Published online Jun 15, 2022. doi: 10.4251/wjgo.v14.i6.1175
Peer-review started: January 14, 2022
First decision: March 13, 2022
Revised: April 1, 2022
Accepted: May 17, 2022
Article in press: May 17, 2022
Published online: June 15, 2022
Neoadjuvant therapy (NT) has increasingly been utilized for patients with localized pancreatic ductal adenocarcinoma (PDAC). It is the recommended approach for borderline resectable (BR) and locally advanced (LA) cancers and an increasingly utilized option for potentially resectable (PR) disease. Despite its increased use, little research has focused on patient-centered metrics among patients undergoing NT, including patient experiences, preferences, and recommendations. A better understanding of all aspects of the patient experience during NT may identify opportunities to design interventions aimed at improving quality of life; it may also facilitate the completion of NT and receipt of surgery, ultimately optimizing long-term outcomes.
To understand the experience of patients initiating and receiving NT to identify opportunities to improve neoadjuvant cancer care delivery.
Semi-structured interviews of patients with localized PDAC during NT were conducted to explore their experience initiating and receiving NT. Interviews took place between August 2020 and October 2021. Due to the descriptive nature of the research, questions were open ended. Interviews were conducted over the phone, audio recorded and then transcribed. All interviews were coded by two inde
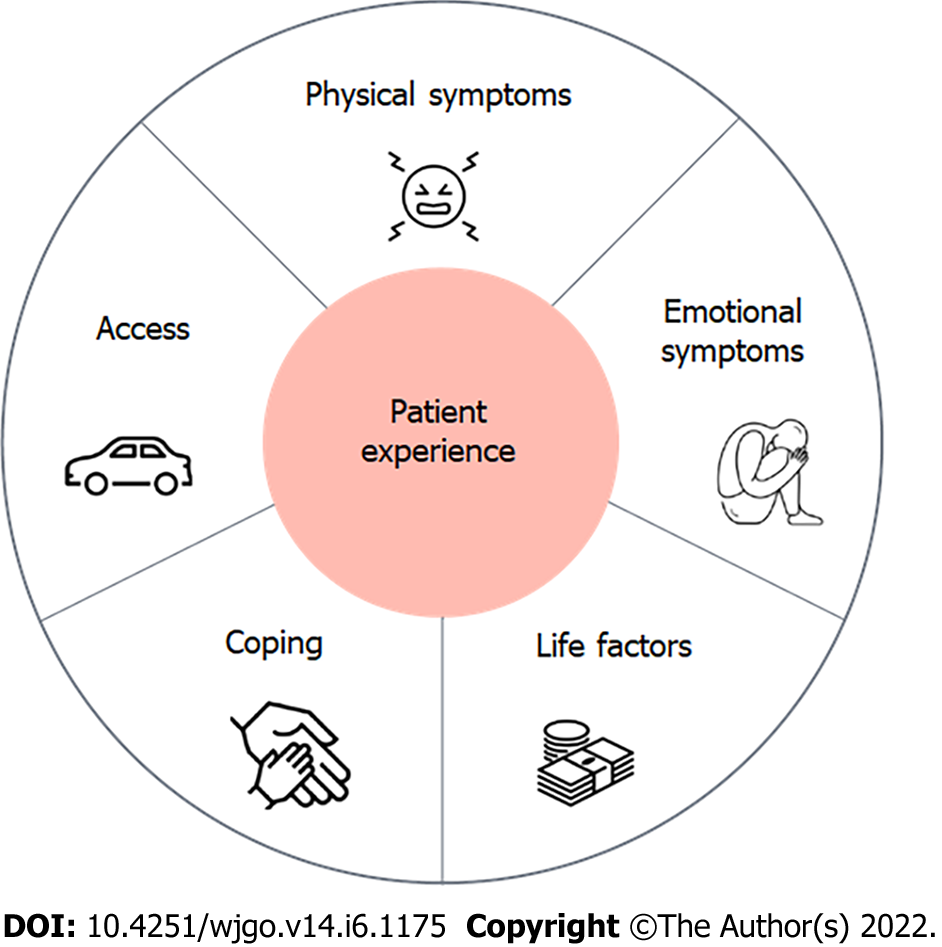
A total of 12 patients with localized PDAC were interviewed. Patients with BR (n = 7), PR (n = 2), and LA (n = 3) cancers participated in the study. All patients indicated that choosing NT was the doctor’s recommendation, while most reported not being familiar with the concept of NT (n = 11) and that NT was presented as the only option (n = 8). Five themes describing the patient experience emerged: physical symptoms, emotional symptoms, coping mechanisms, access to care, and life factors. The most commonly cited recommendation for improving the experience of NT was improved education before and during NT (n = 7). Patients highlighted the need for more information on the rationale behind choosing NT prior to surgery, the anticipated surgery and its likelihood of surgery occurring after NT, as well as general information prior to starting NT treatment. The need for seeing different members of the healthcare team, including ancillary services was also frequently cited as a recommendation for improving the experience of NT (n = 5).
This study provides a framework to allow for a better understanding of the PDAC patient experience during NT and highlights opportunities to improve quality and quantity of life outcomes.
Core Tip: This study aims to understand the experience of localized pancreatic ductal adenocarcinoma (PDAC) patients initiating and receiving neoadjuvant therapy (NT). Semi-structured interviews of patients with localized PDAC during NT were conducted; 12 patients were interviewed. All patients indicated that choosing NT was the doctor’s recommendation. Most reported not being familiar with the concept of NT. Five themes describing the patient experience emerged. This study provides a framework to allow for a better understanding of the patient experience and highlights opportunities to improve quality and quantity of life outcomes for patients with PDAC.
- Citation: Stevens L, Brown ZJ, Zeh R, Monsour C, Wells-Di Gregorio S, Santry H, Ejaz AM, Pawlik TM, Cloyd JM. Characterizing the patient experience during neoadjuvant therapy for pancreatic ductal adenocarcinoma: A qualitative study. World J Gastrointest Oncol 2022; 14(6): 1175-1186
- URL: https://www.wjgnet.com/1948-5204/full/v14/i6/1175.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i6.1175
The delivery of chemotherapy and/or radiation therapy prior to surgery, known as neoadjuvant therapy (NT), is increasingly utilized for patients with pancreatic ductal adenocarcinoma (PDAC)[1,2]. Since a significant proportion of patients are unable to receive all intended adjuvant therapies following major pancreatectomy, NT ensures the receipt of some systemic therapy and leads to improved rates of multimodality therapy. NT also improves margin-negative resection rates, enhances patient selection by ensuring the absence of rapid tumor progression prior to surgery, enables an in vivo test of the efficacy of chemotherapy, and based on emerging evidence from randomized controlled trials, may lead to improved overall survival[3-7]. Based on these advantages, NT is now the recommended approach for borderline resectable (BR) and locally advanced (LA) cancers and an increasingly utilized option for potentially resectable (PR) disease according to national guidelines[3,8,9].
Despite its increasing use in PDAC and other cancer types[2], little is known about the patient experience during NT. Indeed, the neoadjuvant time period might be particularly distressing for patients who must cope with not only the toxicity of treatment, but also side effects from the tumor itself which remains in situ. Furthermore, little is known about the psychosocial impact of NT particularly given many patients’ inherent preference for “just getting the cancer out,” as well as the uncertainty of future surgery[10]. A recent systematic review found scarce data on quality of life (QOL) during NT for PDAC and no existing literature on other aspects of the patient experience[11]. In contrast to immediate surgery, NT is also inherently multi-disciplinary in nature. As such, there may be barriers to effective care initiation and coordination that impede the completion of all scheduled therapy and the receipt of surgical resection[12].
Therefore, the purpose of this qualitative study was to characterize the patient experience during NT for PDAC. Specifically, we sought to understand patient treatment preferences, information needs, the physical and psychosocial impact of treatment, and barriers to successful initiation and delivery of NT. A better understanding of all aspects of the patient experience during NT may identify opportunities to design interventions aimed at improving QOL during NT, facilitating completion of NT and receipt of surgery, and ultimately optimizing the long-term outcomes of patients with PDAC.
Patients with PDAC undergoing NT prior to planned surgery in the future were recruited to participate in this qualitative study. All treatment decisions at our institution are made at a pancreatic cancer specialty specific multidisciplinary clinic and made on an individualized basis. Participants were identified by prospectively screening ambulatory clinics at The Ohio State University Wexner Medical Center and James Comprehensive Cancer Center. Inclusion criteria included receiving at least two cycles of chemotherapy in a neoadjuvant intent, still eligible for surgical resection, and English language speaking, without restrictions on age, race, or disability. Eligible patients were contacted by phone, informed consent was obtained, and an interview was scheduled at the participant’s convenience. Due to the COVID-19 pandemic, interviews were conducted by phone between August 2020 and October 2021.
The interview script was developed using evidence synthesis, stakeholder engagement, and expert opinion. The content of the interviews focused on patient treatment preferences, perspectives on the decision-making process, and all aspects of the patient experience during NT; recommendations on opportunities to improve the delivery of NT were also sought. Questions were open-ended, prompting additional questions depending on the responses of the interviewees (Supplementary material). This type of interview method was selected due to the descriptive nature of the research. Semi-structured interviews allow researchers to discuss topics of interest more in detail by elaborating on emerging themes and asking probing questions. A nominal gift card was given to participants for their participation. This study was approved by the Institutional Review Board of The Ohio State University (IRB# 2019C0155).
All interviews occurred by phone, were audio recorded, and then manually transcribed verbatim by the researchers. Transcripts were then uploaded to NVivo12 (QSR International, Australia) for data extraction, synthesis, and analysis purposes. Data extraction followed an integrated approach, including both an inductive and deductive coding methodology[13]. The following preliminary codes were developed before a more in-depth, inductive coding process took place: Patient Experiences; Patient Perspectives on NT; Solutions, Facilitators and Recommendations; and Sources of Information. Two researchers independently coded the transcripts for sub-themes in an iterative fashion until thematic saturation was achieved[13]. Interviews were then re-reviewed and coded using the final codebook. When coding from both independent researchers was not concordant, these instances were reviewed with a third researcher at team meetings. These sections and codes were discussed until a consensus was reached. Demographic data from participants were summarized and illustrative quotes in each theme were selected.
A total of 12 patients participated in the interviews. On average, patients were 67 years old, ranging from 52 to 81 years. Patients with BR (n = 7, 58%), PR (n = 2, 17%), and LA (n = 3, 25%) cancers participated in the study. A majority of patients (n = 7, 58%) received chemotherapy and radiation therapy before their planned surgery while others (n = 5, 42%) received just chemotherapy. At most recent follow-up, most patients (n = 10, 83%) had completed NT with 8 patients (67%) undergoing surgical resection of their tumor. Complete participant characteristics are reported in Table 1.
| Variable | |
| Age [mean (range), yr] | 67 (52 – 81) |
| Gender | |
| Male | 8 (67) |
| Female | 4 (33) |
| Race | |
| White | 9 (75) |
| Black | 3 (25) |
| Marital status | |
| Married | 7 (58) |
| Single | 4 (33) |
| Divorced | 1 (8) |
| Stage of cancer | |
| PR | 2 (17) |
| BR | 7 (58) |
| LA | 3 (25) |
| Location of tumor | |
| Head | 9 (75) |
| Body | 2 (17) |
| Neck | 1 (8) |
| Type of NT | |
| Chemo | 5 (42) |
| Chemo + XRT | 7 (58) |
| Length of NT1 | |
| < 3 mo | 1 (10) |
| 3-6 mo | 6 (60) |
| > 6 mo | 3 (30) |
| Type of chemo | |
| FOLFIRINOX | 4 (33) |
| Gemcitabine/nab-paclitaxel | 2 (17) |
| Other/both | 6 (50) |
| Major complications during NT2 | |
| Hospitalization | 2 (50) |
| ER visit, no admission | 1 (25) |
| Other | 1 (25) |
| Travel distance | |
| < 15 miles | 4 (33) |
| 15 – 30 miles | 1 (8) |
| 31 – 50 miles | 2 (17) |
| 51 – 100 miles | 3 (25) |
| 100+ miles | 2 (17) |
| Surgical resection | |
| Yes | 8 (67) |
| No | 2 (17) |
| Not scheduled yet | 2 (17) |
| Insurance status | |
| Government | 9 (75) |
| Private | 3 (25) |
| Psychosocial or palliative care counseling | |
| Yes | 2 (17) |
| No | 10 (83) |
Among the 12 patients who participated in the interviews, the vast majority (n = 11, 92%) were not familiar with the concept of NT at the time of initial consultation. All subjects reported that NT was the doctor’s recommendation and most (n = 8, 67%) explained that NT was presented to them as the only option. All interviewees indicated that improving resectability was the main rationale for choosing NT. While some (n = 6, 50%) patients indicated that before meeting with their physicians they did not have a preference for a specific treatment plan, others (n = 4, 33%) expressed that they had hoped to avoid chemotherapy and undergo upfront surgery. All patients indicated that their main source of information were members of their health care team while other sources of information discussed included the internet (n = 4, 33%), family and friends (n = 3, 25%), and educational materials (n = 1, 8%). While most patients (n = 9, 75%) discussed their prognosis in a hopeful manner, some (n = 5, 42%) acknowledged the poor prognosis generally associated with PDAC and others (n = 3, 25%) expressed uncertainty surrounding the prognosis.
Five subthemes of patient experiences during NT emerged: physical symptoms, emotional symptoms, coping mechanisms, access to care, and life factors (Figure 1 and Table 2). All participants reported elements of each of the five subthemes.
| Physical symptoms | |
| Quote No. 1 | “After getting chemo for the next 5 d I’m sick as a dog, weak, losing weight, lost about 40 lbs.” |
| Quote No. 2 | “Side effects of course. You’re gonna be queasy, you’re gonna be lightheaded. Definitely, I ate, but food didn’t taste good, even water didn’t taste good and I didn’t expect that.” |
| Quote No. 3 | “I’m tired all the time. No energy. I sleep a lot. A little diarrhea. Pretty mild, never threw up yet. Only real bad thing is my appetite. Lost it about completely I have to force myself to eat until I start gagging” |
| Emotional symptoms | |
| Quote No. 1 | “Most times I don’t care, I really just don’t want to think about it. I just want to watch a good movie. You know what I mean. Not dwell on it all the time. I love my wife and I want to be around for her. It’s hard.” |
| Quote No. 2 | “There are days that I get a little depressed. Because…I am used to movement. And I just don’t have the stamina, nor the willpower.” |
| Coping and support mechanisms | |
| Quote No. 1 | “…my wife and I, we are Christians and we know it’s up to him, the Lord.” |
| Quote No. 2 | “I have good support from my family and friends. And prayer circles. Getting financial gifts to help us with gas money and things like that. So, they’ve all been very supportive.” |
| Access to care | |
| Quote No. 1 | “And I have had a lot of friends that make sure that I get to my treatments. So, I don’t have any problems there.” |
| Quote No. 2 | “But it got to be a little bit much for [my sister-in-law] so, I had to withdraw money to get me through this [to ride the bus] that was an extra expense, as I am a senior. And I’m on a fixed income. So, I hadn’t counted on that.” |
| Quote No. 3 | “I have no problem with transportation. My wife was always there to give me a ride.” |
| Life factors | |
| Quote No. 1 | “I have help with paying my bills, [grocery store], cleaning my house, things like that, doing laundry. So, I am very lucky.” |
| Quote No. 2 | “I worry about all the damn bills” |
| Quote No. 3 | “I’m retired so really the work thing didn’t come into play, but I have chores around the house that I’m limited in doing” |
Physical symptoms: A few patients (n = 3, 25%) discussed that they did not experience any major side effects and they were tolerating their therapy well (“I have never had any symptoms. No throwing up, no nothing.”). However, most patients reported experiencing major side effects from their treatment. Many patients reported feeling weak (n = 6, 50%). One patient stated: “I’ll say at night, I am going to do this, that and the other and the next day comes and my body says ‘no, we’re not going to do that’.” Others mentioned challenges around weight loss, loss of appetite, and the taste of food (n = 5, 42%), as well as a general feeling of sickness (n = 4, 33%) (“After getting chemo for the next 5 days I’m sick as a dog.”).
Emotional symptoms: In addition to shock experienced during their diagnosis, patients reported varying rates of fear and depression (“… it scared me. It depressed me.”). A few patients (n = 3, 25%) shared concerns for their family and friends’ well-being, regarding uncertainty about next steps in treatment, and about their overall prognosis. One patient stated: “I love my wife and I want to be around for her. It’s hard.” Some (n = 3, 25%) also shared not wanting to think about and dwell too much on their diagnosis and treatment approach, as well as the need for not too much information, as it leads to unnecessary anxiety.
Coping and support mechanism: The main coping and support mechanism cited by most patients (n = 10, 83%) was support from family members. Tangible aspects of support included family members and friends offering rides to appointments, discussing different treatment options, helping with coordinating care and reaching out to the medical team, as well as helping with chores around the house. Patients putting their trust in their religious faith was another coping and support mechanism mentioned by some (n = 5, 42%). One patient stated: “I’m a religious person, so that’s enough said.” Several patients (n = 4, 33%) also mentioned receiving support from different members of the medical team (“So, there is always someone here to answer my questions, which also feels good and gives you comfort.”).
Access to care: For most, access and coordination was an important but feasible aspect of NT. This included minimal obstacles associated with traveling to medical appointments (n = 8, 67%), scheduling appointments (n = 6, 50%), contacting doctors (n = 6, 50%), getting answers to questions (n = 4, 33%), getting insurance to cover treatments (n = 3, 25%), or seeing a doctors and getting referrals (n = 2, 17%). While in general patients did not experience major complications accessing and coordinating care, a minority of patients reported some barriers. A few (n = 3, 25%) highlighted that traveling to appointments was burdensome. One patient explained: “Every time we have to have something done, it’s two hours out of our day, about 2.5 h out of our day just driving to the place. But we made that choice knowing that was the case for the care, the treatment and we’ve been proceeding.”
Life factors: Finally, all patients described the need to integrate their treatment and condition with their normal life circumstances. Several patients discussed the impact of NT on other aspects of their life. NT impacting a patient’s work and financial situation were the most commonly cited sub-themes. Many patients discussed that missing work was not a major challenge they were faced with (n = 5, 42%), and that they did not experience major financial concerns (n = 6, 50%). Yet, some (n = 3, 25%) expressed concern around not being able to work and the burden it placed on them financially (n = 4, 33%). One patient stated: “I’ve been off since all this happened (…) drives me kinda nuts. I used to work all the time. But I got no energy now.” Another patient explained: “I had to withdraw money to get me through this that was an extra expense, as I am a senior. And I’m on a fixed income. So, I hadn’t counted on that.” Other life aspects mentioned were patients having to deal with other health problems (n = 1, 8%) at the same time they are on NT and needing help with daily activities (n = 2, 17%).
The most commonly cited recommendation for improving the experience of NT was to provide better education and more information on NT (n = 7, 58%). Patients highlighted the need for more information on: the rationale behind choosing NT prior to surgery, the anticipated surgery and its likelihood of occurring after NT, as well as general information prior to starting NT treatment. Patients also discussed that more discussions with physicians could potentially be helpful, but also, highlighted the need for information tailoring (not too much vs not too little). The need for seeing different members of the healthcare team, including ancillary services was also frequently cited as a recommendation for improving the experience of NT (n = 5, 42%). Patients discussed the importance of seeing psychologists, palliative care doctors, case workers, physical therapists, and nutritionists. Better coordination and communication (n = 2, 17%) and better treatments (n = 2, 17%) were also offered as potential recommendations.
Pancreatic ductal adenocarcinoma is a highly aggressive malignancy, often thought of as a systemic disease at the time of diagnosis, that requires multimodal therapy with a combination of surgery and chemotherapy in order to achieve meaningful long-term survival[14-17]. NT is being increasingly utilized in patients with localized PDAC[1,3,18]. Previous research on NT for PDAC has focused on its safety, efficacy, and cost-effectiveness with little data on patient-centered preferences or experiences during NT. In this qualitative study of patients actively receiving NT for PDAC, we found several important observations. First, patients are generally unfamiliar with the concept of NT prior to meeting with an oncologist. While many have an inherent preference for upfront surgery, most understand their providers’ recommendation for NT as an attempt to improve resectability (or likelihood of achieving margin-negative resection). Second, patients have unique experiences and care needs during NT that providers should be aware of in order to optimize patient-centered outcomes. A patient-centered approach that supports physical and emotional symptoms and recognizes the importance of life integration is required. Third, specific recommendations for improving the experience of NT prior to surgery were identified.
Interestingly, although patients in our study were actively receiving NT, most were relatively unfamiliar with the concept of NT. All patients expressed that NT was the recommendation of their doctor. While a few patients expressed their desire for a surgery-first approach or to avoid chemotherapy altogether, nevertheless, all patients eventually came to understand and agree with the rationale for NT. This may highlight a disconnect between patients and providers in that systemic chemotherapy is part of the treatment of all patients with pancreatic cancer as even patients with localized cancers who have undergone resection are likely to experience disease recurrence[19,20]. Similar results were found in patients with breast cancer. A study in women with breast cancer who underwent NT found a majority of women understood that chemotherapy was given prior to surgery in order to shrink the tumor but did not grasp the concept that chemotherapy is utilized to treat systemic disease beyond simply local tumor control[21].
Missing from prior studies has been an evaluation of patient-centered preferences and outcomes regarding the use of NT for PDAC. Cancer-related treatment decisions are complex and require consideration of multiple factors; such decisions are often made in the context of shared decision making (SDM), a model in which informed and engaged patients make health-care decisions in conjunction with their providers[22]. The degree to which patients are involved in the SDM process of choosing NT or immediate surgery is unclear. Most patients with cancer desire an active role in making decisions about their care[23] and such patient-centered decision making has been shown to improve patients’ understanding of their treatment options, satisfaction with their health care, and overall quality of life (QOL)[24-26]. Previous research in breast and rectal cancer suggest patient-centered approaches to SDM regarding NT are lacking in clinical practice[27-29]. Indeed, SDM is under-utilized by surgeons in general[30]. Additionally, it is well known that strong emotions and fears may influence treatment decision making[31]. Specifically, emotions may cause behavior or decisions to diverge from more rational or practical decision making consistent with one’s values[32]. For example, patients state their desire to “just get the cancer out” even if this emotional response does not align with one’s values, priorities, or optimal treatment strategy. We found most patients believed NT was their only treatment option moving forward. This is not surprising as a majority of the patients in our study had either BR or LA disease which is currently the preferred treatment strategy based on recent randomized controlled trials[33,34]. Another study has found that most patients believed there was no other treatment option and thus accepted NT[21]. Understanding patient preferences, values, and expectations regarding NT will improve SDM which will lead to not only delivering patient-centered care but also the opportunity to overcome barriers to patient acceptance of NT.
While not previously studied in PDAC, in practice, multiple barriers to the use of NT are often expressed by patients. For example, some patients may have financial concerns secondary to missing work by “delaying” surgery. Others worry about arranging and/or affording transportation for NT due to long travel distances. Additionally, in our study, most, if not all patients, experienced physical and emotional symptoms during NT. Furthermore, the development of toxicities during the course of NT may prove to be a potential barrier that may worsen a patient’s ability to subsequently undergo an operation. A meta-analysis of 38 studies of which 1738 patients received NT found approximately 64% of patients experienced at least grade III toxicity[35]. In fact, this number may be magnified at community hospitals which may not have the same resources as tertiary referral centers to manage toxicities and progress patients through therapy[36-38].
We found that patients with PDAC receiving NT must balance their cancer treatment with other aspects of their lives such as family responsibilities and work in addition to coping with the physical and emotional symptoms that accompany their new diagnosis and treatment (Figure 1). These findings are similar to a previous qualitative study of patients with breast cancer receiving NT. Beaver et al[21] reported five themes among women receiving NT: Coping with the rapid transition from “well” to “ill”, information needs and decision making, needing support and empathy, impact on family, and creating a new “normal”. These findings suggest similar experiences among patients receiving chemotherapy prior to surgery regardless of cancer type. While patients with PDAC certainly have unique challenges such as biliary obstruction, malnutrition, gastric outlet obstruction, as well as cancer-related pain, additional research is needed on supporting the general care needs directly influenced by the neoadjuvant aspects of treatment.
The findings from our study provide a framework to allow for a better understanding of the patient experience during NT and highlight opportunities for inter-disciplinary interventions to improve patient-centered outcomes of those with PDAC. Indeed, many patients who receive NT fail to either complete NT or to undergo subsequent pancreatectomy with common reasons including disease progression or worsening performance status due to toxicity[33,39,40]. Furthermore, since failing to complete therapy or undergo surgical resection is associated with a worse prognosis, having a patient-centered approach to understand potential barriers to completion is essential. As we have demonstrated in this study, patients experience both physical and emotional symptoms during treatment and require a team approach with the help of ancillary services to help complete therapy. Involvement of patient navigators, social workers, nutritionists, and physical therapists to address patient concerns and symptoms may aid to improve the high attrition rate in patients receiving NT. Previous research has highlighted patient dissatisfaction with the lack of access to counseling services, support groups, and educational tools[41].
There are several limitations to our study. Although our study reached theme saturation, the relatively small sample size and single institution design means that the findings may not be generalizable to all patients with PDAC who are receiving NT. Additionally, our study includes patients with PR, BR and LA disease where larger sample sizes are required to investigate if the patient experience differs according to anatomic stage (e.g., patients with LA disease may have lower expectations of undergoing resection and/or greater burden of cancer-related symptoms than patients with PR disease.) Finally, it is unclear if the patient experience is temporal-dependent and since interviews were performed at a single time during NT, future research may focus on longitudinal evaluations of the patient experience.
In conclusion, this is the first qualitative study to characterize the experience of patient’s receiving NT for localized PDAC. Our findings clarify the lack of familiarity with the concept of NT prior to initiating treatment, the unique care needs of patients receiving NT, and recommendations to improve the delivery of cancer care in the neoadjuvant setting. These data provide a framework to allow for a better understanding of the patient experience during NT and highlight opportunities for patient-centered interventions aimed at improving quality and quantity of life outcomes of those with PDAC.
Neoadjuvant therapy (NT) has increasingly been utilized for patients with localized pancreatic ductal adenocarcinoma (PDAC). It is the recommended approach for borderline resectable (BR) and locally advanced (LA) and it has also increasingly been utilized for potentially resectable (PR) disease. However, little research has focused on patient-centered metrics among patients undergoing NT, including patient experiences, preferences, and recommendations.
A better understanding of all aspects of the patient experience during NT may help identify opportunities to design interventions aimed at improving quality of life. It may also facilitate the completion of NT and receipt of surgery, ultimately optimizing long-term outcomes.
This research aims to understand the experience of patients initiating and receiving NT to identify opportunities to improve neoadjuvant cancer care delivery.
Semi-structured, open-ended interviews of patients with localized PDAC during NT were conducted to explore their experience initiating and receiving NT. Interviews were conducted over the phone. All interviews were audio recorded, transcribed, and coded by two independent researchers using NVivo 12, iteratively identifying themes until thematic saturation was achieved.
A total of 12 patients with localized PDAC were interviewed. All patients indicated that choosing NT was the doctor’s recommendation and most reported not being familiar with the concept of NT (n = 11, 92%). Five patient experience themes emerged: physical symptoms, emotional symptoms, coping mechanisms, access to care, and life factors. Improved education before and during NT was the most commonly cited recommendation for improving the experience during NT (n = 7, 58%). Patients highlighted the need for more information on the rationale behind choosing NT prior to surgery, the anticipated surgery and its likelihood of surgery occurring after NT, as well as general information prior to starting NT treatment.
This study provides a framework to allow for a better understanding of the PDAC patient experience during NT and highlights opportunities to improve quality and quantity of life outcomes.
This exploratory research utilizes qualitative interviews to examine the patient experience when initiating and receiving NT.
The authors extend their sincere appreciation to all participants of this study who shared their experience with us. We thank Angela Sarna for her assistance with participant recruitment and administrative support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Falasca M, Australia; Nguyen LT, Viet Nam A-Editor: Xu ZL S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Cloyd JM, Shen C, Santry H, Bridges J, Dillhoff M, Ejaz A, Pawlik TM, Tsung A. Disparities in the Use of Neoadjuvant Therapy for Resectable Pancreatic Ductal Adenocarcinoma. J Natl Compr Canc Netw. 2020;18:556-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Aquina CT, Ejaz A, Tsung A, Pawlik TM, Cloyd JM. National Trends in the Use of Neoadjuvant Therapy Before Cancer Surgery in the US From 2004 to 2016. JAMA Netw Open. 2021;4:e211031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Cloyd JM, Heh V, Pawlik TM, Ejaz A, Dillhoff M, Tsung A, Williams T, Abushahin L, Bridges JFP, Santry H. Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Piperdi M, McDade TP, Shim JK, Piperdi B, Kadish SP, Sullivan ME, Whalen GF, Tseng JF. A neoadjuvant strategy for pancreatic adenocarcinoma increases the likelihood of receiving all components of care: lessons from a single-institution database. HPB (Oxford). 2010;12:204-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | de Geus SW, Eskander MF, Bliss LA, Kasumova GG, Ng SC, Callery MP, Tseng JF. Neoadjuvant therapy versus upfront surgery for resected pancreatic adenocarcinoma: A nationwide propensity score matched analysis. Surgery. 2017;161:592-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Lutfi W, Talamonti MS, Kantor O, Wang CH, Liederbach E, Stocker SJ, Bentrem DJ, Roggin KK, Winchester DJ, Marsh R, Prinz RA, Baker MS. Perioperative chemotherapy is associated with a survival advantage in early stage adenocarcinoma of the pancreatic head. Surgery. 2016;160:714-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Artinyan A, Anaya DA, McKenzie S, Ellenhorn JD, Kim J. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer. 2011;117:2044-2049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, Mohile SG, Mumber M, Schulick R, Shapiro M, Urba S, Zeh HJ, Katz MH. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2541-2556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 9. | Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751-1756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Hamad A, Crossnohere N, Ejaz A, Tsung A, Pawlik TM, Sarna A, Santry H, Wills C, Cloyd JM. Patient Stated Preferences for Neoadjuvant Therapy in Pancreatic Ductal Adenocarcinoma Pancreas. In press. [Cited in This Article: ] |
| 11. | Cloyd JM, Hyman S, Huwig T, Monsour C, Santry H, Wills C, Tsung A, Bridges JFP. Patient experience and quality of life during neoadjuvant therapy for pancreatic cancer: a systematic review and study protocol. Support Care Cancer. 2021;29:3009-3016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Evans DB. The Complexity of Neoadjuvant Therapy for Operable Pancreatic Cancer: Lessons Learned From SWOG S1505. Ann Surg. 2020;272:487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:1758-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1816] [Cited by in F6Publishing: 1925] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 14. | Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, Carter R, Tebbutt NC, Dervenis C, Smith D, Glimelius B, Charnley RM, Lacaine F, Scarfe AG, Middleton MR, Anthoney A, Ghaneh P, Halloran CM, Lerch MM, Oláh A, Rawcliffe CL, Verbeke CS, Campbell F, Büchler MW; European Study Group for Pancreatic Cancer. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 15. | Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1327] [Cited by in F6Publishing: 1242] [Article Influence: 177.4] [Reference Citation Analysis (0)] |
| 16. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1945] [Cited by in F6Publishing: 1828] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 17. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1180] [Cited by in F6Publishing: 1218] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 18. | Cloyd JM, Tsung A, Hays J, Wills CE, Bridges JF. Neoadjuvant therapy for resectable pancreatic ductal adenocarcinoma: The need for patient-centered research. World J Gastroenterol. 2020;26:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, Ko CY, Bentrem DJ. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 20. | Cloyd JM, Katz MH, Prakash L, Varadhachary GR, Wolff RA, Shroff RT, Javle M, Fogelman D, Overman M, Crane CH, Koay EJ, Das P, Krishnan S, Minsky BD, Lee JH, Bhutani MS, Weston B, Ross W, Bhosale P, Tamm EP, Wang H, Maitra A, Kim MP, Aloia TA, Vauthey JN, Fleming JB, Abbruzzese JL, Pisters PW, Evans DB, Lee JE. Preoperative Therapy and Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma: a 25-Year Single-Institution Experience. J Gastrointest Surg. 2017;21:164-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Beaver K, Williamson S, Briggs J. Exploring patient experiences of neo-adjuvant chemotherapy for breast cancer. Eur J Oncol Nurs. 2016;20:77-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood). 2013;32:276-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 498] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 23. | Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp K. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21:1145-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Soc Sci Med. 2001;52:1865-1878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 386] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 25. | Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Psychooncology. 2006;15:9-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 26. | Kehl KL, Landrum MB, Arora NK, Ganz PA, van Ryn M, Mack JW, Keating NL. Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care. JAMA Oncol. 2015;1:50-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 27. | de Ligt KM, Spronk PER, van Bommel ACM, Vrancken Peeters MTFD, Siesling S, Smorenburg CH; Nabon Breast Cancer Audit group. Patients' experiences with decisions on timing of chemotherapy for breast cancer. Breast. 2018;37:99-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Herrmann A, Hall A, Zdenkowski N. Women's Experiences with Deciding on Neoadjuvant Systemic Therapy for Operable Breast Cancer: A Qualitative Study. Asia Pac J Oncol Nurs. 2018;5:68-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 29. | Kunneman M, Engelhardt EG, Ten Hove FL, Marijnen CA, Portielje JE, Smets EM, de Haes HJ, Stiggelbout AM, Pieterse AH. Deciding about (neo-)adjuvant rectal and breast cancer treatment: Missed opportunities for shared decision making. Acta Oncol. 2016;55:134-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | de Mik SML, Stubenrouch FE, Balm R, Ubbink DT. Systematic review of shared decision-making in surgery. Br J Surg. 2018;105:1721-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | D'Agostino TA, Shuk E, Maloney EK, Zeuren R, Tuttle RM, Bylund CL. Treatment decision making in early-stage papillary thyroid cancer. Psychooncology. 2018;27:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Sanders JJ, Curtis JR, Tulsky JA. Achieving Goal-Concordant Care: A Conceptual Model and Approach to Measuring Serious Illness Communication and Its Impact. J Palliat Med. 2018;21:S17-S27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 33. | Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, Oh DY, Chie EK, Lee JM, Heo JS, Park JO, Lim DH, Kim SH, Park SJ, Lee WJ, Koh YH, Park JS, Yoon DS, Lee IJ, Choi SH. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg. 2018;268:215-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 432] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 34. | Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Eijck CH, van Tienhoven G; Dutch Pancreatic Cancer Group. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020;38:1763-1773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 574] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 35. | Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, van Eijck CHJ, Groot Koerkamp B, Rasch CRN, van Tienhoven G; Dutch Pancreatic Cancer Group. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105:946-958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 332] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 36. | Brown ZJ, Cloyd JM. Trends in the utilization of neoadjuvant therapy for pancreatic ductal adenocarcinoma. J Surg Oncol. 2021;123:1432-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Christians KK, Heimler JW, George B, Ritch PS, Erickson BA, Johnston F, Tolat PP, Foley WD, Evans DB, Tsai S. Survival of patients with resectable pancreatic cancer who received neoadjuvant therapy. Surgery. 2016;159:893-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Tzeng CW, Fleming JB, Lee JE, Xiao L, Pisters PW, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR, Fogelman DR, Crane CH, Balachandran A, Katz MH. Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Ann Surg Oncol. 2012;19:2045-2053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Sohal DPS, Duong M, Ahmad SA, Gandhi NS, Beg MS, Wang-Gillam A, Wade JL 3rd, Chiorean EG, Guthrie KA, Lowy AM, Philip PA, Hochster HS. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021;7:421-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 40. | Ye M, Zhang Q, Chen Y, Fu Q, Li X, Bai X, Liang T. Neoadjuvant chemotherapy for primary resectable pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford). 2020;22:821-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 41. | Beesley VL, Janda M, Burmeister EA, Goldstein D, Gooden H, Merrett ND, O'Connell DL, Wyld DK, Chan RJ, Young JM, Neale RE. Association between pancreatic cancer patients' perception of their care coordination and patient-reported and survival outcomes. Palliat Support Care. 2018;16:534-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |