ulcerative colitis. Benign indications for TaTME may include reversal of Hartmann’s procedure, restorative proctocolectomy or completion proctectomy and ileal-pouch anal anastomosis[92].
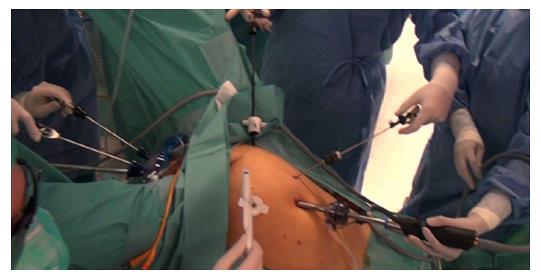
Technical points of the TaTME: Briefly, the procedure is performed in the modified lithotomy (Lloyd-Davies) position. It can be performed by a single team or, as originally described by Lacy, two-team (“Cecil Approach”), which allows for shorter operative times, improved visualization, and better traction and counter-traction to facilitate the resection (Figure 3). The abdominal approach is determined by surgeon preference, and entails full left colon and splenic flexure mobilization, high ligation of the inferior mesenteric artery (with identification and preservation of the pelvic nerve plexuses), and division of the inferior mesenteric vein was divided at the inferior pancreas border, and a TME performed.
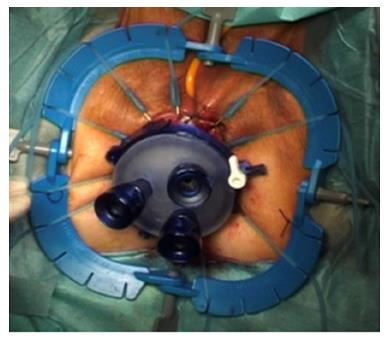
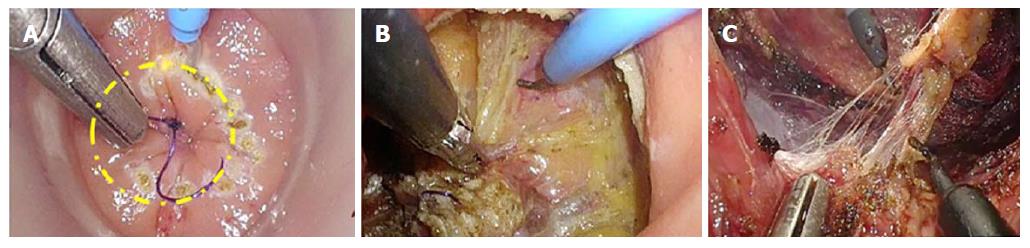
For TaTME, the rectum is irrigated, a purse-string suture placed to occlude the rectum, then the Transanal Access Platform inserted as shown in Figure 4, and pneumorectum established. Performing a tight purse-string suture is imperative to prevent translocation of liquid stool and cancer cells while the dissection is being carried out. Adequate rectal irrigation and the purse-string suture may help reduce the potential for implantation of cancer cells and/or bacteria inherent in the transanal dissection plane that could result in abscesses or local recurrence. While long-term outcomes will need to be assessed for these risks, measures to prevent the risk include standard manipulations and appropriate case selection as well as rectal irrigation with a cytocidal solution[93]. Under endoscopic visualization, the rectum is circumferentially mobilized, and the dissection continued proximally in the avascular TME plane towards the peritoneal reflection to meet the abdominal mobilization (Figure 5)[82,92]. The extraction can be performed transanally, or though a Pfannenstiel or stoma-site incision, depending on the abdominal approach used and the bulk of the specimen.
Safe implementation of TaTME: TaTME may enhance distal rectal access and visualization, allowing optimal margins, adequate lymph node yield, and high quality resection, even in the most difficult patients[94-96]. Denost et al[97] showed that the perineal approach reduces the risk of positive CRM compared to an abdominal approach, and may be an oncologically superior approach for low rectal cancer. Report from the International TaTME Registry suggests the procedure is oncologically safe and effective[83]. Since TaTME is in its infancy, longer follow-up and controlled trials needed. It is important to note that the TaTME is technically challenging, and formal training through a hands-on course is recommended, with active proctoring during the first year and ongoing participation within multicenter registries, for quality improvement and long term follow up[98]. Consensus for standardization of the technique and structured training are ongoing, to facilitate safe, appropriate implementation into clinical practice[92,98-101].
Advantages of TaTME: In general, transanal approaches allow better visualization of the distal rectum and clearly demonstrates the distal resection margin. The TaTME furthers these benefits, uniquely allowing deep pelvic dissection without the need for traction on the rectum. The plane of resection is clearly identified, even in obese and male patients with narrow pelvis, which were considered unfavorable conditions for laparoscopic TME[102].
Oncological benefits: The major potential benefit of TaTME is its theoretical ability to obtain a higher quality TME specimen. Results from the international TaTME registry showed complete or almost complete mesorectal excision rate of 96%, CRM positive rate of 2.4% and DRM positive rate of 0.3%[83]. Similarly, Xu et al[103] and colleagues concluded that TaTME provided lower rate of positive CRM compared to laparoscopic TME (OR: 0.34, 95%CI: 0.12-0.93; I2 = 0%). A recent meta-analysis reinforced the previous results, demonstrating that TaTME attained significantly higher rate of complete and near complete mesorectal excision than laparoscopic TME[104]. Additionally, TaTME had wider CRM with a significantly lower number of patients with positive CRM (OR: 0.39, P = 0.02). However, more controlled trials including larger number of patients are required to validate the oncologic and pathologic outcomes with TaTME.
Functional benefits: Bowel, bladder, and sexual dysfunctions are among the most common and devastating complications of rectal cancer surgery. TaTME decreases the number of permanent stomas, but at the cost of increasing the rate of coloanal anastomoses. With this, there is the theoretical risk of impaired continence and functional outcomes. Few studies have addressed long-term functional outcomes to date. Preliminary results demonstrate similar postoperative sphincter function when compared with laparoscopic or open TME[105-107]. A recent review of 30 patients evaluating functional outcomes 6 mo after TaTME showed acceptable quality of life and functional outcomes, comparable to published results after conventional laparoscopic low anterior resection[108]. In this study, deterioration for all domains was observed at one month after surgery compared to baseline, but returned to baseline at 6 mo for all areas except social function and anal pain. More studies with larger sample sizes and longer follow up are needed. A lower rate of urinary dysfunction has been observed after TaTME, which can be attributable to the enhanced visualization that improves definition of anatomic landmarks and allows nerve-sparing dissection in the presacral plane[109].
The risk of urethral injury is a real concern, and a unique complication of the procedure; Studies have shown an incidence of more than 10%, in addition, injury of the urethral sphincter can lead to urinary incontinence and dysfunction[83]. The membranous urethra is put at risk if the posterior prostatic lobe is deflected downwards inadvertently, or during the perineal phase of an abdominoperineal resection. Urethral injury may be prevented with adequate training and mentoring of the technique and following a meticulous technique of dissection in the anterior plane[110]. Methods to better identify the urethra intraoperatively and reduce injury rates, such as with fluorescence imaging, have been described and may also be beneficial with this new technique[111,112].
Technical benefits of TaTME include having significantly shorter operation time than laparoscopic TME[103]. A plausible explanation is that the bottom-up approach overcomes the technical limitations associated with laparoscopic TME, enabling surgeons to proceed more easily and efficiently. Also, the simultaneous two-team technique can help reducing the operation time significantly[25]. Another technical advantage of TaTME is having lower rates of conversion to open surgery compared to laparoscopic TME (OR: 0.29, P = 0.02)[104]. The overall conversion rate of laparoscopic TME was almost four-times that of TaTME (8.6% vs 2.6%). On analysis of the reasons for conversion, technical difficulties accounted for 25% of conversions in the TaTME group vs 47% in the laparoscopic TME group. Technical difficulties necessitating conversion in the laparoscopic group were related to high BMI and narrow pelvis as previously implied. TaTME also allows for transanal specimen extraction, thus decreasing the need for an abdominal assist incision.
Safety: The safety and feasibility of TaTME for short and midterm outcomes has been extensively described[80,89,91,113-115]. A report from the TaTME International Registry reported postoperative morbidity, anastomotic leakage and mortality rates of 32.6%, 6.7% and 2.6%, respectively[83]. A pooled analysis in a recent systematic review had similar rates of intraoperative complications and lower rate of postoperative morbidity compared to laparoscopic TME, with no significant difference between the TaTME and laparoscopic TME in regards to anastomotic leak[104]. However, there remain some concerns about the rapid development of this new technique and critics would point to the more catastrophic complications including prostate and urethral injuries. But this had led to design and implementation of detailed national training programs which have been initiated in the United States and Europe. This may help safe expansion of the technique and mitigate the safety issues.
Although TaTME has achieved promising oncological and functional results in treatment of rectal cancer as reported in several studies, the technique does have certain limitations that need to be addressed. Firstly, the bottom-to-up dissection approach followed in TaTME can be quite difficult since the majority of surgeons are not familiar with such different anatomical perspective for dissection, therefore adequate training under expert supervision is imperative before employing the technique in practice. Secondly, with new techniques new complications may arise, this is true with TaTME as a number of complications were recognized after the procedure. Complications specific to TaTME include formation of local collection or abscess secondary to bacterial contamination due to transection of the rectum at the start of the procedure. In one report[116], TaTME was found to be associated with positive cultures in more than one-third of the patient, with development of pre-sacral abscess in 17% of the patients.
As aforementioned, the risk of injury of the urethra and urethral sphincter, which can occur in up to 10% of patients, is a unique complication of TaTME compared to the abdominal approaches for rectal cancer[84]. The risk of urinary retention and transient urinary dysfunction was previously reported and minimal detrusor activity was documented in urodynamic studies implying neurogenic bladder dysfunction[82]. It is also worthy to note that the CO2 insufflation used to aid dissection might expose planes beyond the scope for dissection particularly during lateral and posterior dissection of the mid rectum which can lead to extending the dissection too deep into the pre-sacral space which carries a significant risk of injuring the autonomic nerves and venous plexus in this plane[96,117].
In order to establish the efficacy of a new surgical treatment, well-designed controlled experiments must be performed. Trials such as the COLOR III[118] and the TaTME trial in United States are currently recruiting, with the primary outcome of non-inferiority for local recurrence. While we wait for the definitive outcomes from large-scale controlled trials, multicentre registries are valuable for quality assurance and audits to optimize and standardize outcomes. The international TaTME registry, in which worldwide surgeons performing TaTME are invited to join, is a secure online database funded by the Pelican Cancer Foundation (https://tatme.medicaldata.eu). The analysis of this large population-based cohort is a joint of effort with the objective of improving research and care of the patients with rectal cancer treated with TaTME.
In the last few years, there has been an increase in availability, quality, and utilization of online and social media resources for surgery. These platforms best feature offer instant and unlimited medical knowledge. Tools such as the online Advances in Surgery (AIS) Channel (https://aischannel.com) or iLappSurgery Foundation app (http://www.ilappsurgery.com) have gained favour in the surgical community. They have taken the next step by providing high-quality surgical education, which is clearly one of the keys to raise the standards of training. These two platforms are focused specifically on laparoscopic surgery and colorectal procedures, with TaTME being one of its cornerstones.
All these initiatives for TaTME research, training and education have experienced a great acceptance. This is based on the obvious theoretical benefits that can overcome problems such as the risk for increased non-complete specimens obtained by laparoscopy and the longer operative times and higher costs associated with robotics. The international TaTME registry, AIS Channel and iLappSurgery Foundation have been developed for being guidance not only for trainees but also for experienced surgeons. TaTME is a complex procedure to learn, so continuous quality improvement from data analysis as well as high-quality training programs are needed for correct standardization and safe implementation of the technique[98,100,101].
The survival outcomes with respect to disease recurrence in rectal cancer surgery are directly related to the quality of resection[3], and thus the success of TaTME must be held against this quality assurance measure to ensure oncological parity and perhaps superiority. This new technique is complex and requires exceptional anatomical knowledge to perform an unfamiliar dissection. Previous laparoscopic colorectal experience and a high case volume are essential to reach a standard in an acceptable amount of time. Nursing/operating room staff and anaesthesiology also require specific training to become familiar with the new set up, particularly when performing a two-team approach, where the coordination among all operating room staff is crucial to avoid a potentially dangerous situation.
The learning curve of TaTME is yet to be established; however, according to estimation by expert groups approximately 20 consecutive cases are sufficient to develop an adequate learning curve for a surgeon proficient in laparoscopic and transanal surgery. In accordance with this appraisal, a minimum of five proctored cases is recommended in order to achieve an optimal level for the TaTME performance. However, establishing centres of excellence would allow surgeons to increase volume of cases and allow training of more junior surgeons in a safe manner. Different training courses taught by expert groups are available, which generally include didactic lessons, live cases, and hands-on cadaver labs. After completion of the courses, proctoring in the origin institutions should be the next step in the adoption process. Mentors should travel and proctor cases along with the trainees, to show on the spot the tips and tricks as well as adjusting the technique to the site´s intrinsic characteristics. Validation and accreditation of the technique are also under development and a matter of discussion in the international surgical societies[98,100].
The TaTME was developed from existing platforms and as an attempt to resolve the challenges of minimally invasive low rectal cancer surgery. As evidence for safety and feasibility accumulates, and with the implantation of structured training programs in order to standardize training, teaching, and safe expansion, TaTME seems on course for further uptake. The improved visibility of the pelvic structures and better accessibility for ultralow anastomoses may render the transanal approach ideal for a wide variety of cases. The indications for TaTME are currently expanding beyond mid and low rectal cancers, and open up new possibilities to use the approach for different diseases. Although the initial results of TaTME are promising and encouraging, further controlled clinical trials including larger number of patients with long-term follow-up are required to validate the oncologic and pathologic outcomes of TaTME. With the international registry and ongoing controlled trials, we look forward to long-term outcomes with this innovative approach.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Elmore U, Solomon MJ, Wang ZN S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA Cancer J Clin. 1971;21:361-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 198] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1985] [Cited by in F6Publishing: 1818] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 3. | Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O’Callaghan C, Myint AS, Bessell E, Thompson LC. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 739] [Cited by in F6Publishing: 694] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 4. | Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1037] [Cited by in F6Publishing: 1022] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 5. | Initial report from a Swedish multicentre study examining the role of preoperative irradiation in the treatment of patients with resectable rectal carcinoma. Swedish Rectal Cancer Trial. Br J Surg. 1993;80:1333-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 149] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Wibe A, Møller B, Norstein J, Carlsen E, Wiig JN, Heald RJ, Langmark F, Myrvold HE, Søreide O; Norwegian Rectal Cancer Group. A national strategic change in treatment policy for rectal cancer--implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum. 2002;45:857-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 423] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 7. | Kapiteijn E, Kranenbarg EK, Steup WH, Taat CW, Rutten HJ, Wiggers T, van Krieken JH, Hermans J, Leer JW, van de Velde CJ. Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Prospective randomised trial with standard operative and histopathological techniques. Dutch ColoRectal Cancer Group. Eur J Surg. 1999;165:410-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3104] [Cited by in F6Publishing: 2972] [Article Influence: 129.2] [Reference Citation Analysis (0)] |
| 9. | Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358:1291-1304. [DOI] [Cited in This Article: ] [Cited by in Crossref: 681] [Cited by in F6Publishing: 647] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 10. | Randomised trial of surgery alone versus surgery followed by radiotherapy for mobile cancer of the rectum. Medical Research Council Rectal Cancer Working Party. Lancet. 1996;348:1610-1614. [PubMed] [Cited in This Article: ] |
| 11. | Wolmark N, Wieand HS, Hyams DM, Colangelo L, Dimitrov NV, Romond EH, Wexler M, Prager D, Cruz AB Jr, Gordon PH, Petrelli NJ, Deutsch M, Mamounas E, Wickerham DL, Fisher ER, Rockette H, Fisher B. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 337] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4342] [Cited by in F6Publishing: 4228] [Article Influence: 211.4] [Reference Citation Analysis (1)] |
| 13. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC; EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1993] [Cited by in F6Publishing: 1942] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 14. | Hong YS, Nam BH, Kim KP, Kim JE, Park SJ, Park YS, Park JO, Kim SY, Kim TY, Kim JH. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15:1245-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 270] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 15. | Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1474] [Cited by in F6Publishing: 1337] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 16. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1089] [Cited by in F6Publishing: 1037] [Article Influence: 69.1] [Reference Citation Analysis (2)] |
| 17. | Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2606] [Cited by in F6Publishing: 2453] [Article Influence: 122.7] [Reference Citation Analysis (0)] |
| 18. | Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H; Clinical Outcomes of Surgical Therapy Study Group. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655-662; discussion 662-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 789] [Cited by in F6Publishing: 760] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 19. | Bonjer HJ, Hop WC, Nelson H, Sargent DJ, Lacy AM, Castells A, Guillou PJ, Thorpe H, Brown J, Delgado S. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg. 2007;142:298-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 397] [Cited by in F6Publishing: 438] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 20. | Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224-2229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1901] [Cited by in F6Publishing: 1768] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 21. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM; COlon cancer Laparoscopic or Open Resection Study Group (COLOR). Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1691] [Cited by in F6Publishing: 1611] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 22. | Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet. 2000;356:93-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in F6Publishing: 619] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 23. | Leroy J, Jamali F, Forbes L, Smith M, Rubino F, Mutter D, Marescaux J. Laparoscopic total mesorectal excision (TME) for rectal cancer surgery: long-term outcomes. Surg Endosc. 2004;18:281-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 307] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 24. | Dulucq JL, Wintringer P, Stabilini C, Mahajna A. Laparoscopic rectal resection with anal sphincter preservation for rectal cancer: long-term outcome. Surg Endosc. 2005;19:1468-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Bretagnol F, Lelong B, Laurent C, Moutardier V, Rullier A, Monges G, Delpero JR, Rullier E. The oncological safety of laparoscopic total mesorectal excision with sphincter preservation for rectal carcinoma. Surg Endosc. 2005;19:892-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Wibe A, Endreseth BH. [Surgical treatment of rectal cancer in Norway]. Tidsskr Nor Laegeforen. 2007;127:2950-2953. [PubMed] [Cited in This Article: ] |
| 27. | Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 561] [Cited by in F6Publishing: 588] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 28. | Color II Study Group. Buunen M, Bonjer HJ, Hop WC, Haglind E, Kurlberg G, Rosenberg J, Lacy AM, Cuesta MA, D’Hoore A, Fürst A, Lange JF, Jess P, Bulut O, Poornoroozy P, Jensen KJ, Christensen MM, Lundhus E, Ovesen H, Birch D, Iesalnieks I, Jäger C, Kreis M, van riet Y, van der Harst E, Gerhards MF, Bemelman WA, Hansson BM, Neijenhuis PA, Prins HA, Balague C, Targarona E, Luján Mompeán JA, Franco Osorio JD, Garcia Molina FJ, Skullman S, Läckberg Z, Kressner U, Matthiessen P, Kim SH, Poza AA. COLOR II. A randomized clinical trial comparing laparoscopic and open surgery for rectal cancer. Dan Med Bull. 2009;56:89-91. [PubMed] [Cited in This Article: ] |
| 29. | Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J; ALaCaRT Investigators. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 708] [Cited by in F6Publishing: 675] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 30. | Fleshman J1, Branda M2, Sargent DJ2, Boller AM3, George V4, Abbas M5, Peters WR Jr6, Maun D7, Chang G8, Herline A9, Fichera A10, Mutch M11, Wexner S12, Whiteford M13, Marks J14, Birnbaum E11, Margolin D15, Larson D2, Marcello P16, Posner M10, Read T16, Monson J17, Wren SM18, Pisters PW8, Nelson H19. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 725] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 31. | Row D, Weiser MR. An update on laparoscopic resection for rectal cancer. Cancer Control. 2010;17:16-24. [PubMed] [Cited in This Article: ] |
| 32. | Young M, Pigazzi A. Total mesorectal excision: open, laparoscopic or robotic. Recent Results Cancer Res. 2014;203:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Xiong B, Ma L, Huang W, Zhao Q, Cheng Y, Liu J. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis of eight studies. J Gastrointest Surg. 2015;19:516-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 34. | Bianchi PP, Ceriani C, Locatelli A, Spinoglio G, Zampino MG, Sonzogni A, Crosta C, Andreoni B. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc. 2010;24:2888-2894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 35. | D’Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P, Alfano G. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27:1887-1895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 36. | Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med. 2010;363:701-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 590] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 37. | Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA. 2017;318:1569-1580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 727] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 38. | Baxter NN, Garcia-Aguilar J. Organ preservation for rectal cancer. J Clin Oncol. 2007;25:1014-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Kidane B, Chadi SA, Kanters S, Colquhoun PH, Ott MC. Local resection compared with radical resection in the treatment of T1N0M0 rectal adenocarcinoma: a systematic review and meta-analysis. Dis Colon Rectum. 2015;58:122-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Nash GM, Weiser MR, Guillem JG, Temple LK, Shia J, Gonen M, Wong WD, Paty PB. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum. 2009;52:577-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Blumberg D, Paty PB, Guillem JG, Picon AI, Minsky BD, Wong WD, Cohen AM. All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum. 1999;42:881-885. [PubMed] [Cited in This Article: ] |
| 42. | Madbouly KM, Remzi FH, Erkek BA, Senagore AJ, Baeslach CM, Khandwala F, Fazio VW, Lavery IC. Recurrence after transanal excision of T1 rectal cancer: should we be concerned? Dis Colon Rectum. 2005;48:711-719; discussion 719-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Bentrem DJ, Okabe S, Wong WD, Guillem JG, Weiser MR, Temple LK, Ben-Porat LS, Minsky BD, Cohen AM, Paty PB. T1 adenocarcinoma of the rectum: transanal excision or radical surgery? Ann Surg. 2005;242:472-477; discussion 477-479. [PubMed] [Cited in This Article: ] |
| 44. | You YN, Baxter NN, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245:726-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 45. | Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, García-Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064-1071; discussion 1071-1074. [PubMed] [Cited in This Article: ] |
| 46. | Stitzenberg KB, Sanoff HK, Penn DC, Meyers MO, Tepper JE. Practice patterns and long-term survival for early-stage rectal cancer. J Clin Oncol. 2013;31:4276-4282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Rectal Cancer, Version 3.2017, cited 2017-12-22; Published March. 2017; Available from: https://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf. [Cited in This Article: ] |
| 48. | Atallah S, Keller D. Why the Conventional Parks Transanal Excision for Early Stage Rectal Cancer Should Be Abandoned. Dis Colon Rectum. 2015;58:1211-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Buess G, Mentges B, Manncke K, Starlinger M, Becker HD. Technique and results of transanal endoscopic microsurgery in early rectal cancer. Am J Surg. 1992;163:63-69; discussion 69-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 195] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Langer C, Liersch T, Süss M, Siemer A, Markus P, Ghadimi BM, Füzesi L, Becker H. Surgical cure for early rectal carcinoma and large adenoma: transanal endoscopic microsurgery (using ultrasound or electrosurgery) compared to conventional local and radical resection. Int J Colorectal Dis. 2003;18:222-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Moore JS, Cataldo PA, Osler T, Hyman NH. Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum. 2008;51:1026-1230; discussion 1030-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 52. | Winde G, Nottberg H, Keller R, Schmid KW, Bünte H. Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum. 1996;39:969-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 265] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 53. | Clancy C, Burke JP, Albert MR, O’Connell PR, Winter DC. Transanal endoscopic microsurgery versus standard transanal excision for the removal of rectal neoplasms: a systematic review and meta-analysis. Dis Colon Rectum. 2015;58:254-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 54. | Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol. 2014;18:775-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 55. | Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc. 2010;24:2200-2205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 361] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 56. | McLemore EC, Weston LA, Coker AM, Jacobsen GR, Talamini MA, Horgan S, Ramamoorthy SL. Transanal minimally invasive surgery for benign and malignant rectal neoplasia. Am J Surg. 2014;208:372-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Maglio R, Muzi GM, Massimo MM, Masoni L. Transanal minimally invasive surgery (TAMIS): new treatment for early rectal cancer and large rectal polyps—experience of an Italian center. Am Surg. 2015;81:273-277. [PubMed] [Cited in This Article: ] |
| 58. | Lee L, Kelly J, Nassif GJ, Keller D, Debeche-Adams TC, Mancuso PA, Monson JR, Albert MR, Atallah SB. Establishing the learning curve of transanal minimally invasive surgery for local excision of rectal neoplasms. Surg Endosc. 2018;32:1368-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Albert MR, Atallah SB, deBeche-Adams TC, Izfar S, Larach SW. Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum. 2013;56:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 60. | Khoo RE. Transanal excision of a rectal adenoma using single-access laparoscopic port. Dis Colon Rectum. 2010;53:1078-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Stipa F, Lucandri G, Ferri M, Casula G, Ziparo V. Local excision of rectal cancer with transanal endoscopic microsurgery (TEM). Anticancer Res. 2004;24:1167-1172. [PubMed] [Cited in This Article: ] |
| 62. | Suppiah A, Maslekar S, Alabi A, Hartley JE, Monson JR. Transanal endoscopic microsurgery in early rectal cancer: time for a trial? Colorectal Dis. 2008;10:314-327; discussion 327-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117. [PubMed] [Cited in This Article: ] |
| 64. | Autorino R, Yakoubi R, White WM, Gettman M, De Sio M, Quattrone C, Di Palma C, Izzo A, Correia-Pinto J, Kaouk JH. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Whiteford MH, Denk PM, Swanström LL. Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc. 2007;21:1870-1874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 66. | Sylla P, Willingham FF, Sohn DK, Gee D, Brugge WR, Rattner DW. NOTES rectosigmoid resection using transanal endoscopic microsurgery (TEM) with transgastric endoscopic assistance: a pilot study in swine. J Gastrointest Surg. 2008;12:1717-1723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 67. | Lacy AM, Delgado S, Rojas OA, Almenara R, Blasi A, Llach J. MA-NOS radical sigmoidectomy: report of a transvaginal resection in the human. Surg Endosc. 2008;22:1717-1723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernández M, Delgado S, Sylla P, Martínez-Palli G. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “down-to-up” total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc. 2013;27:3165-3172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 69. | Chouillard E, Chahine E, Khoury G, Vinson-Bonnet B, Gumbs A, Azoulay D, Abdalla E. NOTES total mesorectal excision (TME) for patients with rectal neoplasia: a preliminary experience. Surg Endosc. 2014;28:3150-3157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | de Sousa LH, de Sousa JA, de Sousa Filho LH, de Sousa MM, de Sousa VM, de Sousa AP, Zorron R. Totally NOTES (T-NOTES) transvaginal cholecystectomy using two endoscopes: preliminary report. Surg Endosc. 2009;23:2550-2555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Leroy J, Cahill RA, Asakuma M, Dallemagne B, Marescaux J. Single-access laparoscopic sigmoidectomy as definitive surgical management of prior diverticulitis in a human patient. Arch Surg. 2009;144:173-179; discussion 179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 72. | Leroy J, Diana M, Perretta S, Wall J, De Ruijter V, Marescaux J. Original technique to close the transrectal viscerotomy access in a NOTES transrectal and transgastric segmental colectomy. Surg Innov. 2011;18:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Leroy J, Barry BD, Melani A, Mutter D, Marescaux J. No-scar transanal total mesorectal excision: the last step to pure NOTES for colorectal surgery. JAMA Surg. 2013;148:226-230; discussion 231. [PubMed] [Cited in This Article: ] |
| 74. | Zornig C, Mofid H, Emmermann A, Alm M, von Waldenfels HA, Felixmüller C. Scarless cholecystectomy with combined transvaginal and transumbilical approach in a series of 20 patients. Surg Endosc. 2008;22:1427-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 75. | Zorron R, Phillips HN, Coelho D, Flach L, Lemos FB, Vassallo RC. Perirectal NOTES access: “down-to-up” total mesorectal excision for rectal cancer. Surg Innov. 2012;19:11-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Zorron R, Phillips HN, Wynn G, Neto MP, Coelho D, Vassallo RC. “Down-to-Up” transanal NOTES Total mesorectal excision for rectal cancer: Preliminary series of 9 patients. J Minim Access Surg. 2014;10:144-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Marks JH, Myers EA, Zeger EL, Denittis AS, Gummadi M, Marks GJ. Long-term outcomes by a transanal approach to total mesorectal excision for rectal cancer. Surg Endosc. 2017;31:5248-5257. [PubMed] [Cited in This Article: ] |
| 78. | Marks JH, Valsdottir EB. (2015) Total mesorectal excision with coloanal anastomosis: laparoscopic technique. In: Mulholland M (ed) Operative techniques in surgery, vol 2. Lippincott Williams and Wilkins, Philadelphia, pp 1177-1189. . [Cited in This Article: ] |
| 79. | Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 507] [Cited by in F6Publishing: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 80. | Lacy AM, Tasende MM, Delgado S, Fernandez-Hevia M, Jimenez M, De Lacy B, Castells A, Bravo R, Wexner SD, Heald RJ. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J Am Coll Surg. 2015;221:415-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 81. | Sylla P, Bordeianou LG, Berger D, Han KS, Lauwers GY, Sahani DV, Sbeih MA, Lacy AM, Rattner DW. A pilot study of natural orifice transanal endoscopic total mesorectal excision with laparoscopic assistance for rectal cancer. Surg Endosc. 2013;27:3396-3405. [PubMed] [Cited in This Article: ] |
| 82. | Burke JP, Martin-Perez B, Khan A, Nassif G, de Beche-Adams T, Larach SW, Albert MR, Atallah S. Transanal total mesorectal excision for rectal cancer: early outcomes in 50 consecutive patients. Colorectal Dis. 2016;18:570-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 83. | Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; TaTME Registry Collaborative. Transanal Total Mesorectal Excision: International Registry Results of the First 720 Cases. Ann Surg. 2017;266:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 304] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 84. | Muratore A, Mellano A, Marsanic P, De Simone M. Transanal total mesorectal excision (taTME) for cancer located in the lower rectum: short- and mid-term results. Eur J Surg Oncol. 2015;41:478-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 85. | Chen WH, Kang L, Luo SL, Zhang XW, Huang Y, Liu ZH, Wang JP. Transanal total mesorectal excision assisted by single-port laparoscopic surgery for low rectal cancer. Tech Coloproctol. 2015;19:527-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Buchs NC, Wynn G, Austin R, Penna M, Findlay JM, Bloemendaal AL, Mortensen NJ, Cunningham C, Jones OM, Guy RJ. A two-centre experience of transanal total mesorectal excision. Colorectal Dis. 2016;18:1154-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 87. | Buchs NC, Nicholson GA, Yeung T, Mortensen NJ, Cunningham C, Jones OM, Guy R, Hompes R. Transanal rectal resection: an initial experience of 20 cases. Colorectal Dis. 2016;18:45-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Marks JH, Montenegro GA, Salem JF, Shields MV, Marks GJ. Transanal TATA/TME: a case-matched study of taTME versus laparoscopic TME surgery for rectal cancer. Tech Coloproctol. 2016;20:467-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 89. | Veltcamp Helbach M, Deijen CL, Velthuis S, Bonjer HJ, Tuynman JB, Sietses C. Transanal total mesorectal excision for rectal carcinoma: short-term outcomes and experience after 80 cases. Surg Endosc. 2016;30:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 90. | Chen CC, Lai YL, Jiang JK, Chu CH, Huang IP, Chen WS, Cheng AY, Yang SH. Transanal Total Mesorectal Excision Versus Laparoscopic Surgery for Rectal Cancer Receiving Neoadjuvant Chemoradiation: A Matched Case-Control Study. Ann Surg Oncol. 2016;23:1169-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 91. | Caycedo-Marulanda A, Jiang HY, Kohtakangas EL. Outcomes of a Single Surgeon-Based Transanal-Total Mesorectal Excision (TATME) for Rectal Cancer. J Gastrointest Cancer. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Mizrahi I, Sands D. Total mesorectal excision for rectal cancer: A review. Ann Laparosc Endosc Surg. 2017;2:144. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Sun G, Tang Y, Li X, Meng J, Liang G. Analysis of 116 cases of rectal cancer treated by transanal local excision. World J Surg Oncol. 2014;12:202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Arroyave MC, DeLacy FB, Lacy AM. Transanal total mesorectal excision (TaTME) for rectal cancer: Step by step description of the surgical technique for a two-teams approach. Eur J Surg Oncol. 2017;43:502-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 95. | Atallah S. Transanal total mesorectal excision: full steam ahead. Tech Coloproctol. 2015;19:57-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 96. | Heald RJ. A new solution to some old problems: transanal TME. Tech Coloproctol. 2013;17:257-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 97. | Denost Q, Adam JP, Rullier A, Buscail E, Laurent C, Rullier E. Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg. 2014;260:993-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 98. | Francis N, Penna M, Mackenzie H, Carter F, Hompes R; International TaTME Educational Collaborative Group. Consensus on structured training curriculum for transanal total mesorectal excision (TaTME). Surg Endosc. 2017;31:2711-2719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 99. | Motson RW, Whiteford MH, Hompes R, Albert M, Miles WF; Expert Group. Current status of trans-anal total mesorectal excision (TaTME) following the Second International Consensus Conference. Colorectal Dis. 2016;18:13-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 100. | Penna M, Hompes R, Mackenzie H, Carter F, Francis NK. First international training and assessment consensus workshop on transanal total mesorectal excision (taTME). Tech Coloproctol. 2016;20:343-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 101. | McLemore EC, Harnsberger CR, Broderick RC, Leland H, Sylla P, Coker AM, Fuchs HF, Jacobsen GR, Sandler B, Attaluri V. Transanal total mesorectal excision (taTME) for rectal cancer: a training pathway. Surg Endosc. 2016;30:4130-4135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 102. | Fernández-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, Díaz del Gobbo G, DeLacy B, Balust J, Lacy AM. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261:221-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 103. | Xu W, Xu Z, Cheng H, Ying J, Cheng F, Xu W, Cao J, Luo J. Comparison of short-term clinical outcomes between transanal and laparoscopic total mesorectal excision for the treatment of mid and low rectal cancer: A meta-analysis. Eur J Surg Oncol. 2016;42:1841-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 104. | Ma B, Gao P, Song Y, Zhang C, Zhang C, Wang L, Liu H, Wang Z. Transanal total mesorectal excision (taTME) for rectal cancer: a systematic review and meta-analysis of oncological and perioperative outcomes compared with laparoscopic total mesorectal excision. BMC Cancer. 2016;16:380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 105. | Bretagnol F, Rullier E, Laurent C, Zerbib F, Gontier R, Saric J. Comparison of functional results and quality of life between intersphincteric resection and conventional coloanal anastomosis for low rectal cancer. Dis Colon Rectum. 2004;47:832-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 106. | Dumont F, Ayadi M, Goéré D, Honoré C, Elias D. Comparison of fecal continence and quality of life between intersphincteric resection and abdominoperineal resection plus perineal colostomy for ultra-low rectal cancer. J Surg Oncol. 2013;108:225-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Rouanet P, Saint-Aubert B, Lemanski C, Senesse P, Gourgou S, Quenet F, Ycholu M, Kramar A, Dubois J. Restorative and nonrestorative surgery for low rectal cancer after high-dose radiation: long-term oncologic and functional results. Dis Colon Rectum. 2002;45:305-313; discussion 313-315. [PubMed] [Cited in This Article: ] |
| 108. | Koedam TW, van Ramshorst GH, Deijen CL, Elfrink AK, Meijerink WJ, Bonjer HJ, Sietses C, Tuynman JB. Transanal total mesorectal excision (TaTME) for rectal cancer: effects on patient-reported quality of life and functional outcome. Tech Coloproctol. 2017;21:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 109. | Wolthuis AM, de Buck van Overstraeten A, D’Hoore A. Laparoscopic natural orifice specimen extraction-colectomy: a systematic review. World J Gastroenterol. 2014;20:12981-12992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 80] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 110. | Atallah SB, DuBose AC, Burke JP, Nassif G, deBeche-Adams T, Frering T, Albert MR, Monson JRT. Uptake of Transanal Total Mesorectal Excision in North America: Initial Assessment of a Structured Training Program and the Experience of Delegate Surgeons. Dis Colon Rectum. 2017;60:1023-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 111. | Atallah S, Mabardy A, Volpato AP, Chin T, Sneider J, Monson JRT. Surgery beyond the visible light spectrum: theoretical and applied methods for localization of the male urethra during transanal total mesorectal excision. Tech Coloproctol. 2017;21:413-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 112. | Barnes TG, Penna M, Hompes R, Cunningham C. Fluorescence to highlight the urethra: a human cadaveric study. Tech Coloproctol. 2017;21:439-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 113. | Kneist W, Wachter N, Paschold M, Kauff DW, Rink AD, Lang H. Midterm functional results of taTME with neuromapping for low rectal cancer. Tech Coloproctol. 2016;20:41-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Rasulov AO, Mamedli ZZ, Gordeyev SS, Kozlov NA, Dzhumabaev HE. Short-term outcomes after transanal and laparoscopic total mesorectal excision for rectal cancer. Tech Coloproctol. 2016;20:227-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 115. | Kang L, Chen WH, Luo SL, Luo YX, Liu ZH, Huang MJ, Wang JP. Transanal total mesorectal excision for rectal cancer: a preliminary report. Surg Endosc. 2016;30:2552-2562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Velthuis S, Veltcamp Helbach M, Tuynman JB, Le TN, Bonjer HJ, Sietses C. Intra-abdominal bacterial contamination in TAMIS total mesorectal excision for rectal carcinoma: a prospective study. Surg Endosc. 2015;29:3319-3323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 117. | Buchs NC, Nicholson GA, Ris F, Mortensen NJ, Hompes R. Transanal total mesorectal excision: A valid option for rectal cancer? World J Gastroenterol. 2015;21:11700-11708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 118. | Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange-de Klerk ES, Sietses C, Tuynman JB, Lacy AM, Hanna GB, Bonjer HJ. COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc. 2016;30:3210-3215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |