Published online Apr 26, 2014. doi: 10.4252/wjsc.v6.i2.82
Revised: November 29, 2013
Accepted: January 13, 2014
Published online: April 26, 2014
Mesenchymal stem cell (MSC) therapy is entering a challenging phase after completion of many preclinical and clinical trials. Among the major hurdles encountered in MSC therapy are inconsistent stem cell potency, poor cell engraftment and survival, and age/disease-related host tissue impairment. The recognition that MSCs primarily mediate therapeutic benefits through paracrine mechanisms independent of cell differentiation provides a promising framework for enhancing stem cell potency and therapeutic benefits. Several MSC priming approaches are highlighted, which will likely allow us to harness the full potential of adult stem cells for their future routine clinical use.
- Citation: Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J Stem Cells 2014; 6(2): 82-93
- URL: https://www.wjgnet.com/1948-0210/full/v6/i2/82.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i2.82
Human bone marrow mesenchymal stem cells (MSCs) are currently being investigated in clinical trials for immune, cardiovascular, neurodegenerative, gastrointestinal, bone/cartilage and blood disorders (http://clinicaltrials.gov). The clinical utility of MSCs is in part due to their lack of significant immunogenicity, permitting safe allogeneic cell transplantation without the need for immunosuppression. However, these clinical trials have thus far demonstrated moderate and at times inconsistent benefits, indicating an urgent need to optimize the therapeutic platform and enhance stem cell potency[1-3]. Along this line, parallel preclinical studies have identified several potentially useful and logistically feasible strategies that may be employed to achieve more robust clinical efficacy of MSC therapy. On the other hand, risk factors associated with MSC therapy cannot be overlooked because long-term safety data remain lacking and unanticipated side effects may appear much later. Potential risks related to disease transmission and activation of latent viruses in allogeneic cell transplantation also highlight the importance of continued surveillance post MSC therapy. Thus, future success of MSC therapy will lie in rational optimization of therapeutic strategies in conjunction with an adequate assessment of benefit and risk factors.
While early preclinical MSC studies suggested therapeutic mechanisms mediated by MSC trans-differentiation or fusion, these mechanisms do not occur in sufficiently high frequency to account for the observed functional improvement after stem cell administration. Current evidence indicates that although MSCs exhibit prominent multi-lineage differentiation potential, this cellular feature bears little relevance to their therapeutic effects. Instead, production of multiple paracrine factors by MSCs provides the underlying regenerative mechanism[4-6]. Therapeutically, the MSC-derived soluble mediators, which include many cytokines and growth factors, are functionally redundant and synergistic, contributing to cytoprotection, angiogenesis, tissue repair, normalization of extracellular matrix (ECM) and alleviation of inflammation. Preclinical studies have indeed highlighted the central role of MSC-derived interleukin (IL)-6-type cytokines, vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in the treatment for heart failure and multiple sclerosis[6-8]. MSCs also interact with cells of both the innate and adaptive immune systems, leading to functionally relevant immunomodulation[9]. Of note, MSCs are widely present in vivo and their perivascular origin in multiple organs have been demonstrated[5,10]. This apparent in vivo“drug store” function of MSCs constitutes the primary therapeutic underpinning of MSC therapy.
Current clinical trial data do not yet support routine use of MSC therapy for the prevention and treatment of organ dysfunction or tissue degeneration. Robust cell therapy is likely dictated by at least two key competence factors affecting both the transplanted stem cells and the treated host tissue. This view necessitates a complete understanding of the cell-tissue crosstalk mechanism and the adoption of an integrative approach in maximizing therapeutic efficacy regardless of the organ system being targeted. Since the mechanisms of action of MSCs in tissue regeneration are likely multifaceted, cell competency can be dictated by the abilities of the injected MSCs to migrate, engraft, survive, differentiate and produce functional paracrine mediators. Tissue competency reflects the ability of the host tissues to favorably respond to the injected MSCs and MSC-derived paracrine factors, resulting in activation of the endogenous regenerative machinery[11]. While the exogenous repair mechanism is imparted by the implanted MSCs and is often short-lived, the endogenous repair mechanism conferred by the host stem/progenitor cell niches can exert a powerful and long-lasting regenerative benefit. Integration of the exogenous and endogenous repair mechanisms in clinical trial design will prove instrumental in transitioning toward future routine clinical use of adult stem cells. In considering the strategies for boosting the competency factors in MSC therapy, we will focus primarily on non-genetically based methods because genetically modified MSCs will likely pose some concerns and safety issues for clinical application. Given that MSC therapy is being used to target a wide spectrum of diseases in diverse patient populations, the logistical aspects of MSC therapy will also be considered.
MSCs from different donors may exhibit different degrees of competence due to varying factors such as gender, disease status and age[12,13]. Limited information indicates that female stem cells may possess a more pronounced regenerative potential than male stem cells[14], which is in line with the finding that female patients typically exhibit certain cardioprotective phenomenon from acute myocardial infarction and better outcome after the incidence compared to male patients[15]. Although the gender influence is thought to be mediated through differential sex hormone receptor signaling, a recent study shows that female rodent MSCs produce a higher level of VEGF than male rodent MSC in response to hypoxia[13]. Given the critical role of paracrine factors in MSC therapy, additional study is warranted to determine whether female MSCs are indeed more robust in production of multiple paracrine factors and should be selected for the use of allogeneic MSCs.
Aside from the gender effect, studies have further revealed disease- and age-associated functional impairment of various types of adult stem cells[16,17]. While the basal hematopoietic capacity is maintained throughout life, the ability of hematopoietic stem cells (HSCs) to respond to stress and differentiation cues appears to decrease with age[18,19]. The use of autologous MSCs is not always desirable or feasible because patients can exhibit declined stem cell quality and/or quantity[20-22]. For instance, diabetes can negatively impact MSCs by reducing angiogenic capacity and therapeutic potential[23]. Certain disease-causing genotypes may preclude therapeutic use of autologous MSCs due to the inherent genetic defects[24,25]. Even chemotherapy can induce MSC damage and reduce cell yields in patients with hematological malignancy[26]. Thus, the use of allogeneic MSCs from healthy donors is gaining acceptance. The use of allogeneic MSCs isolated from healthy donors offers a major advantage because these adult stem cells can be thoroughly tested and formulated into off-the-shelf medicine in advance. MSCs are particularly well suited for this application due to their immune privileged status.
Lessons learned from HSC therapy following myeloablation have revealed that administration of sufficient HSCs promotes faster cell recovery and reduces hospitalizations[27]. Clinical trials of stem cell therapy for regenerative repair have also demonstrated the importance of administering a sufficient cell dose[28,29]. Using the hamster heart failure model, we evaluated the relationship between the injected MSC doses [(0.1-40) × 106 cells/kg body weight] and cardiac therapeutic benefits as determined by echocardiography, morphometry, gene expression analysis and histochemistry[30,31]. The series of pharmacodynamic studies established the minimal cell dose, i.e., about 1 × 106 cells/kg (Table 1), which is necessary for achieving quantifiable but weak benefits for the failing hamster heart. The studies also revealed that the most prominent therapeutic benefits were achieved by about 40 × 106 cells/kg, which however approximates 2.8 billion cells per 70-kg human! Notably, published clinical trials of MSC therapy have largely relied on injections of about 1 × 106 cells/kg[32-36], which appears suboptimal based on our cell dose study. Since the effective treatment dose is influenced by the body size, biodistribution of the MSC-induced paracrine factors in the human body is likely much less efficient than in the small rodent. Given the large body weight difference between rodents and humans, obtaining sufficient MSCs necessary for mounting a prominent therapeutic response in humans constitutes a daunting challenge. In particular, obtaining sufficient MSCs to achieve maximum clinical benefits may not be economically viable as elaborated in Table 1.
| Cell number/animal | Cell number/kg | Cardiac repair | Cell number/70-kg human |
| 0.01 × 106 | 0.1 × 106 | - (no) | 7 × 106 |
| 0.1 × 106 | 1 × 106 | + (weak) | 70 × 106 |
| 1 × 106 | 10 × 106 | ++ (moderate) | 700 × 106 |
| 4 × 106 | 40 × 106 | +++ (robust) | 2800 × 106 |
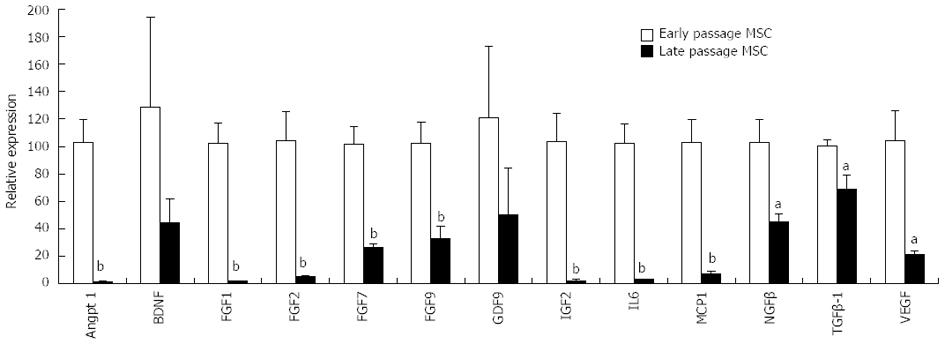
Normal mitotic somatic cells gradually cease division after continuous expansion in culture and enter a state referred to as replicative aging or senescence, exhibiting a Hayflick limit of 50 population doublings[37,38]. Embryonic stem cells (ESCs) however proliferate indefinitely in culture, which correlates with their high telomerase activity and long telomeres[39]. MSCs constitute a minor population of the nucleated cells (0.01%-0.001%) in the adult human bone marrow. Unlike hematopoietic stem cell transplantation, which is a well-established therapeutic regimen for hematological disorders[40], it is necessary to amplify MSCs in culture to generate sufficient cells required for therapeutic applications. This ex vivo cell amplification step unavoidably creates many issues that can confound MSC therapeutics. Long-term in vitro passaging alters bone marrow and adipose MSCs[41]. Prolonged culturing of MSCs from several species causes senescence and prominent changes in gene expression[42,43]. Down-regulation of genes involved in DNA repair during MSC senescence[44] can potentially cause genomic instability. Our study of porcine MSCs shows that late-passage MSCs exhibit significantly reduced expression of many paracrine factors compared to early-passage MSCs (Figure 1). Since cellular aging is a rapid and continuous process in culture, the use of ex vivo amplified MSCs, even those derived from early-passages, can generate inconsistent therapeutic effects.
Our MSC and growth factor therapy for hamster heart failure have revealed several major factors critical for tissue repair such as IL-6-type cytokines, VEGF and HGF[6,7,30,31,45]. We show that MSC therapy increases the levels of paracrine factors present in the serum and multiple organs, suggesting a systemic distribution mode for the soluble mediators at least in the rodent. We further sought to engineer an MSC phenotype exhibiting enhanced expression of paracrine factors, aiming to lower the effective treatment cell dose. We turned our attention to the pattern recognition receptor (PRR) pathway of the innate immune system, which is capable of overproducing many immunomodulatory cytokines, most notably IL-6, upon activation[46,47]. Distinct immune cell PRRs initiate the cytokine cascade through interacting with a variety of molecular patterns conserved among microbial pathogens. The Toll-like receptor (TLR) pathway is the best characterized PRR system and engagement of TLRs stimulates production of many immunomodulatory cytokines from antigen-presenting cells. TLR3 in particular recognizes double-stranded (ds) RNA, and is activated by the dsRNA mimetic polyinosinic-polycytidylic acid or poly(I:C)[48,49]. MSCs also express several members of the TLR family[50], including TLR3, which is an endolysosomal receptor protein.
We initially treated MSCs with three different concentrations of poly(I:C) for 24 h to examine the downstream effect on expression of trophic factors[31]. Gene expression assays revealed a prominent induction of IL-6 and IL-6-type cytokines by 0.8-20 g/mL poly(I:C). For instance, a 10 fold increase in IL-6 mRNA and 40 fold increase in secreted IL-6 were observed. A less than 2 fold induction of IL-11 mRNA and ~4 fold induction of secreted IL-11 were also observed. Leukemia inhibitory factor (LIF), another member of the IL-6-type cytokines, was also induced. SDF1, VEGF and HGF, all of which are activated by IL-6/JAK/STAT3 signaling, were significantly induced by poly(I:C). Interestingly, the anti-inflammatory cytokine IL-10 was significantly induced. The inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) were only induced by the highest poly(I:C) concentration (20 g/mL). This finding prompted us to adopt an MSC-boosting protocol based on 4 g/mL poly(I:C) for 24 h, which induced IL-6, IL-10, IL-11, LIF, VEGF, SDF1 and HGF without induction of the inflammatory cytokines. Longer treatment of MSCs with poly(I:C), e.g., 2 d, was found to be cytotoxic.
Upon testing the potency of the PRR-primed MSCs using the hamster heart failure model, we found that the super MSCs reduced the effective therapeutic cell dose by 40 fold (Table 1) through actively recruiting cardiac progenitor cells and decreasing myocardial inflammation, culminating in a 50% reduction in myocardial fibrosis, a 40% reduction in apoptosis and a 50% increase in ventricular function. This pioneering study of engaging the MSC PRR axis for reducing cell dose requirement in heart failure therapy was recently featured in an AJP editorial[51]. Although the function of immune cell PRRs has been well established, their role in stem cell function is just beginning to be unraveled. Prolonged TLR activation of the immune system is invariably associated with chronic inflammation. Interestingly, Cole et al[52] demonstrated an unexpected beneficial role for TLR3 in the arterial wall upon systemic administration of poly(I:C). Further, Packard et al[53] found poly(I:C) administration to be protective against cerebral ischemia-reperfusion injury. Since MSCs are widely present in vivo and their perivascular origin in multiple human organs appears certain[5,10], it is possible that these prophylactic benefits of poly(I:C) may be mediated through its trophic stimulatory effect on the endogenous MSC niches.
Recognition of various pathogen-associated molecular patterns by immune PRRs leads to transcriptional activation of distinct gene targets, and sets forth a diverse array of pathways that determine the magnitude, duration, and type of the host inflammatory response. Immune cell TLR2 and TLR4 are major PRRs responding to bacterial invasion, and their activation leads to increased IL-6 and a host of other cytokines similar to the anti-viral response mediated by dsRNA-sensing PRRs[46]. Given the prominent roles of IL-6 in stem cell maintenance and cardiac regeneration[6,54-56], transient low-dose priming of MSC TLR2/4 may also represent a physiologically significant mechanism for tissue repair. It has indeed been shown recently that TLR2/6-dependent stimulation of MSCs promotes angiogenesis in vitro and in vivo in bone tissue engineering[57]. TLR2 forms functional heterodimers with TLR1 and TLR6, and is activated by peptidoglycan. Immune TLR4 upon activation by lipopolysaccharide (LPS) causes elevated levels of IL-6, IL-8, IL-10, IL-12, IL-15, TNFα, IL-1 and TGFβ. Potential effects of TLR2 and TLR4 engagement on MSC paracrine profiles can therefore be tested by treating cells with low-dose peptidoglycan and LPS (1-20 g/mL each). However, since TLR2 and TLR4 are also known to be involved in tissue inflammation triggered by ischemia/reperfusion injury[58], it is unclear whether transient low-dose priming of MSC TLR2/4 may favorably impact the failing heart as demonstrated for MSC TLR3.
Unlike TLR3, TLR2 and TLR4 are present on the plasma membrane, recruiting the adaptor protein MyD88 for signal transduction. Since TLR activation in the absence of MyD88 generally results in delayed kinetics[59], the difference in the paracrine cascades can be expected to influence MSC therapeutics. Notably, MSCs have been found to be differentially primed by TLR4 and TLR3 ligands to adopt a pro-inflammatory (MSC1) and anti-inflammatory (MSC2) status, respectively[60]. The MSC1 and MSC2 phenotypes were further found to attenuate and promote tumor growth/metastasis, respectively[61]. These studies thus indicate that the cytokine secretion profile of MSCs plays a decisive role in dictating the therapeutic potency and treatment outcome, and warrants special consideration in the design of stem cell therapy.
Rapid loss of the implanted MSCs has been frequently observed and may be caused in part by hypoxic stress, which triggers apoptosis[62-64]. The bone marrow environment contains oxygen tensions ranging from 1% to 7%. However, most in vitro cell culture work is performed at a pO2 level of 142 mmHg or 20% O2, which is much higher than that of the in vivo environment[65]. The implanted MSCs are expected to experience reduced oxygen levels as they attempt to establish contacts with the ECM environment. Preconditioning MSCs by brief hypoxia prior to cell administration may thus allow the cells to better adapt to the lower pO2 tissue environment and promote cell engraftment. Typically, MSCs are cultured in normoxia (95% air and 5% CO2) as control and in hypoxia (1% oxygen, 5% CO2, and 94% nitrogen) for 2 d. Assay of cultured MSCs for cell surface phosphatidylserine, which is a sensitive method for detecting early apoptosis, can be used to determine whether an increase in MSC apoptosis after hypoxic exposure may be induced.
In addition to induction of many angiogenic growth factors, hypoxia is known to induce SDF-1 and its cognate receptor CXCR4[66,67]. Indeed, low oxygen has been shown to increase expression of CXCR4 and CX3CR1 and promote MSC engraftment[68,69]. A hypoxia-regulated heme oxygenase-1 vector modification of MSCs was found to enhance the tolerance of engrafted MSCs to hypoxic injury and improves their viability in ischemic hearts[70]. Note however that although hypoxia promoted MSC proliferation in vitro, it unexpectedly attenuated MSC osteogenic potential[71], suggesting that the utility of hypoxia preconditioning may be application specific. Additional relevant preconditioning strategies intended to enhance MSC survival have been based on the use of unique compounds such as prolyl hydroxylase inhibitor[62], lysophosphatidic acid[72], HMG-CoA reductase inhibitor[73,74], eNOS enhancer[75] and sphingosine-1-phosphate[76]. Whether this pharmacological approach may also reduce the effective MSC dose as observed for TLR3-activated MSCs remains to be determined. These pharmacological strategies may also find their application in tackling the issues of host tissue deficiency related to aging and disease (see below). This is because the function and competence of the endogenous host tissue progenitor cell niche also dictates the therapeutic outcome.
Rapid loss of the injected cells is perceived as a major hurdle in stem cell therapy[63,64,77] and may be caused in part by inadequate ECM engagement. Expression of chemokines and their receptors is known to be regulated by cytokines and this phenomenon has been explored to facilitate MSC engraftment after cell implantation[78,79]. Intervention through the use of growth factors and/or cytokines is appealing because the trophic factor network is typically marked by cross-talk mechanisms enabling mutual induction of gene expression. Priming MSCs with a cocktail of growth factors and cytokines has indeed been found to enhance the cardiac therapeutic efficacy[80]. In this study, a cocktail of growth factors containing 50 ng/mL FGF-2, 2 ng/mL IGF-1 and 10 ng/mL BMP-2 was used for MSC pretreatment and its effect on the viability under hypoxia and paracrine profiles of MSCs were evaluated. The growth factor pretreatment was found to enhance expression of cardiac transcription factors and promote cell viability under hypoxia. Transplantation of the pretreated MSCs resulted in smaller infarct size and better cardiac function than transplantation of untreated MSCs. This cytokine preconditioning approach is particularly relevant because MSCs are adherent cells and depend on adequate ECM engagement for growth and survival. Anoikis is initiated when trypsinized MSCs are forced into suspension for injections[81]. Along this line, plasminogen activator inhibitor-1 (PAI-1) has been found to promote anoikis, and PAI-1 null MSCs exhibit enhanced in vivo survival after implantation[64].
Many cytokines are known to exhibit cell adhesion-promoting activities including HGF, IGF-1, SDF-1, TGF-β and VEGF and interestingly these trophic factors are also produced by MSCs, suggesting that MSCs can be regulated by diverse autocrine mechanisms. These cytokines act in part by affecting the integrin and matrix metalloproteinase (MMP) systems. In particular, EGF can promote activation of MMP-2 and cell migration[82]. TGF-1 can stimulate MMP-9-mediated cell migration[83]. SDF-1 can increase V 3 integrin expression, cell migration, and therapeutic potentials of EPCs[84,85]. We also demonstrated that human MSCs overexpressing VEGF exhibited significantly enhanced cardiac repair capacity[7]. Since no cell retention and survival enhancement strategies have translated to the clinic, strategies aimed at promoting long-term maintenance of the injected cells are worth pursuing, which may ultimately lead to the production of more potent stem cells that can be delivered in lesser quantity.
Host tissue competence can greatly influence the outcome of MSC therapy because it is increasingly been recognized that aging and disease can adversely affect the tissue milieu into which MSCs are introduced[86]. The parabiosis study exposing old mice to factors present in young mouse serum[87] indicates that the age-related decline of muscle satellite cell activity is modulated by systemic factors that change with age. This is because stem cell activity is profoundly influenced by the supporting ECM and cells in the immediate vicinity[88]. The presence of ECM breakdown products and the extra lamina caused by the deposition of collagens in aged muscle tissue can potentially interfere with paracrine signaling. Aged muscle for instance exhibits increased Wnt signaling and fibrosis[89,90], which can impinge unfavorably on the functional paracrine cascade initiated by the implanted MSCs. Importantly, although the intrinsic regenerative potential of aged muscle appears to be largely intact, critical factors such as the Notch ligand Delta required for regeneration appear limiting[87,91,92].
Increasing age has been found to be associated with adverse prognosis in the setting of ischemic injury, coronary angioplasty, and cardiac surgery[93-97]. Although the adult heart contains resident cardiac stem cells capable of supporting limited myocardial regeneration[98], age-associated fibrotic remodeling and senescence of cardiac stem cells lead to contractile dysfunction and gradual loss of cardiomyocytes[99,100], and the aged heart exhibits significant structural deteriorations including fibrosis and poor angiogenic capacity[101,102]. Thus, the aged heart is more refractory to regenerative therapy[103,104]. The harmful host tissue milieu present in the aged tissue may interfere with the trophic actions of MSCs. Several tissue proteases such as elastase, cathepsin and dipeptidylpeptidase (DPP) are known to cleave and inactivate cytokines. Elevated activities of these proteases in the aged tissue may destabilize the trophic factors induced by MSC therapy, rendering the therapy ineffective. Therapeutic efficacy may thus be improved by optimizing tissue retention and stability of the delivered proteins[105-107]. For instance, administration of Diprotin A, a pharmacologic inhibitor of DPP, enhanced the stability of SDF-1, which increased myocardial homing of CXCR4+ progenitor cells and function[108,109]. Thus, a potential strategy to boost the trophic response of the older tissue is to inject non-toxic protease inhibitor(s) into the host tissue prior to MSC administration. This tissue preconditioning strategy is aimed at promoting trophic factor stability by attenuating abnormally elevated local or systemic protease activities.
The bone marrow compartment harbors many populations of primitive progenitor/stem cells that are mobilized by various chemokines. Of note, a lack of bone marrow support for cardiac repair in aged animals has been documented[110], indicating that the MSC-initiated healing process may be compromised by the impaired tissue cross-talk mechanism, leading to a greater susceptibility of the old heart to ischemic injury and an inefficient response to protective interventions. IL-6 deficiency, for instance, affects bone marrow stromal precursor cells, resulting in defective hematopoietic support[54]. This host tissue impairment represents a significant hurdle to regenerative medicine because most preclinical therapeutic studies are based on the use of young animals, but stem cell therapy typically targets the elderly. Development of suitable preconditioning strategies targeting MSCs and aged host tissue is thus expected to lead to more efficacious regenerative treatment regimens.
Routes of drug administration are major considerations in pharmacokinetic and pharmacodynamics studies and applications. The choices are however fairly limited for cell-based medicine as cell viability needs to be preserved. Since diseased tissue is often associated with ischemia, inflammation, and fibrosis, which can impair cell survival, therapeutic delivery of stem cells to areas away from the damaged tissue offers an advantage. Intravenously (iv) infused MSCs are currently being adopted for clinical trials of neurodegenerative and heart diseases[36,111], highlighting the significance of formulating a minimally invasive stem cell delivery approach for patient care. Although iv MSCs are largely distributed to the lungs, this systemic cell delivery method appears feasible with MSCs because their therapeutic benefits are largely mediated by paracrine mechanisms independent of stemness[5,6]. Thus, intracoronary infusion of MSCs for heart therapy, which retained only 1%-2% of the infused cells in the porcine myocardium, was found to result in significant functional improvement in the hibernating myocardium[112,113].
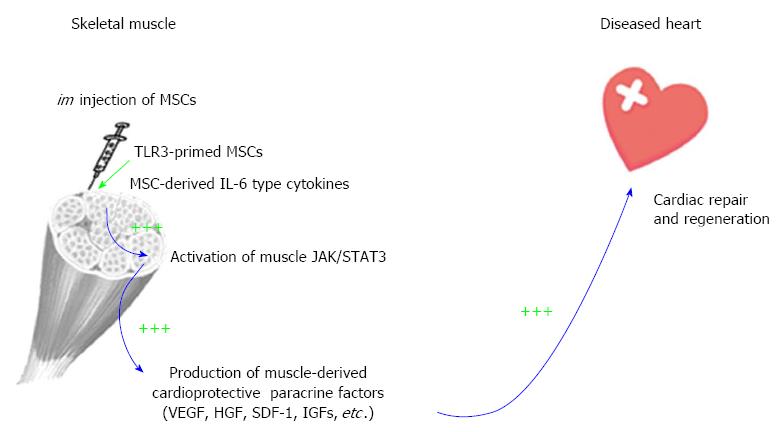
The recognition that IL-6 and IL-6-type cytokines are abundantly produced by MSCs[6,55] and that skeletal muscle actively induces IL-6 during exercise[56,114] prompted us to pioneer an intramuscular (im) MSC delivery route for cardiac repair[6,30,115]. This im MSC therapeutic strategy is coupled to the inherent ability of skeletal muscle to produce beneficial trophic factors in response to exercise and injury[116-118], and therefore represents an integrative physiological approach. The skeletal muscle is capable of regeneration after injury, and this ability is coupled to its production of many cardioprotective factors such as VEGF and HGF, which have been used in preclinical or clinical trials for cardiovascular therapy[119,120]. Although im MSCs are trapped in the local musculature, their trophic actions promote increased growth factor levels in the quadriceps, liver, and brain, suggesting a possible global physiological effect[6,30]. We further demonstrated that blocking JAK/STAT3 signaling abrogated the therapeutic effects of MSCs, indicating the functional relevance of MSC IL-6-type cytokines in initiating the paracrine cascade[6].
As depicted in Figure 2, MSC-derived IL-6 and IL-6-type cytokines activate the injected muscle through JAK/STAT3 signaling, inducing downstream trophic factor genes such as VEGF, HGF, SDF-1 and IGFs. These factors mediate mobilization of bone marrow progenitor cells, cardioprotective signaling and activation of cardiac progenitor cells, resulting in decreased myocardial fibrosis and inflammation and increased cardiac regeneration and function. Notably, im MSCs also induce Suppressor of Cytokine Signaling 3 (SOCS3), which functions in a negative feedback loop to terminate cytokine signaling[6]. Since excessive and prolonged IL-6 activity can cause tissue inflammation, induction of SOCS3 by im MSCs reduces the risk of this adverse reaction. The induced paracrine factors further enhance the expression of myocardial growth factors, activating the pro-survival signaling pathways in the diseased heart. Given that exercise is known to increase production of several beneficial trophic factors from the contracting skeletal muscle[121-123], preventing coronary artery disease and cognitive decline[124,125], our findings illustrate an im MSC-mediated cardioprotective paracrine mechanism mimicking the trophic action of exercise.
MSC therapy is entering a new era shifting the focus from initial feasibility study to optimization of therapeutic regimen and enhancement of treatment potency. Since tissue degeneration is often complex in nature and likely entails a therapeutic intervention strategy targeting multiple pathogenic mechanisms, the multiple paracrine factors released by MSCs and the injected host tissue acting in synergy are well suited as a regenerative medicine. Complete identification and understanding of these trophic factors can eventually lead to the development of cell-free trophic factor cocktails ideal for the treatment of tissue injury and degeneration, which may eliminate the concern associated with potential MSC transformation. Major challenges exist, however, regarding suboptimal stem cell potency and age/disease-related host tissue impairment, which may dampen enthusiasm for translational application of stem cells in general. The strategies outlined in this review offer a testable platform to launch innovative clinical trials based on rational design of MSC therapy.
P- Reviewers: Chakrabarti S, Gazdag G, Grof P S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Malliaras K, Kreke M, Marbán E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther. 2011;90:532-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Allison M. Genzyme backs Osiris, despite Prochymal flop. Nat Biotechnol. 2009;27:966-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology Am Soc Hematol Educ Program. 2011;2011:280-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Lee T. Stem cell therapy independent of stemness. World J Stem Cells. 2012;4:120-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1127] [Cited by in F6Publishing: 1144] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 6. | Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G, Lee T. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol. 2010;299:H1428-H1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Zisa D, Shabbir A, Suzuki G, Lee T. Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun. 2009;390:834-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 311] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 9. | Tyndall A, Walker UA, Cope A, Dazzi F, De Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K. Immunomodulatory properties of mesenchymal stem cells: a review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res Ther. 2007;9:301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2900] [Cited by in F6Publishing: 2768] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 11. | Lee T. Host tissue response in stem cell therapy. World J Stem Cells. 2010;2:61-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Phinney DG, Kopen G, Righter W, Webster S, Tremain N, Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75:424-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1). J Mol Cell Cardiol. 2007;42:142-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Ray R, Novotny NM, Crisostomo PR, Lahm T, Abarbanell A, Meldrum DR. Sex steroids and stem cell function. Mol Med. 2008;14:493-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res. 2003;58:390-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2631] [Cited by in F6Publishing: 2543] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 18. | Schlessinger D, Van Zant G. Does functional depletion of stem cells drive aging? Mech Ageing Dev. 2001;122:1537-1553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Van Zant G. Genetic control of stem cells: implications for aging. Int J Hematol. 2003;77:29-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Keymel S, Kalka C, Rassaf T, Yeghiazarians Y, Kelm M, Heiss C. Impaired endothelial progenitor cell function predicts age-dependent carotid intimal thickening. Basic Res Cardiol. 2008;103:582-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 664] [Cited by in F6Publishing: 617] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 22. | Lehrke S, Mazhari R, Durand DJ, Zheng M, Bedja D, Zimmet JM, Schuleri KH, Chi AS, Gabrielson KL, Hare JM. Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res. 2006;99:553-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Kim YS, Kwon JS, Hong MH, Kang WS, Jeong HY, Kang HJ, Jeong Mh, Ahn Y. Restoration of angiogenic capacity of diabetes-insulted mesenchymal stem cells by oxytocin. BMC Cell Biol. 2013;14:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Wallace SR, Oken MM, Lunetta KL, Panoskaltsis-Mortari A, Masellis AM. Abnormalities of bone marrow mesenchymal cells in multiple myeloma patients. Cancer. 2001;91:1219-1230. [PubMed] [Cited in This Article: ] |
| 25. | Li Y, Zhang C, Xiong F, Yu MJ, Peng FL, Shang YC, Zhao CP, Xu YF, Liu ZS, Zhou C. Comparative study of mesenchymal stem cells from C57BL/10 and mdx mice. BMC Cell Biol. 2008;9:24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Kemp K, Morse R, Wexler S, Cox C, Mallam E, Hows J, Donaldson C. Chemotherapy-induced mesenchymal stem cell damage in patients with hematological malignancy. Ann Hematol. 2010;89:701-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Mohty M, Duarte RF, Croockewit S, Hübel K, Kvalheim G, Russell N. The role of plerixafor in optimizing peripheral blood stem cell mobilization for autologous stem cell transplantation. Leukemia. 2011;25:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 29. | Friis T, Haack-Sørensen M, Mathiasen AB, Ripa RS, Kristoffersen US, Jørgensen E, Hansen L, Bindslev L, Kjær A, Hesse B, Dickmeiss E, Kastrup J. Mesenchymal stromal cell derived endothelial progenitor treatment in patients with refractory angina. Scand Cardiovasc J. 2011;45:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296:H1888-H1897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Mastri M, Shah Z, McLaughlin T, Greene CJ, Baum L, Suzuki G, Lee T. Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency. Am J Physiol Cell Physiol. 2012;303:C1021-C1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 33. | Kuzmina LA, Petinati NA, Parovichnikova EN, Lubimova LS, Gribanova EO, Gaponova TV, Shipounova IN, Zhironkina OA, Bigildeev AE, Svinareva DA. Multipotent Mesenchymal Stromal Cells for the Prophylaxis of Acute Graft-versus-Host Disease-A Phase II Study. Stem Cells Int. 2012;2012:968213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 443] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 35. | Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang X, Shao Y, Yang S, Han ZC. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med. 2011;5:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1006] [Cited by in F6Publishing: 957] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 37. | Sedivy JM. Can ends justify the means?: telomeres and the mechanisms of replicative senescence and immortalization in mammalian cells. Proc Natl Acad Sci USA. 1998;95:9078-9081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 141] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4095] [Cited by in F6Publishing: 3887] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 39. | Albert M, Peters AH. Genetic and epigenetic control of early mouse development. Curr Opin Genet Dev. 2009;19:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Hansen JA, Gooley TA, Martin PJ, Appelbaum F, Chauncey TR, Clift RA, Petersdorf EW, Radich J, Sanders JE, Storb RF. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 484] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 41. | Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229-4238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 42. | Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205:194-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 43. | Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675-682. [PubMed] [Cited in This Article: ] |
| 44. | Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 812] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 45. | Zisa D, Shabbir A, Mastri M, Taylor T, Aleksic I, McDaniel M, Suzuki G, Lee T. Intramuscular VEGF activates an SDF1-dependent progenitor cell cascade and an SDF1-independent muscle paracrine cascade for cardiac repair. Am J Physiol Heart Circ Physiol. 2011;301:H2422-H2432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5434] [Cited by in F6Publishing: 6001] [Article Influence: 428.6] [Reference Citation Analysis (0)] |
| 47. | O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1836] [Cited by in F6Publishing: 1910] [Article Influence: 112.4] [Reference Citation Analysis (0)] |
| 48. | Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4510] [Cited by in F6Publishing: 4489] [Article Influence: 195.2] [Reference Citation Analysis (0)] |
| 49. | Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun. 2002;293:1364-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 356] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 50. | DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm. 2010;2010:865601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 51. | Zimmermann O. Mesenchymal stem cells and cardiac regeneration: a sophisticated approach depends on trophic effects--what’s left over? Focus on “Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency”. Am J Physiol Cell Physiol. 2012;303:C1004-C1005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Cole JE, Navin TJ, Cross AJ, Goddard ME, Alexopoulou L, Mitra AT, Davies AH, Flavell RA, Feldmann M, Monaco C. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci USA. 2011;108:2372-2377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 53. | Packard AE, Hedges JC, Bahjat FR, Stevens SL, Conlin MJ, Salazar AM, Stenzel-Poore MP. Poly-IC preconditioning protects against cerebral and renal ischemia-reperfusion injury. J Cereb Blood Flow Metab. 2012;32:242-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Rodríguez Mdel C, Bernad A, Aracil M. Interleukin-6 deficiency affects bone marrow stromal precursors, resulting in defective hematopoietic support. Blood. 2004;103:3349-3354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 2009;108:577-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 56. | Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7:33-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 568] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 57. | Grote K, Petri M, Liu C, Jehn P, Spalthoff S, Kokemüller H, Luchtefeld M, Tschernig T, Krettek C, Haasper C. Toll-like receptor 2/6-dependent stimulation of mesenchymal stem cells promotes angiogenesis by paracrine factors. Eur Cell Mater. 2013;26:66-79; discussion 79. [PubMed] [Cited in This Article: ] |
| 58. | Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 59. | Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 60. | Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 798] [Cited by in F6Publishing: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 61. | Waterman RS, Henkle SL, Betancourt AM. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 2012;7:e45590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 62. | Liu XB, Wang JA, Ogle ME, Wei L. Prolyl hydroxylase inhibitor dimethyloxalylglycine enhances mesenchymal stem cell survival. J Cell Biochem. 2009;106:903-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation. 2010;121:325-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 64. | Copland IB, Lord-Dufour S, Cuerquis J, Coutu DL, Annabi B, Wang E, Galipeau J. Improved autograft survival of mesenchymal stromal cells by plasminogen activator inhibitor 1 inhibition. Stem Cells. 2009;27:467-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Tokuda Y, Crane S, Yamaguchi Y, Zhou L, Falanga V. The levels and kinetics of oxygen tension detectable at the surface of human dermal fibroblast cultures. J Cell Physiol. 2000;182:414-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 66. | Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem. 2005;280:22473-22481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 67. | Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 604] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 68. | Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, Eliopoulos N, Galipeau J, Béliveau R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21:337-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 69. | Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2:e416. [PubMed] [Cited in This Article: ] |
| 70. | Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 289] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 71. | Pattappa G, Thorpe SD, Jegard NC, Heywood HK, de Bruijn JD, Lee DA. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods. 2013;19:68-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 72. | Liu X, Hou J, Shi L, Chen J, Sang J, Hu S, Cong X, Chen X. Lysophosphatidic acid protects mesenchymal stem cells against ischemia-induced apoptosis in vivo. Stem Cells Dev. 2009;18:947-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow--derived endothelial progenitor cells. J Clin Invest. 2001;108:399-405. [PubMed] [Cited in This Article: ] |
| 74. | Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885-2890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 704] [Cited by in F6Publishing: 669] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 75. | Sasaki K, Heeschen C, Aicher A, Ziebart T, Honold J, Urbich C, Rossig L, Koehl U, Koyanagi M, Mohamed A. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA. 2006;103:14537-14541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, Spyridopoulos I, Chun J, Brinkmann V, Keul P. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 77. | Gargioli C, Coletta M, De Grandis F, Cannata SM, Cossu G. PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med. 2008;14:973-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Tang J, Wang J, Guo L, Kong X, Yang J, Zheng F, Zhang L, Huang Y. Mesenchymal stem cells modified with stromal cell-derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol Cells. 2010;29:9-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 80. | Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 81. | Zvibel I, Smets F, Soriano H. Anoikis: roadblock to cell transplantation? Cell Transplant. 2002;11:621-630. [PubMed] [Cited in This Article: ] |
| 82. | Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem Biophys Res Commun. 2009;379:445-450. [PubMed] [Cited in This Article: ] |
| 83. | Sun L, Diamond ME, Ottaviano AJ, Joseph MJ, Ananthanarayan V, Munshi HG. Transforming growth factor-beta 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Mol Cancer Res. 2008;6:10-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 84. | Lai TH, Fong YC, Fu WM, Yang RS, Tang CH. Stromal cell-derived factor-1 increase alphavbeta3 integrin expression and invasion in human chondrosarcoma cells. J Cell Physiol. 2009;218:334-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Zemani F, Silvestre JS, Fauvel-Lafeve F, Bruel A, Vilar J, Bieche I, Laurendeau I, Galy-Fauroux I, Fischer AM, Boisson-Vidal C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 86. | Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 310] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 87. | Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1544] [Cited by in F6Publishing: 1534] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 88. | Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 518] [Cited by in F6Publishing: 494] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 89. | Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 567] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 90. | Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1084] [Cited by in F6Publishing: 1083] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 91. | Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 92. | Zacks SI, Sheff MF. Age-related impeded regeneration of mouse minced anterior tibial muscle. Muscle Nerve. 1982;5:152-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Latting CA, Silverman ME. Acute myocardial infarction in hospitalized patients over age 70. Am Heart J. 1980;100:311-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Wennberg DE, Makenka DJ, Sengupta A, Lucas FL, Vaitkus PT, Quinton H, O’Rourke D, Robb JF, Kellett MA, Shubrooks SJ. Percutaneous transluminal coronary angioplasty in the elderly: epidemiology, clinical risk factors, and in-hospital outcomes. The Northern New England Cardiovascular Disease Study Group. Am Heart J. 1999;137:639-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Tu JV, Jaglal SB, Naylor CD. Multicenter validation of a risk index for mortality, intensive care unit stay, and overall hospital length of stay after cardiac surgery. Steering Committee of the Provincial Adult Cardiac Care Network of Ontario. Circulation. 1995;91:677-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 279] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 96. | Ivanov J, Weisel RD, David TE, Naylor CD. Fifteen-year trends in risk severity and operative mortality in elderly patients undergoing coronary artery bypass graft surgery. Circulation. 1998;97:673-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 97. | Mariani J, Ou R, Bailey M, Rowland M, Nagley P, Rosenfeldt F, Pepe S. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg. 2000;120:660-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 98. | Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2692] [Cited by in F6Publishing: 2408] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 99. | Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 452] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 100. | Biernacka A, Frangogiannis NG. Aging and Cardiac Fibrosis. Aging Dis. 2011;2:158-173. [PubMed] [Cited in This Article: ] |
| 101. | Loubani M, Ghosh S, Galiñanes M. The aging human myocardium: tolerance to ischemia and responsiveness to ischemic preconditioning. J Thorac Cardiovasc Surg. 2003;126:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479-1487. [PubMed] [Cited in This Article: ] |
| 103. | Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 588] [Cited by in F6Publishing: 628] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 104. | Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 105. | Post MJ, Laham R, Sellke FW, Simons M. Therapeutic angiogenesis in cardiology using protein formulations. Cardiovasc Res. 2001;49:522-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 106. | Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 257] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 107. | Veronese FM, Harris JM. Introduction and overview of peptide and protein pegylation. Adv Drug Deliv Rev. 2002;54:453-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 108. | Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 109. | Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 399] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 110. | Sopko NA, Turturice BA, Becker ME, Brown CR, Dong F, Popović ZB, Penn MS. Bone marrow support of the heart in pressure overload is lost with aging. PLoS One. 2010;5:e15187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 111. | Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 662] [Cited by in F6Publishing: 646] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 112. | Leiker M, Suzuki G, Iyer VS, Canty JM, Lee T. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant. 2008;17:911-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 113. | Suzuki G, Iyer V, Lee TC, Canty JM. Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res. 2011;109:1044-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 114. | Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379-1406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1310] [Cited by in F6Publishing: 1355] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 115. | Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy by nonautologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation. 2009;87:1275-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 116. | Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 488] [Cited by in F6Publishing: 459] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 117. | Hughes RA, Sendtner M, Goldfarb M, Lindholm D, Thoenen H. Evidence that fibroblast growth factor 5 is a major muscle-derived survival factor for cultured spinal motoneurons. Neuron. 1993;10:369-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 111] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 118. | Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppänen P, Turunen MP, Markkanen JE, Arve K. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol. 2002;160:1393-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 119. | Testa U, Pannitteri G, Condorelli GL. Vascular endothelial growth factors in cardiovascular medicine. J Cardiovasc Med (Hagerstown). 2008;9:1190-1221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 120. | Nakamura T, Matsumoto K, Mizuno S, Sawa Y, Matsuda H, Nakamura T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2131-H2139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214:337-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 388] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 122. | Wu G, Rana JS, Wykrzykowska J, Du Z, Ke Q, Kang P, Li J, Laham RJ. Exercise-induced expression of VEGF and salvation of myocardium in the early stage of myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H389-H395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 123. | Trenerry MK, Carey KA, Ward AC, Cameron-Smith D. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol (1985). 2007;102:1483-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 124. | Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 2003;107:3109-3116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1330] [Cited by in F6Publishing: 1283] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 125. | Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2010;30:493-503. [PubMed] [Cited in This Article: ] |
| 126. | Zisa D, Shabbir A, Mastri M, Suzuki G, Lee T. Intramuscular VEGF repairs the failing heart: role of host-derived growth factors and mobilization of progenitor cells. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1503-R1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |