Device description
Name and manufacturer: Mitralign Transcatheter Annuloplasty System; Mitralign Inc., Tewksbury, MA, USA.
Approval status: CE mark study completed; CE marking anticipated in 2015.
Platform: Transcatheter retrograde annuloplasty system.
Specific design: Retrograde femoral access, placement of one or two Bident catheters for controlled, direct annuloplasty of the posterior mitral annulus.
Delivery system: Wire delivery catheter over articulating guiding catheter, Bident catheter, pledget catheter.
Device sizes/lengths: 14 Fr guiding catheter, Bident catheter with varying span (10, 14, 17, 21 mm).
Procedural details
The procedure is performed under general anaesthesia, guided by 2D/3D transoesophageal echocardiography and fluoroscopy, with an ACT of 250 s maintained throughout the procedure.
After femoral access, the 14 Fr deflectable guiding catheter is introduced into the left ventricle and directed towards the posterior annulus (Figure 1A). Through the guide catheter, an articulating wire delivery catheter is advanced to steer a crossing wire to the targeted position of the posterior mitral annulus (Figure 1A). An insulated 0.019’’ interventional crossing wire with radiofrequency (RF) facilitates the crossing of the wire through the annulus into the left atrium. Over the first wire, a Bident catheter with an appropriate span (10, 14, 17 or 21 mm) is advanced to the annulus and a second crossing wire is introduced through the Bident arm for annular crossing with RF energy (Figure 1B).
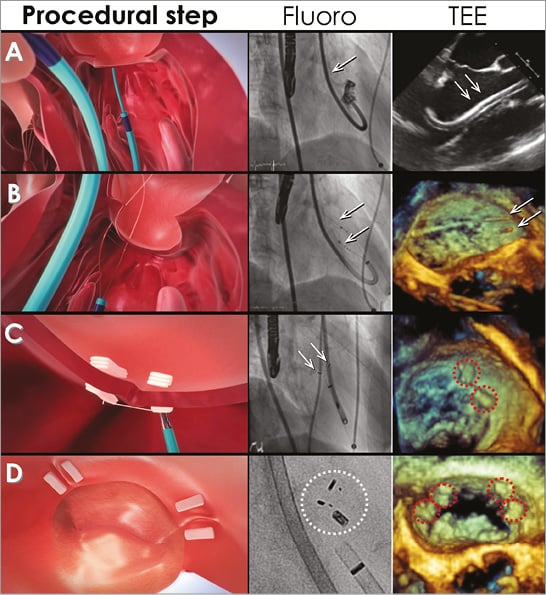
Figure 1. Percutaneous mitral annuloplasty with the Mitralign system. A) 14 Fr guide catheter (arrows) at the mitral annulus. B) Bident catheter and wire crossing of the mitral annulus (arrows). C) Pledget delivery at P1/P2 scallops of the posterior mitral annulus. D) Final result after pledget plication and suture cut with two pairs of pledgets delivered on the posterior mitral annulus. Fluoro: fluoroscopy; TEE: transoesophageal echocardiography
Thereafter, a pledget delivery catheter is introduced over each wire across the annulus into the left atrium. Half of the polyester pledget is delivered at the atrial site, and the ventricular pledget part is delivered after pledget delivery catheter removal (Figure 1C). Finally, the pledgets are released and attached, leaving two sutures exiting the guide catheter (Figure 1D).
Over the sutures a dedicated plication lock device is advanced to one of the pledgets. Once the required plication is achieved, a stainless steel lock is placed, maintaining the plication of the mitral annulus (Figure 1D). As a final step, the sutures are cut approximately 4 mm from the lock. If a second pair of pledgets is needed, the steps are repeated in the P1 and P3 scallop regions of the posterior mitral annulus (Figure 1D). Post-procedural antithrombotic regimen consists of aspirin for a minimum of six months and clopidogrel 75 mg for a minimum of 30 days.
Clinical data
The first-in-man multicentre study has been completed and study data on six-month outcomes were presented at EuroPCR 20151. The study prospectively included 51 patients with moderate to severe functional mitral regurgitation (FMR) (mean age 67.7±11 years, left ventricular ejection fraction [LVEF] 34±8%), on optimal medical treatment. All patients were followed for safety and efficacy outcomes based on clinical assessment and echocardiographic core lab evaluation.
Concerning safety outcomes, no intraprocedural death occurred; 30-day rates for all-cause mortality, stroke and myocardial infarction were 2.8%, 5.9% and 2.0%, respectively; the six-month death rate was described as 12.2%.
Echocardiography proved the effectiveness of annuloplasty with a significant reduction of anterior-posterior and septal-lateral dimensions of the mitral annulus, accompanied by decreased MV tenting area and an increase in MV coaptation depth. This effect led to a significant reduction in the percentage of patients presenting with severe mitral regurgitation before interventional annuloplasty and was accompanied by significant evidence for reverse left ventricular remodelling after six months of follow-up. Echocardiography showed a reduction of LV end-diastolic diameters and LV volumes.
Ongoing studies
A clinical phase II study for further evaluation of the Mitralign system will be started soon, including approximately 50 patients with FMR.
Recently, the Mitralign procedure has been used successfully for the treatment of functional tricuspid regurgitation on a compassionate use basis2.
Unique features
– Fully percutaneous solution for the treatment of FMR.
– Customisable to adjust for patient anatomy and either symmetric or asymmetric annular enlargement.
– Minimal invasive approach preserves future clinical options such as combination with interventional edge-to-edge repair.
Potential improvements
Current drawbacks of the procedure are as follows:
– Severe LV enlargement and systolic movement limit the stability of the flexible guide catheter.
– An atypical anatomy of the papillary muscles possibly hinders the steering ability of the pledget delivery system.
These drawbacks could be overcome with either a wider variation of flexible guide catheters, or refinement of patient selection.
Changing the interventional access route to an antegrade transvenous, transseptal approach would avoid LV anatomical interactions.
Conflict of interest statement
G. Nickenig and C. Hammerstingl received speakers honoraria from Mitralign Inc.




