Abstract
Aims: The percutaneous treatment of coronary bifurcation lesions remains hampered by suboptimal results, mainly in the side branch (SB), even with the use of drug-eluting stents. Dedicated bifurcation stents could provide an attractive alternative to improve early outcomes and reduce SB restenosis. We aimed to assess in a prospective single-arm multicentre registry, safety and effectiveness at 6-month clinical follow-up of the Tryton dedicated side branch (SB) stent.
Methods and results: In this prospective international registry, patients with coronary bifurcation lesions underwent treatment with a dedicated stenting technique using the Tryton stent, in conjunction with a “workhorse” main branch (MB) stent (drug-eluting or bare metal). The Tryton stent is specifically designed for bifurcations, with minimal strut/vessel ratio in the proximal MB, providing full strut coverage at the SB ostium, with short stent length in the SB and with the ability to adapt to the wide spectrum of bifurcation angles and sizes. The primary endpoint was 6-month major adverse cardiac events (MACE: cardiac death, myocardial infarction and target lesion revascularisation). Secondary endpoints were technical and procedural success (respectively defined as successful implantation of Tryton at the intended lesion and successful performance of the whole procedure with Tryton without in-hospital MACE). A total of 302 patients were enrolled. Of these, 296 had 6-month data available for evaluation. Technical and procedural success occurred in 98.0% (95% confidence interval: 95.7%-99.1%) and 94.4% (91.2%-96.5%) patients, respectively. The cumulative 6-month MACE rate was 6.4% (4.2%-9.7%), including 4.7% (2.9%-7.7%) myocardial infarctions (3.7% periprocedural), and 3.4% (2.0%-6.1%) target lesion revascularisations (2.4% in the MB, 0.7% in the SB, and 0.3% in both MB and SB). One stent thrombosis (0.3% [0%-1.6%]) occurred.
Conclusions: The treatment of bifurcation lesions with a dedicated Tryton stent is safe and feasible, with good technical and procedural success, very low revascularisation, MACE and stent thrombosis rates at 6-month clinical follow-up.
Introduction
Coronary bifurcation lesions account for 15-20% of all percutaneous coronary interventions1. When compared to non-bifurcation lesions, this lesion subset is considered complex to treat, with inferior angiographic and clinical results (acutely and at follow-up)2.
Currently, using standard “workhorse” stents two treatment strategies have been explored: a single stent technique with a stent in the main branch (MB) and balloon only in the side branch (SB) (“provisional-T”), and various double stent techniques with stents in both branches. The growth of literature has failed to show a benefit with the planned use of a double stent technique, which is also associated with increased procedural time and expenses. As a result, the single “provisional-T” stent technique has emerged as the preferred treatment3-6. However, independently from the technique of choice, results are still suboptimal due to high rates of restenosis in the SB. However, clinical practice is different from trials as most operators consider an elective double stenting technique the best solution to achieve superior acute angiographic results in bifurcations with severe SB disease4,7. The late outcomes of double stenting techniques such as (mini-)crush, culotte, reverse-T and double kissing crush are still suboptimal probably due to the stents themselves, which are not designed for bifurcation lesions. To address these problems, specifically dedicated bifurcation stents have been developed. The Tryton (Tryton Medical, Inc., Newton, MA, USA) stent is such a dedicated bifurcation stent, which is using a double stenting, “culotte-like” technique. The Tryton is positioned in the proximal MB across the carina into the SB with a second “workhorse” stent positioned sequentially in the MB. However, as compared to the “classical” culotte technique, the Tryton makes the procedure easier, due its distinctive design.
Recently, the Tryton stent has shown promising results in the first-in-man studies8,9.
The present study aimed at confirming and extending the first-in-man findings, in a larger and less selected cohort of patients.
Methods
The E-Tryton/Benelux Study is a pooled analysis of two prospective multicentre international registries, with the same design and concurrent enrolment time, aimed at assessing the efficacy and safety of the Tryton SB stent in unselected patients with bifurcation lesions. The study, carried out according to the Declaration of Helsinki, has been approved by the ethics committees of all participating centres, and signed informed consent was obtained from all patients.
PATIENT SELECTION
All patients referred with stable or unstable coronary artery disease and scheduled to undergo percutaneous coronary intervention (PCI) of de novo coronary artery lesions located at the level of a bifurcation, were eligible. Eligible lesions were defined as having a MB diameter between 2.5 mm and 4.0 mm and a SB diameter between 2.25 mm and 3.5 mm. Besides the size of the vessels, the only pre-specified exclusion criterion was a contraindication to dual antiplatelet therapy (DAPT).
TRYTON DEVICE
The Tryton stent is a balloon-expandable slotted tube, thin strut (diameter: 76.2 µm) cobalt-chromium stent. The stent has a unique design, consisting of three zones: 1. MB zone; 2. Transition zone; 3. SB zone. The MB zone is designed with minimal metal to artery ratio, composing three longitudinal fronds which terminate proximally in two circumferential “wedding bands”. This design allows definitive strut coverage at the SB ostium while allowing use of a standard drug-eluting stent (DES) in the MB with minimal strut overlap. The transition zone between the MB zone and the SB zone is composed of three panels which provide conformal carina coverage. The SB zone has an open cell design providing standard proximal SB scaffolding. During the study period, the Tryton stent was available mounted on three stent delivery balloons: one straight (2.5 mm diameter for both MB and SB) and two stepped with distal SB tapering (3.0 or 3.5 mm MB diameter and 2.5 mm SB diameter). The stent delivery system has a total of four markers. In addition to standard markers delineating the proximal and distal stent edges, there are two additional middle markers which delineate the transition zone. Stent placement is achieved by straddling the middle markers across the SB ostium, i.e., with the proximal middle marker in the MB and the distal middle marker in the SB (Figure 1). A single stent length was available (19 mm, with 10 mm MB zone, 4 mm transition zone and 5 mm SB zone), which was able to address a large spectrum of bifurcation lesions with MB diameters ranging between 2.5 and 3.5 mm and SB diameter of 2.5 mm.
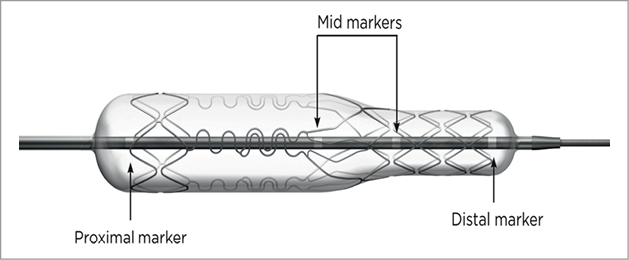
Figure 1. A Tryton stent premounted on a balloon, which is tapered distally at the location of the side branch zone. Stent placement is achieved by straddling the mid-markers across the side branch ostium, i.e., with the proximal of the two mid-markers in the main branch and the distal of the two mid-markers in the side branch.
INTERVENTIONAL PROCEDURE
All study patients were treated with acetylsalicylic acid (80-325 mg per day) and clopidogrel (300-600 mg loading-dose before the procedure, if needed, and 75 mg per day maintenance). Heparin was administered intravenously in boluses to maintain an activated clotting time ≥250 seconds during the procedure. Administration of glycoprotein IIB/IIIA inhibitors was left at the physician’s discretion.
Predilatation of the MB and/or the SB was left at the operator’s discretion. The Tryton stent was then positioned and deployed across the SB origin as described above. The SB wire was then redirected by withdrawing it into the proximal MB and advancing it into the distal MB. Sequentially, after predilatation of the MB stent struts, a standard “work-horse” stent was deployed in the MB. The procedure was completed after recommended final kissing balloon inflation (Figure 2 and Figure 3). The use of a DES in the MB was recommended but not mandatory.
Acetylsalicylic acid was continued indefinitely after the procedure, and clopidogrel was continued for 12-months.
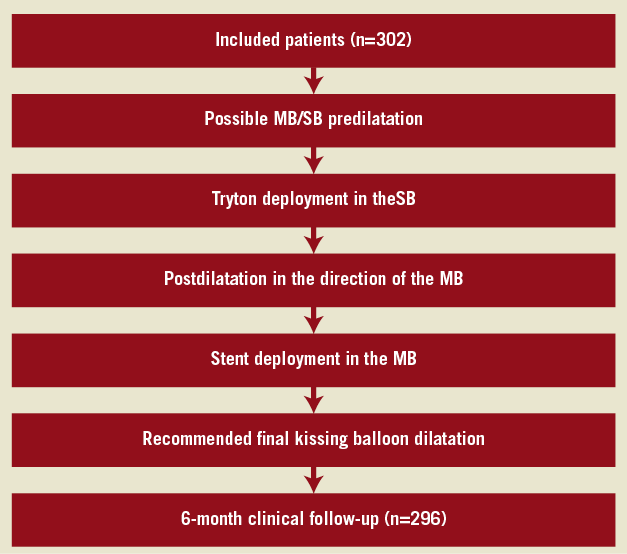
Figure 2. Flow-chart of the consecutive procedures. MB: main branch; SB: side branch
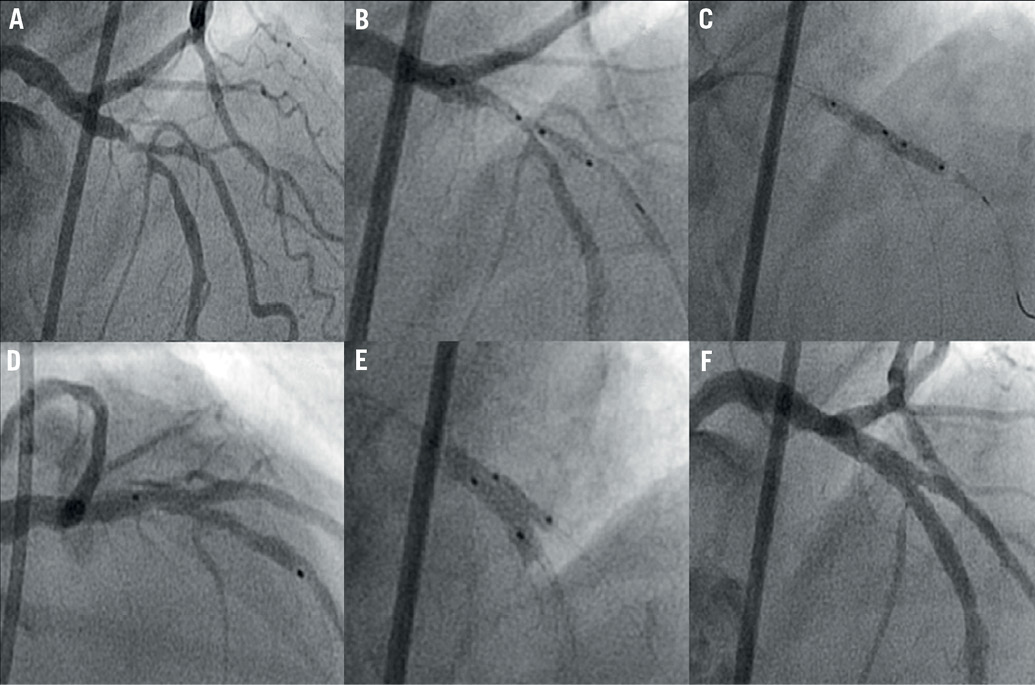
Figure 3. Angiographic overview of the procedure with the Tryton stent: A. Significant bifurcation stenosis of left anterior descending artery/diagonal branch (Medina 1,1,1); B. Positioning of Tryton stent from the proximal main branch in the direction of the side branch; C. Deployment of the Tryton stent, D. Drug-eluting stent placement in main branch applying a “modified coulotte” technique with the proximal part of the Tryton; E. Final kissing balloon inflation of the main and side branch; F. Final result.
FOLLOW-UP AND STUDY ENDPOINTS
Patients underwent clinical evaluation prior to hospital discharge and at six months.
The primary endpoint was the incidence of 6-month major adverse cardiac events (MACE), defined as a combined hierarchical endpoint of cardiac death, myocardial infarction, and target lesion revascularisation (TLR). Secondary endpoints were technical success, and procedural success.
Definitions follow the Academic Research Consortium criteria10. Briefly, spontaneous myocardial infarction was defined as any elevation of troponin (or other cardiac enzymes if troponin was lacking) combined with ischaemic chest pain. Periprocedural myocardial infarction was defined as an elevation of myocardial markers >3 times the upper limit of normal (troponin or creatine kinase-MB depending on availability at the specific study site). The relationship of the myocardial infarction to the target vessel was based on electrocardiographic changes or angiographic assessment when available. Target lesion revascularisation was defined as any repeat percutaneous or surgical coronary intervention due to a stenosis in the treated segment (including the whole stented segment plus 5 mm proximal and distal in the MB, and 5 mm distal in the SB). Technical success was defined as successful implantation of the Tryton stent at the intended lesion site. Procedural success was defined as technical success plus further treatment in the rest of the target lesion without the occurrence of in-hospital MACE. All patients’ files were monitored by the company itself and all outcomes were adjudicated by a clinical events committee consisting of physicians involved in the study.
STATISTICAL ANALYSIS
Continuous variables are presented as means ±standard deviations, categorical variables are presented as counts and percentages. For the most important endpoints of the study, 95% confidence intervals of the single point estimates were calculated using the dedicated Confidence Interval Analysis software (CIA, version 2.0.0).
Results
PATIENT AND PROCEDURAL CHARACTERISTICS
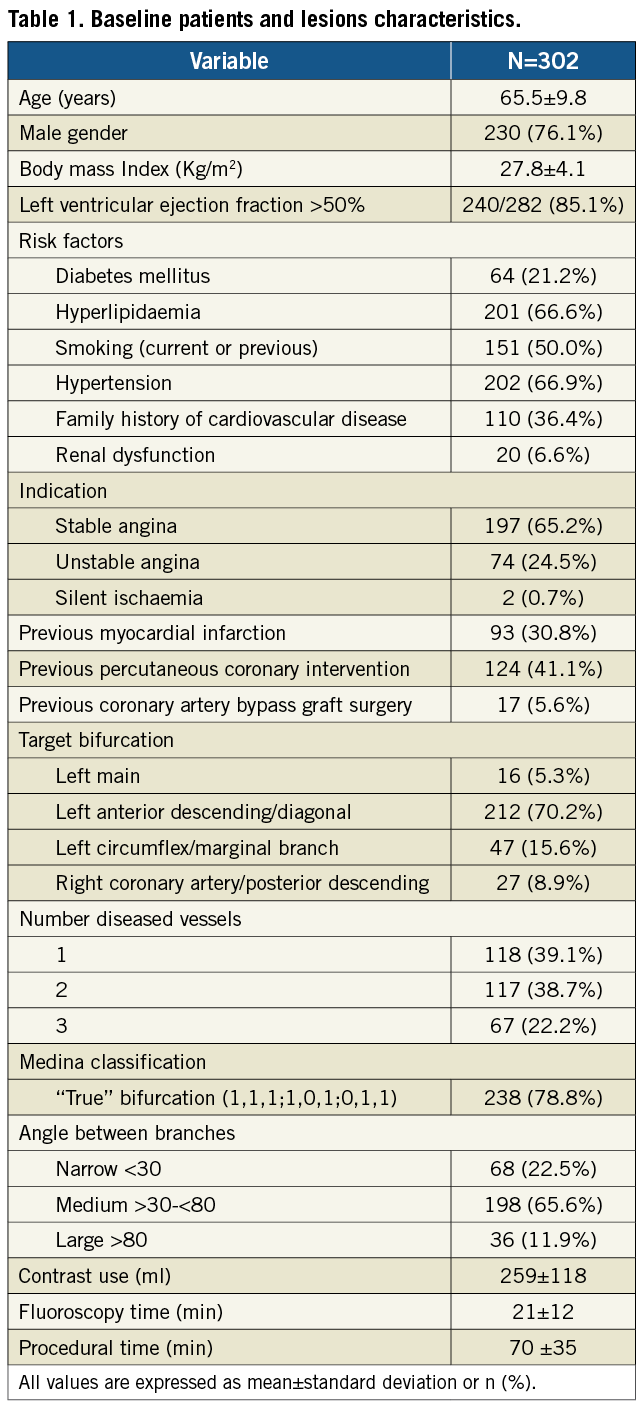
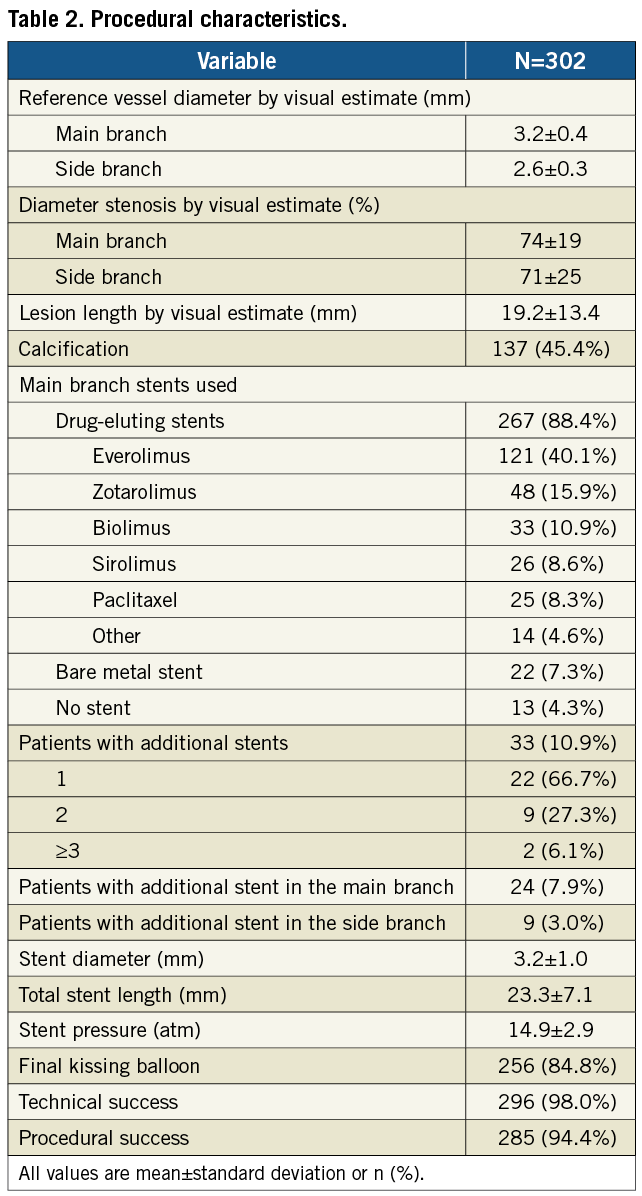
A total of 302 consecutive patients were enrolled in the study between January 2009 and October 2010 at 15 centres in The Netherlands, Belgium, Luxemburg, Ireland, Poland, Latvia, France and Spain. Baseline clinical characteristics are shown in Table 1. Procedural and angiographic characteristics are shown in Table 1 and Table 2.
IN-HOSPITAL ADVERSE EVENTS
Technical success and procedural success were achieved in 98% (95% confidence interval: 95.7%-99.1%) and 94.4% (91.2%-96.5%) of the patients, respectively. Of the 17 patients without procedural success, six were due to technical device failure (three failures to deliver stent, two stent detachments from the balloon, and one shaft breakage), none of them with clinical sequelae. Furthermore, 11 patients (3.7%) met the criteria for in-hospital MACE, all periprocedural myocardial infarctions. Eight myocardial infarctions were due to elevations of troponin without concomitant elevation of creatine kinase-MB, electrocardiographic changes or clinical sequelae. The other three myocardial infarctions were associated with significant creatine kinase-MB elevation, including one patient with untreatable SB occlusion (due to aggressive predilatation of an heavily calcified lesion, before unsuccessful attempt to deploy the Tryton stent), one patient with four overlapping stents in the MB (the left anterior descending artery) to treat a long diffuse disease with subsequent symptomatic occlusion of the septal branches, and one patient with periprocedural iatrogenic dissection involving the left main artery (non-target lesion) treated with stent placement with an otherwise good angiographic outcome.
ADVERSE EVENTS BETWEEN DISCHARGE AND 6-MONTH FOLLOW-UP
Follow-up at six months was available in 296 patients (98%). The clinical events are shown in Table 3. After discharge, two deaths, both non-cardiac, and three (1.0%) myocardial infarctions, respectively two, three and six weeks after the index procedure, were reported. The first patient with a myocardial infarction had a definite early stent thrombosis in the target lesion under DAPT, treated with a successful percutaneous revascularisation. This patient was known with previous coronary artery bypass grafting (CABG) and renal dysfunction. At baseline, a Medina (1,1,0) bifurcation lesion without severe calcifications was successfully treated with Tryton stent in the SB and DES in the MB, finishing with kissing balloon post-dilatation.
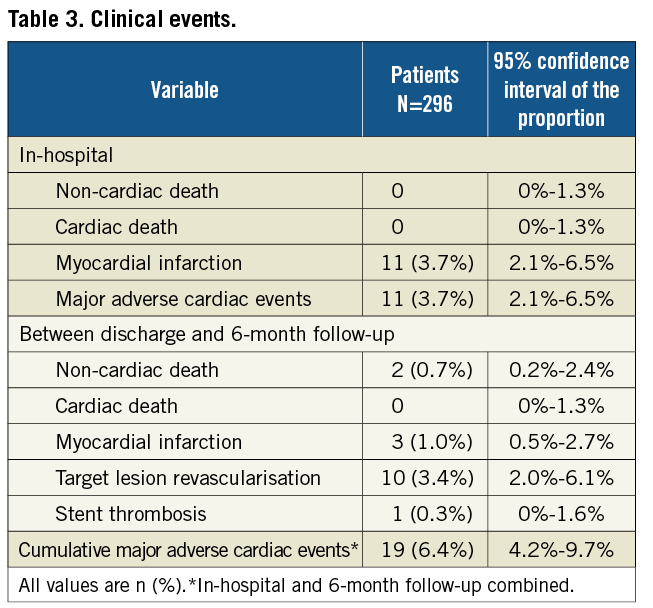
The second patient had a periprocedural myocardial infarction after staged PCI of a non-target vessel (the same patient underwent a TLR two months later). The third patient had a periprocedural myocardial infarction after a CABG for target lesion restenosis and progression of disease in other segments. In total, 10 TLR (3.4% [2.0%-6.1%]) occurred: eight were restenosis, one was stent thrombosis and one was a residual dissection initially untreated which was stented five days following the index procedure because of recurrent angina. In the TLR cases, the involved branches were the MB in seven cases, the SB in two cases and both the MB and SB in one case. Two patients underwent CABG, eight repeat PCI. The MACE rate between discharge and 6-month follow-up was 3.4%. The cumulative per-patient MACE rate at 6-month follow-up was 6.4% [4.2%-9.7%], counting 19 patients with 27 MACE events.
Discussion
The main findings of this registry are: 1) high procedural and technical success rates of the Tryton dedicated bifurcation stent, 2) low cumulative midterm MACE and TLR rates (6.4% and 3.4%, respectively), and 3) promising safety profile with only one stent thrombosis.
Bifurcations, which account for 15-20% of all lesions treated percutaneously1, remain hampered by procedural difficulties, post-procedural complications and suboptimal long-term results11. Recently, clinical results have improved with the introduction of DES. Still, in 10 studies (involving 1,452 patients) a single stenting strategy with DES showed a pooled weighted MACE rate of 10.9%3-5,12-18. On the other hand, a double stenting strategy with conventional DES did not improve outcomes: in 24 studies (involving 2,731 patients) it showed a pooled weighted MACE rate of 14.5%3-6,12,13,15,17,19-29.
Dedicated bifurcation stents have recently been introduced with the aim to simplify treatment and improve early and late outcomes following stenting of bifurcation lesions. Among these devices, the Tryton SB stent allows for an easier and improved culotte stenting approach.
As compared to the “classical” culotte technique, the Tryton makes the procedure easier due a distinctive design consisting of three zones: 1. an MB zone, with minimal metal to artery ratio allowing for easier re-crossing in the direction of the distal MB, easier successive deployment of a second stent in the MB and minimised amount of metal in the overlapping zone; 2. a transition zone, providing radial strength, effective coverage of the SB ostium and conformability to different bifurcation angles; and 3. an SB zone, scaffolding the proximal part of the SB in a conventional way.
Several specific aspects of this device deserve attention to explain the results of this study. First, Tryton allows for a “safety positioning” margin during stent placement provided by a generous 4 mm long transition zone (delineated by two radiopaque markers) which are placed across the SB origin. Because of its length and clearly defined margins, good angiographic results can be achieved even when the Tryton stent is deployed more proximally or distally than initially planned. Second, the low metal to vessel ratio of the Tryton in the proximal MB results in less overall metal in the proximal MB, once the MB stent is placed. Third, as the SB is routinely scaffolded with metal, this may permit more aggressive SB post-dilatation, potentially leading to improved stent apposition and to clinical advantages such as reduced restenosis and stent thrombosis. This expectation was confirmed in this registry with only 0.3% observed stent thrombosis at 6-months. Fourth, the specific stent design allows for a broad adaptability to different anatomies. Moreover, the transition zone, due to its mechanical properties, allows for improved conformability to different bifurcation angles.
Other dedicated bifurcation devices that have recently been introduced (Biosensors Axxess, Cappella Sideguard, Stentys, TAXUS Petal and Medtronic bifurcation system), showed interesting results30-34. However, these devices may be less adaptable to several of the bifurcation anatomies as they have less conformability to different bifurcation angles (Axxess), are more dependent on an exact deployment for a good acute angiographic result (Sideguard), provide a less accurate scaffolding of the SB ostium in case of additional provisional SB stenting (Stentys), and provide relatively low acute device success (<90%) due to technical difficulties during deployment (TAXUS Petal and Medtronic bifurcation system). The Tryton stent, due to its properties, may overcome these technical issues. However, no side-by-side comparison has been performed between Tryton and other dedicated bifurcation stents, hence no firm conclusion can be drawn.
Findings of the present study yield very promising results, confirming earlier reports8,9 of this current larger study. Although all these studies with the Tryton stent seemingly showed an improvement of bifurcation treatment, direct comparison of these findings with historical data from previous randomised data with DES treatment is difficult, since this registry did not use stringent exclusion and inclusion criteria as used in those trials. Therefore, a large randomised trial, necessary to put the promising results of this large registry in perspective, has been recently started, involving more than 700 patients (http://www.clinicaltrials.gov/ct2/show/NCT01258972?term=tryton&rank=1).
Study limitations
All limitations of a registry design apply to this study. Although in principle almost all patients with bifurcation lesions should have been enrolled in this registry, selection bias may have occurred when identifying patients for treatment with a Tryton or other available strategies. Second, the nature of this registry does not allow for direct comparison with a reference technique. Thus, randomised trials still have to be performed to place our findings in perspective. Third, there was no independent monitoring of data and clinical event committee. This may have introduced inaccuracies in the data. However, attention was paid to the registration and adjudication of data and monitoring. Fourth, the Tryton stent was already commercially available during this registry: this could have resulted in the use of the device without registration, leading to an underreporting of its use. Finally, the lack of systematic angiographic follow-up likely resulted in the under-detection of angiographic restenosis. However, angiographic follow-up is not routinely performed in clinical practice, resembling more closely a real-world setting. Besides, angiographic follow-up tends to increase the number of clinically unjustified revascularisations35,36.
Conclusion
In this large prospective registry, bifurcation stenting with the Tryton dedicated SB stent is safe and feasible, with high technical and procedural success rates. Moreover, midterm outcomes are very promising with very low TLR and MACE rates and with only one stent thrombosis at 6-month follow-up.
Sources of funding
Tryton medical supported all centres by providing the study stent.
Conflict of interest statement
P. R. Stella serves as member of the steering board of the ongoing Tryton IDE randomised clinical trial. All the other authors have no conflicts of interest to declare.




