Abstract
Aims: To assess outcomes with a new fully repositionable and retrievable valve for transcatheter aortic valve replacement (TAVR).
Methods and results: The Lotus Aortic Valve System is designed to facilitate precise positioning and minimise paravalvular regurgitation. REPRISE I enrolled symptomatic, high-surgical-risk patients with severe aortic stenosis. The primary endpoint (clinical procedural success) included successful implantation without major adverse cardiovascular or cerebrovascular events (MACCE). In all patients (N=11) the first Lotus Valve was successfully deployed. Partial resheathing to facilitate accurate placement was attempted and successfully performed in four patients; none required full retrieval. The primary endpoint was achieved in 9/11 with no in-hospital MACCE in 10/11. There was one major stroke; in another patient, discharge mean aortic gradient was 22 mmHg (above the primary endpoint threshold of 20 mmHg), but improved to 15 mmHg at 30 days. The cohort’s mean aortic gradient decreased from 53.9±20.9 mmHg at baseline to 15.4±4.6 mmHg (p<0.001) at one year; valve area increased from 0.7±0.2 cm2 to 1.5±0.2 cm2 (p<0.001). Discharge paravalvular aortic regurgitation, adjudicated by an independent core laboratory, was mild (n=2), trivial (n=1), or absent (n=8). Four patients required a permanent pacemaker post-procedure. There were no deaths, myocardial infarctions or new strokes through one year.
Conclusions: Initial results support proof-of-concept with the Lotus Valve for TAVR.
Introduction
Transcatheter aortic valve replacement (TAVR) has become a viable alternative for the treatment of severe symptomatic aortic stenosis in selected patients who are poor candidates for surgical valve replacement1. Encouraging short- and longer-term data on prosthetic valve function and clinical outcomes have been reported in a number of large observational registries from various countries2-8. In the randomised Placement of AoRTic TraNscathetER Valves (PARTNER) trial, patients unsuitable for surgical valve replacement who underwent TAVR experienced significant reductions in mortality and repeat hospitalisation compared to those receiving conventional medical therapy through two years9; high-surgical-risk patients receiving either TAVR or surgical replacement had a similar mortality risk10.
Notwithstanding these favourable results, TAVR with early-generation devices has been associated with increased stroke risk versus surgical valve replacement10,11, and paravalvular regurgitation more commonly seen with TAVR compared to surgery may be associated with higher early and late mortality7,10,12,13. While judicious patient selection may serve to mitigate these risks14-16, device design improvements may enable more precise placement and minimise or eliminate paravalvular regurgitation. The transcatheter Lotus™ Aortic Valve (Boston Scientific Corporation, Natick, MA, USA) is fully retrievable and repositionable with a unique adaptive seal designed to minimise paravalvular regurgitation. The first human implantation of the initial Lotus Valve has been described previously17. We describe here the one-year results with a later version of the Lotus prosthesis in the prospective, single-arm REPRISE I feasibility study which was designed to assess the acute safety and performance of this novel system in patients at high risk for surgical intervention (ClinicalTrials.gov registration number NCT01383720).
Methods
DEVICE DESCRIPTION
The Lotus™ Aortic Valve Replacement System (Figure 1; Boston Scientific Corporation) has been described previously18. Briefly, the system includes a bioprosthetic aortic valve implant consisting of three bovine pericardial leaflets attached to a braided nitinol frame with a radiopaque marker and a catheter-based system for introduction and retrograde delivery via the femoral artery. One valve size, 23 mm, was available for this study. The valve is pre-attached to the delivery system. The Lotus Valve functions early in deployment, aiding controlled, precise initial positioning, repositioning or full retrieval at any point prior to release if required. Rapid pacing is not required during the implant procedure. The valve is designed to expand radially as the valve shortens during deployment. An adaptive seal surrounds the inflow portion of the device and is designed to minimise paravalvular regurgitation. The device was introduced percutaneously through a dedicated introducer sheath (outer diameter the same size as a conventional 18 Fr sheath) via the femoral artery using conventional percutaneous catheterisation techniques or via a surgical cut-down. Patients with a femoral artery lumen diameter ≥6.0 mm were eligible for inclusion in the trial.
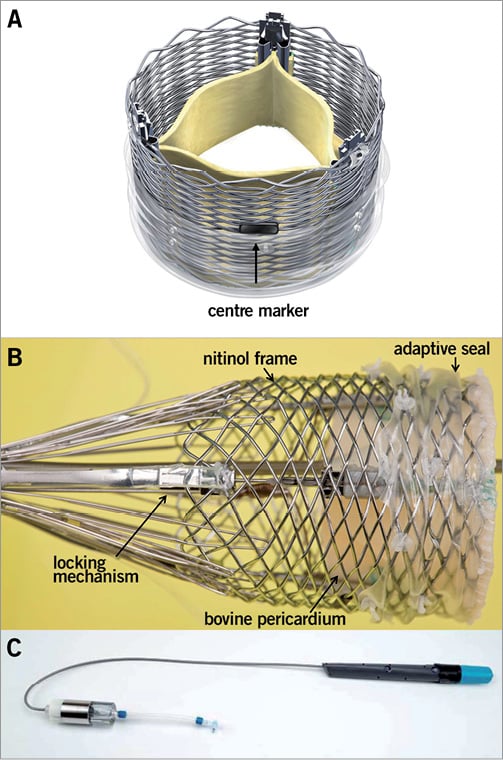
Figure 1. Lotus Aortic Valve Replacement System. Pictured is the 23 mm Lotus Valve with three bovine pericardial tissue leaflets and a central radiopaque marker to aid positioning (A), a polyurethane/polycarbonate outer seal to minimise paravalvular leakage and a braided nitinol frame with post- and buckle- locking mechanisms for valve stabilisation in vivo (B). The braided structure foreshortens and expands radially when delivered and is locked in position using the post- and buckle- locking mechanism. The Lotus Delivery Catheter has a handle with one control used to deploy the valve, a second control to detach the deployed valve and a guard to prevent inadvertent release (C).
PATIENT SELECTION
Enrolled patients had symptomatic aortic valve stenosis with New York Heart Association (NYHA) functional Class ≥II and documented calcific aortic valve stenosis, with an initial aortic valve area (AVA) of <1.0 cm2 (or AVA index of <0.6 cm2/m2), and either a mean pressure gradient >40 mmHg, or a jet velocity >4 m/s, as measured by transthoracic echocardiography (TTE). Patients were deemed high-risk based on a Society of Thoracic Surgery score ≥8%19, a logistic EuroSCORE ≥20%20 or multidisciplinary Heart Team (including an interventional cardiologist and a cardiothoracic surgeon) agreement that frailty and/or coexisting comorbidities would be associated with a high surgical risk. Patients had a documented aortic annulus size between 19 and 22 mm (able to accommodate the 23 mm Lotus Valve). Key exclusion criteria included congenital unicuspid or bicuspid aortic valve, acute myocardial infarction (MI), transient ischaemic attack (TIA) or stroke within the previous six months or any permanent neurological defect, severe renal insufficiency, pre-existing prosthetic heart valve (aortic or mitral) or a prosthetic ring in any position, more than moderate (>2+) mitral or aortic regurgitation, untreated clinically significant coronary artery disease likely to require revascularisation after the procedure and documented left ventricular ejection fraction below 30%. Complete inclusion and exclusion criteria are provided in Online Appendix A.
The study was approved by the ethics committee at each participating centre and all patients signed written informed consent before undergoing any study-specific tests or procedures. Screening materials from patients identified by the investigators as having met the inclusion and exclusion criteria were reviewed by a Case Review Committee to assess and confirm eligibility. The committee included the study principal investigator (PI), other investigators experienced with TAVR and sponsor representatives. Patients were considered enrolled once an attempt was made to insert the Lotus introducer sheath into the femoral artery.
PROTOCOL
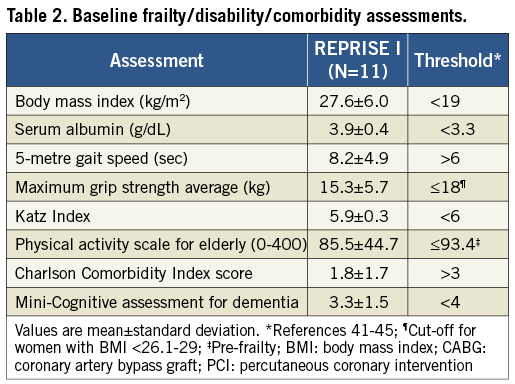
Screening data reviewed by the Case Review Committee included TTE, coronary angiography, computed tomography angiography of the aortic valve and entire aorta, computed tomography angiography or invasive angiography of the iliofemoral system, Society of Thoracic Surgery score, logistic EuroSCORE, modified Rankin Scale score and the Heart Team assessment. Comprehensive frailty assessments were made prospectively including number of falls in the past six months, average maximum grip strength, 5-metre gait speed21, Katz Index22, Physical Activity Scale for Elderly23, Charlson comorbidity index score24, and the Mini-Cognitive assessment for dementia25.
All operators and medical personnel completed comprehensive training prior to implanting patients and received on-site proctorship during implant procedures from a trained proctor experienced in TAVR.
Unfractionated heparin was administered before the procedure started and all implants were performed under general anaesthesia with transoesophageal echocardiographic guidance and with a temporary pacing wire inserted into the right ventricle. The Lotus introducer sheath was passed through the femoral and iliac arterial system and positioned in the descending aorta. A super stiff guidewire (Amplatz; Boston Scientific Corporation) was advanced across the aortic valve and into the left ventricle under fluoroscopic guidance. Balloon valvuloplasty was carried out with rapid ventricular pacing and the Lotus Valve subsequently positioned in the aortic valve annulus. Rapid ventricular pacing was not performed during valve implantation. A case study from REPRISE I with images and a detailed description of the Lotus Valve implantation procedure has been published18. Valve position was assessed by TTE and contrast aortography and the valve repositioned if necessary prior to final release. After hospital discharge, clinical follow-up was scheduled for 30 days, 3 months, 6 months, 12 months and then annually from two to five years.
Aspirin (≥150 mg) and clopidogrel (75 mg) once a day for five days prior to the procedure or a loading dose of ≥300 mg pre-implant were required. After the procedure, daily aspirin (100 mg) was required for three months and recommended indefinitely. Clopidogrel (75 mg) once a day was required for three months.
DATA MANAGEMENT, ENDPOINTS AND ADDITIONAL MEASUREMENTS
Study monitors verified all case report form data on site. An independent echocardiography core laboratory (Victor Davila-Román, MD; CVR Consulting, PC; St. Louis, MO, USA) reviewed all images for qualitative and quantitative analysis. All 12-lead electrocardiograms were sent to a core laboratory (Peter J. Zimetbaum, MD; Harvard Clinical Research Institute; Boston, MA, USA) for independent analysis.
The primary endpoint was clinical procedural success, defined as successful implantation of a Lotus Valve (device success) without in-hospital major adverse cardiovascular and cerebrovascular events (MACCE, including all-cause mortality, periprocedural MI ≤72 hours, major stroke, urgent/emergent conversion to surgery or repeat procedure for valve-related dysfunction) through discharge or seven days post-procedure, whichever came first. Device success, MI, and stroke were defined in accordance with Valve Academic Research Consortium definitions (VARC-1)26. Pre-specified secondary endpoints included aortic valve regurgitation and successful repositioning and retrieval of the Lotus Valve System, if attempted.
An independent Clinical Events Committee composed of interventional cardiologists, cardiothoracic surgeons and a neurologist adjudicated death, MI, neurologic events, valve-related dysfunction leading to urgent/emergent conversion to surgery or repeat procedure, bleeding, acute kidney injury, vascular complications, symptomatic coronary obstruction, valve thrombosis, endocarditis, new conduction disturbances and cardiac arrhythmias, requirements for new permanent pacemaker and repeat hospitalisation due to valve- or procedure-related clinical deterioration. Additional measurements at clinical follow-up included valve performance as assessed by TTE, cardiac function as measured by echocardiography, NYHA functional class and health status as evaluated by SF-12 and EQ-5D Quality of Life questionnaires.
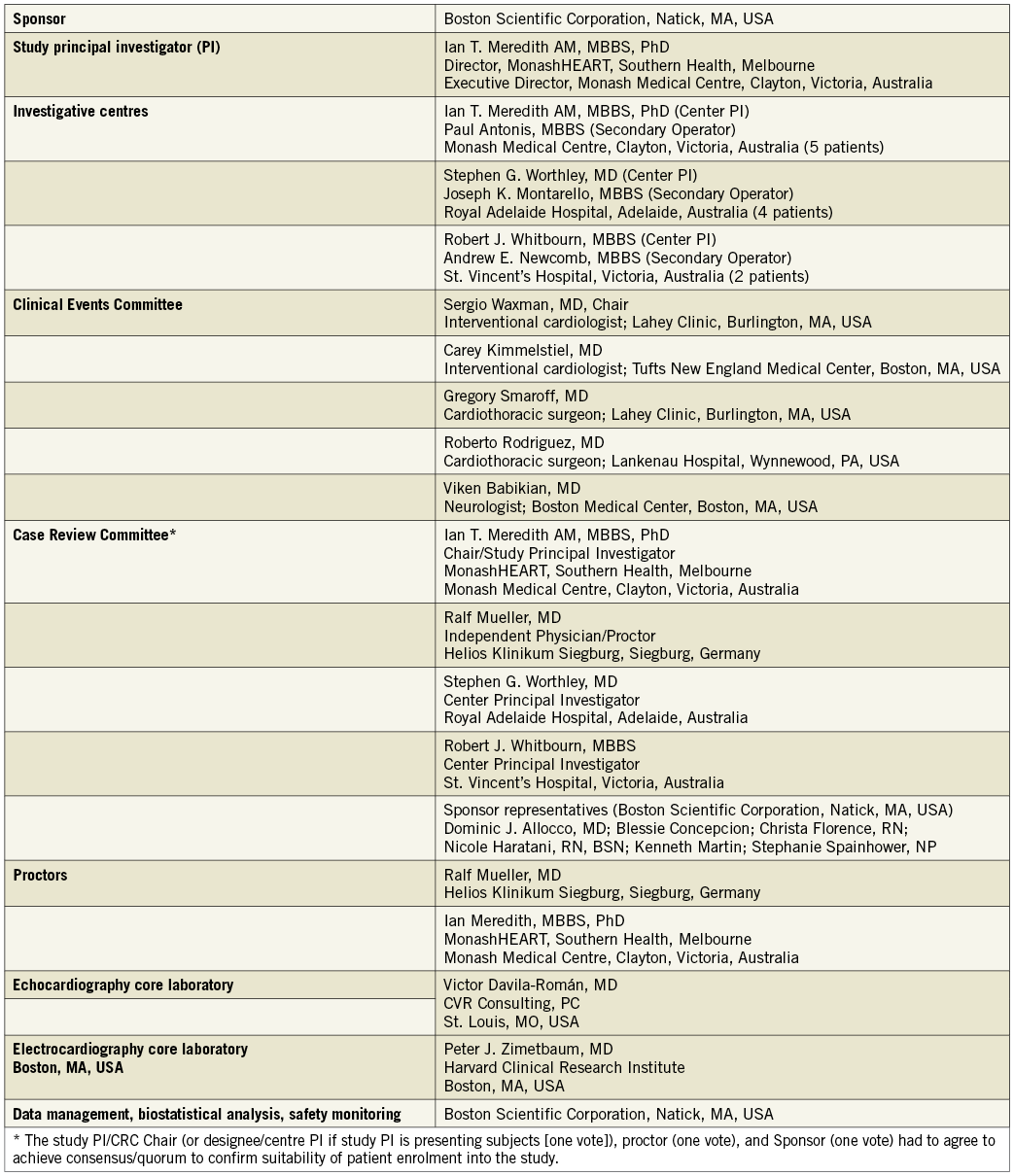
Endpoints and measurements are listed in Online Appendix B and major endpoint definitions are provided in Online Appendix C. Study organisation and oversight committee membership are provided in Online Appendix D.
STATISTICAL METHODS
No formal statistical testing was performed for the primary endpoint in this single-arm feasibility study. Subject demographics, clinical history, risk factors, device performance and safety outcomes are summarised using descriptive statistics for continuous variables and frequency tables for discrete variables. P-values for continuous variables are from the paired Student t-test; p-values for comparison of NYHA class distribution are from the Wilcoxon signed rank test for paired data; p-values for the comparison of repeated measures such as mean aortic gradient are from the repeated measures and random effects ANOVA model. All statistical analyses were undertaken with SAS software (version 8.2 or above; SAS Institute, Inc., Cary, NC, USA).
Results
PATIENTS
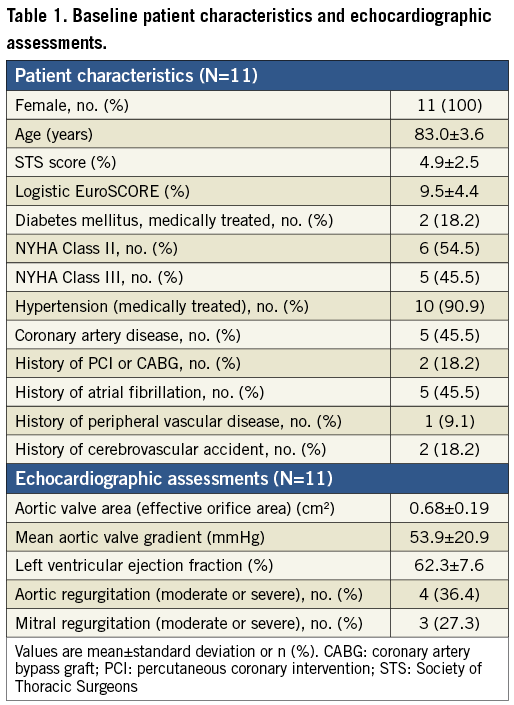
Between April 14 and April 20, 2012, 11 patients were enrolled at three investigative sites in Australia (Online Appendix D). There were 15 patients assessed for inclusion and four were deemed not suitable by the Case Review Committee due to aortic annulus too big for a 23 mm valve (n=1), very low take-off of the coronary arteries (n=1), and/or vascular access vessels too small (n=2). Table 1 shows baseline patient characteristics and echocardiographic assessments. All patients were female and mean age was 83.0±3.6 years. The mean Society of Thoracic Surgeons (STS) score and logistic EuroSCORE were 4.9±2.5% and 9.5±4.4%, respectively. All patients were confirmed by the Heart Team to be at high risk for surgery due to frailty or associated comorbidities (Table 2).
OUTCOMES
PROCEDURE
In all patients the first Lotus Valve was successfully deployed. Limited recapture to facilitate accurate final positioning was easily accomplished in all cases attempted (n=4). Valve retrieval was not required and thus not attempted in any patient. Procedure and fluoroscopy time were 110.4±34.7 minutes and 36.9±8.8 minutes, respectively, with 200±74.3 cc of contrast used.
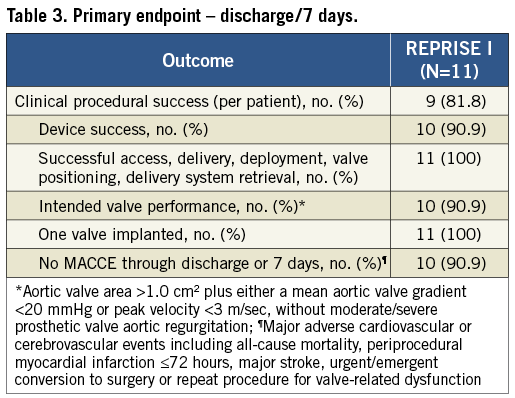
PRIMARY ENDPOINT
The primary endpoint was met in 9/11 patients (Table 3); one patient (Patient A) experienced an in-hospital stroke and one patient (Patient B) experienced device failure. The major ischaemic stroke occurred two days post-index procedure; the modified Rankin Scale score in this patient was 0 at baseline and 3 at one year. Patient B experienced a device failure based on mean aortic valve gradient of 22.1 mmHg and peak velocity of 328 cm/s. Although this event met one of the VARC-1 criteria for device failure, the core lab noted that the valve appeared to be functioning well and the mildly elevated gradient and velocity were likely related to increased flow across the aortic valve. The AVA was 1.6 cm2 and the left ventricular outflow tract/ascending aorta time-velocity integral ratio was 0.51 at discharge. At 30-day and one-year follow-up, the mean transvalvular gradients were 15.0 and 20.0 mmHg, respectively, and the peak velocities were 279 cm/s and 301 cm/s, respectively.
CLINICAL OUTCOMES
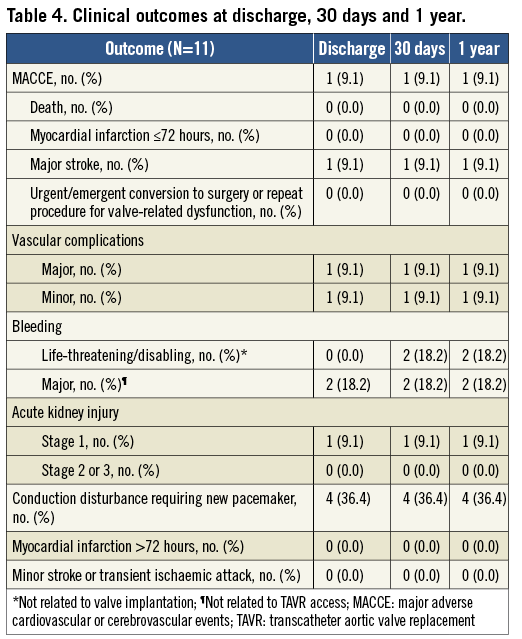
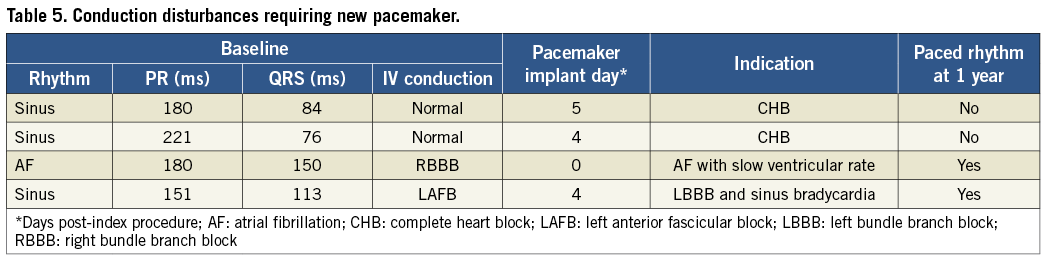
Table 4 shows clinical outcomes at discharge, 30 days, and one year. There were no additional MACCE events beyond the primary endpoint. The VARC-1 combined safety endpoint, including MACCE, life-threatening/disabling bleeding, major vascular complications, and Stage 3 acute kidney injury26, was 3/11 through one year. Patient A experienced a small left femoral dissection that was successfully treated with balloon inflation during the procedure but qualified as a VARC-1 major vascular complication due to the balloon inflation. There were two life-threatening/disabling bleeds through 30 days; neither was related to valve implantation and both resolved. In one case, the patient was successfully treated with pericardiocentesis on day 14 for an event considered related to permanent pacemaker implantation on day four. Another patient had a gastrointestinal bleed on day 20 and received transfusion of multiple units of packed red blood cells. There were also two major bleeding events that occurred in the periprocedural period; neither was associated with TAVR access. One patient developed a haematoma at the site of a left brachial arterial line and another developed one at the site of the right internal jugular sheath. In Patient B and three other patients, conduction disturbances led to implantation of a permanent pacemaker before discharge; two of these four patients had paced rhythm at one year (Table 5). A single REPRISE I patient experienced new-onset atrial fibrillation, which occurred at day 52 post-procedure. While all REPRISE I patients were NYHA Class II (n=6) or III (n=5) at baseline, this distribution was significantly improved between baseline and 30 days (three in Class I, seven in Class II, one in Class III; p=0.02) and baseline and one year (five in Class I, six in Class II; p=0.004).
ECHOCARDIOGRAPHY
Valve performance data as determined by independent core lab analyses of TTE outcomes are shown in Table 6 and Figure 2- Figure 4. Changes in peak aortic velocity, mean and peak aortic valve gradient and effective orifice area were statistically significant from baseline to discharge, baseline to 30 days and baseline to one year (p<0.001 for each paired analysis). Figure 2 shows mean aortic valve gradient and Figure 3 shows effective orifice area by patient at baseline and at each time point. There was no moderate or severe paravalvular aortic regurgitation observed in any patient at any time point (Figure 4). At one year, one patient had mild regurgitation, one patient had trivial and nine patients had none.
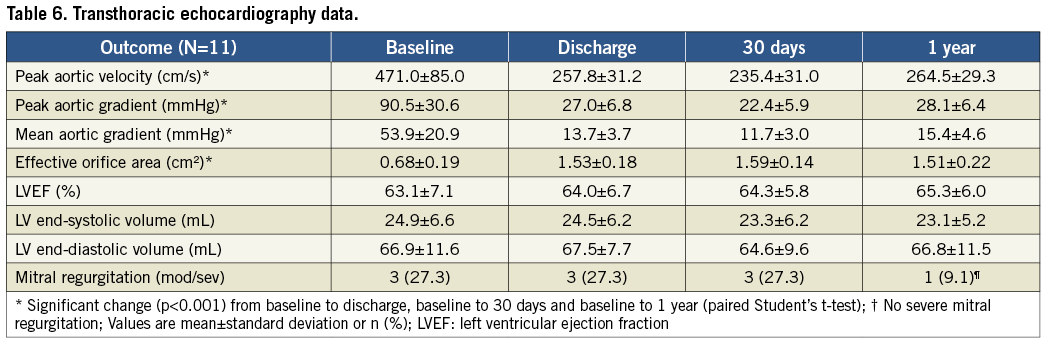
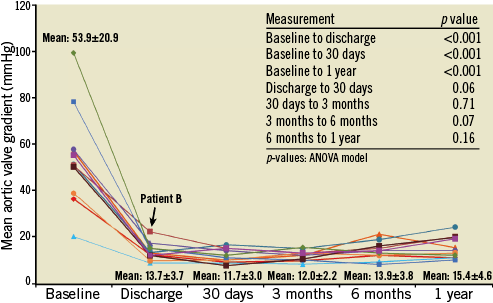
Figure 2. Mean aortic valve gradient per patient by transthoracic echocardiography. Data are shown for each of the 11 patients at the five time points; cohort mean values at each time point include standard deviation.
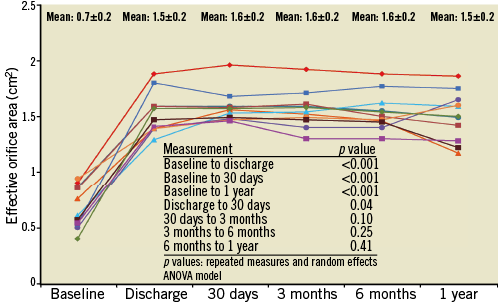
Figure 3. Effective orifice area per patient by transthoracic echocardiography. Data are shown for each of the 11 patients at the five time points; cohort mean values at each time point include standard deviation.
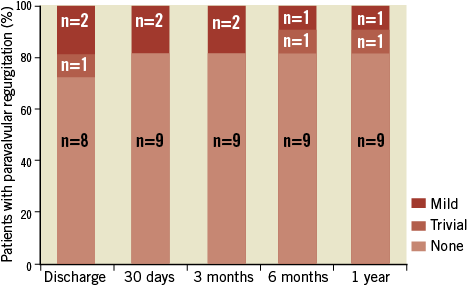
Figure 4. Paravalvular aortic regurgitation. There were no cases of moderate or severe paravalvular regurgitation.
Discussion
This feasibility study assessed the acute safety and performance of the fully repositionable transcatheter Lotus Aortic Valve Replacement System in symptomatic high-risk surgical patients with calcific aortic valve stenosis. The prosthetic valve was positioned successfully in all patients (N=11) with no moderate or severe aortic regurgitation after placement or through one year. Clinical procedural success was achieved in 9/11 patients (one major stroke and one device failure based on a slightly elevated aortic gradient/velocity which improved with time), with no additional MACCE through one year. All patients were alive at one year and there was a marked improvement in functional class across the cohort compared to baseline. Thus, these results support proof of concept with the Lotus Valve in TAVR in this patient subset.
PATIENT SELECTION
As existing risk scores imperfectly characterise risk, each centre’s Heart Team considered other comorbidities and patient frailty (Table 2) in addition to using STS and EuroSCORE. While not captured well by any of the standard risk scores, these added measures helped to more fully characterise a patient population that potentially benefits from TAVR.
VALVE FUNCTION
The novel Lotus Valve System incorporates several features intended to improve upon early-generation devices. The valve functions early in deployment, providing haemodynamic stability and allowing controlled, precise deployment, recapture and subsequent repositioning/redeployment or removal as necessary. Repositioning was successful in all attempted cases (n=4), with no requirements for full retrieval. As described in a REPRISE I case study, the capacity to retrieve and reposition the device easily allowed for more optimal annular positioning even when initial valve placement was considered acceptable27. Clinical procedural success was high (9/11) and comparable to results reported with other bioprosthetic aortic valves2,3,28-30. The one patient with device failure had a gradient that was slightly above the VARC-1 threshold, and this failure was likely due to increased flow across the aortic valve as opposed to any valve dysfunction. Mean transvalvular gradient and peak velocity for this patient improved at later time points. In the overall cohort, there was a significant improvement in valve function from a mean aortic gradient of 53.9±20.9 mmHg at baseline to 15.4±4.6 mmHg at one year (p<0.001).
PARAVALVULAR REGURGITATION
Implantation of the Lotus Valve with its adaptive seal designed to mitigate paravalvular regurgitation resulted in 8/11 patients with no paravalvular regurgitation at discharge and trivial or mild regurgitation in the others. This result was maintained at one year as one patient had trivial, one had mild, and nine had no regurgitation (Figure 4). Reported moderate or severe aortic regurgitation after TAVR has ranged from 6% to 21%31. In the FRANCE 2 registry8, 30-day paravalvular regurgitation of grade 2 or more (on a scale of 0 to 4) post-implantation of commercially available TAVR devices was an independent predictor of one-year mortality. In the Italian CoreValve registry7, post-procedural paravalvular leak ≥2+ independently predicted mortality between 30 days and one year. While the effect of paravalvular regurgitation on mortality was proportional to its severity in the PARTNER trial, an increased rate of late deaths through two years was seen with even mild regurgitation10. The current results with the Lotus Valve compare very favourably to aortic regurgitation outcomes in the first-human-use study of another repositionable valve32 as well as with other commercially available transcatheter valves3,4,6-8,33.
PACEMAKER IMPLANTATION
Reported rates for early conduction abnormalities and the need for pacemaker implantation after TAVR have ranged from 3% to 40%1,6-8,11,33-39. Of the four REPRISE I patients who required implantation of a new permanent pacemaker before discharge, three had conduction abnormalities at baseline; only two patients had paced rhythm at one year. Pre-existing conduction disease has been identified as a predictor of permanent pacemaker implantation post TAVR40. A recent account following transfemoral TAVR noted similar 12-month clinical outcomes among patients with periprocedural permanent pacemaker implantation compared to those without39.
Study limitations
Limitations of this study include the small number of patients typical of a human feasibility study which was insufficient to provide accurate estimates of clinical event rates, and the absence of a randomised control group. The currently used surgical risk scores imperfectly characterise surgical risk. The centre Heart Team assessment thus included factors not accounted for by these risk scores (Table 2). The absence of mortality through one year could be due, at least in part, to the inclusion of lower-risk patients. The study was conducted with a single, small valve size and as a result all participants were female; thus the results of this trial may not be representative of outcomes in an unselected population. Greater understanding of the impact of this technology on paravalvular leak and the need for permanent pacemakers can only be drawn from a larger study approximating a more normal distribution of aorto-annular dimensions.
Conclusions
In this small feasibility study, the Lotus Valve could be positioned precisely and successfully with minimal aortic regurgitation after placement. Haemodynamic and clinical benefits achieved upon valve implant have been sustained out to one year with a low rate of adverse events in this patient population. The larger REPRISE II study in high-risk surgical patients will further evaluate the safety and efficacy of this novel bioprosthetic aortic valve.
Acknowledgements
The authors thank Carola Alfaro, M.S., and Edmund McMullen, M. Math, for assistance with statistical analyses, Ruth M. Starzyk, Ph.D, for assistance with drafting and editing the manuscript and Blessie Concepcion for critical review of the manuscript (all from Boston Scientific Corporation, Natick, MA, USA). REPRISE I is sponsored and funded by Boston Scientific Corporation.
Funding source
Boston Scientific Corporation, Natick, MA, USA.
Conflict of interest statement
I.T. Meredith reports receiving consultant fees and honoraria from Abbott Vascular, Boston Scientific, and Medtronic. S.G. Worthley reports receiving consultant fees and honoraria from Medtronic and St. Jude. A.E. Newcomb reports receiving unrestricted educational funding from Boston Scientific. S. Lockwood reports receiving consulting fees from Boston Scientific. Ms. Haratani was a full-time employee of Boston Scientific and a stockholder of Sadra Medical and Boston Scientific Corporation. D.J. Allocco and K.D. Dawkins are full-time employees and stockholders of Boston Scientific Corporation. R.J. Whitbourn, P. Antonis, and J.K. Montarello have no conflicts of interest to declare.
The references can be found in the online version of the paper.
Online data supplement
Online Appendix A. REPRISE I inclusion and exclusion criteria.
Online Appendix B. REPRISE I endpoints and additional measurements.
Online Appendix C. REPRISE I endpoint definitions (major endpoints).
Online Appendix D. REPRISE I study organisation and processes.
Appendix A. REPRISE I inclusion and exclusion criteria.
Appendix B. REPRISE I endpoints and additional measurements.
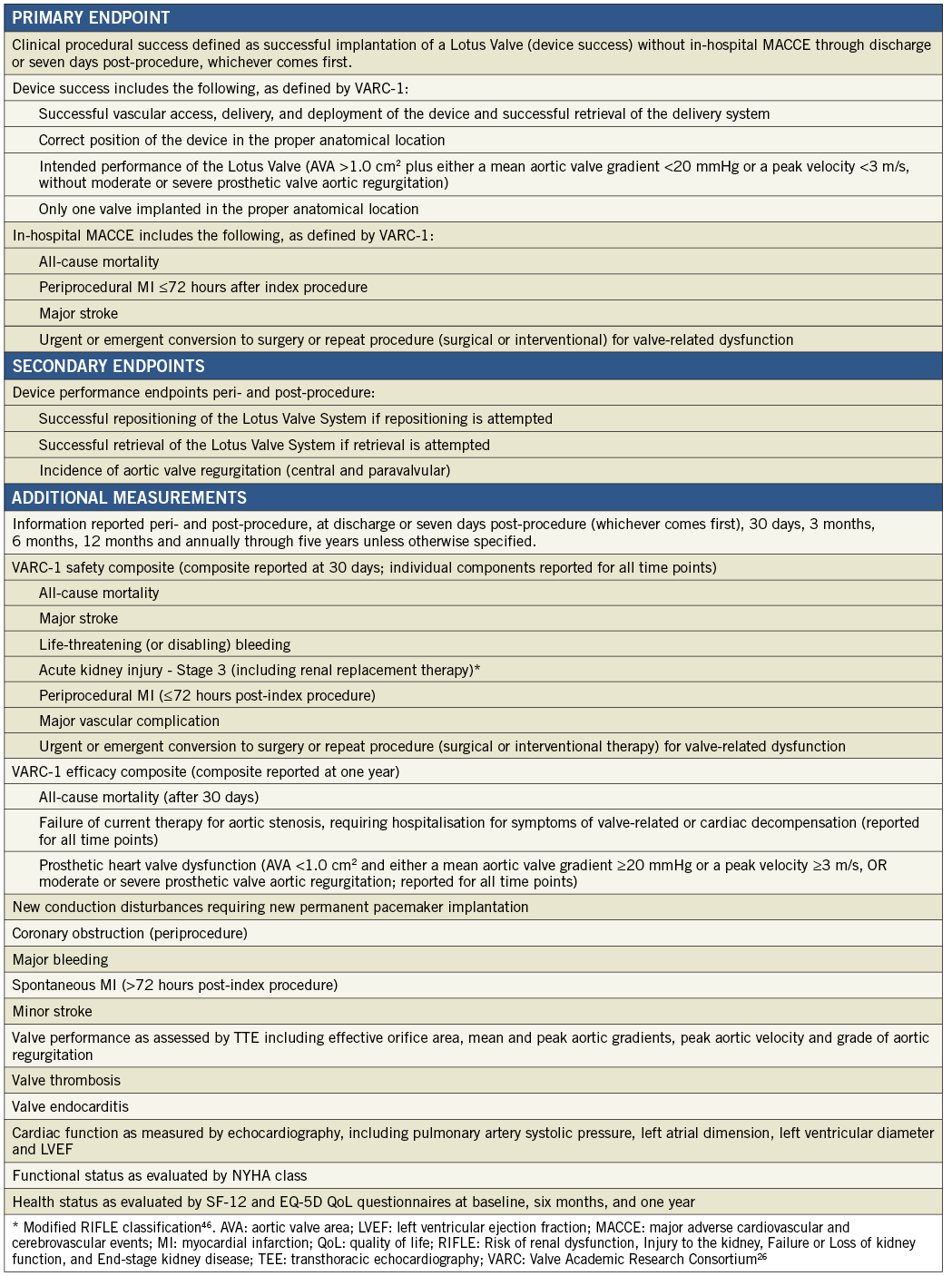
Appendix C. REPRISE I endpoint definitions (major endpoints).

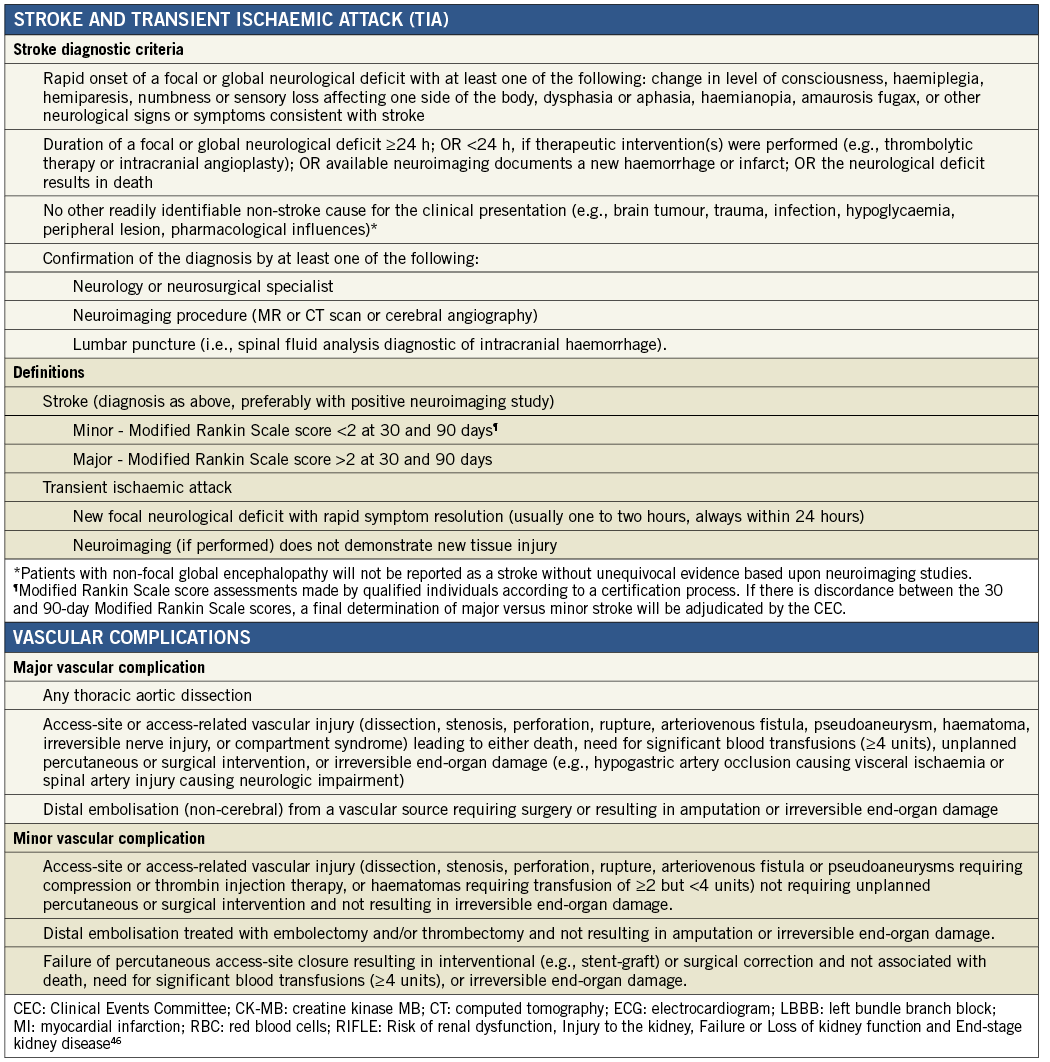
Appendix D. REPRISE I study organisation and processes.