Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.253
Peer-review started: July 29, 2014
First decision: October 16, 2014
Revised: November 8, 2014
Accepted: November 27, 2014
Article in press: December 1, 2014
Published online: March 26, 2015
The tumor microenvironment (TME) is complex and constantly evolving. This is due, in part, to the crosstalk between tumor cells and the multiple cell types that comprise the TME, which results in a heterogeneous population of tumor cells and TME cells. This review will focus on two stromal cell types, the cancer-associated adipocyte (CAA) and the cancer-associated fibroblast (CAF). In the clinic, the presence of CAAs and CAFs in the TME translates to poor prognosis in multiple tumor types. CAAs and CAFs have an activated phenotype and produce growth factors, inflammatory factors, cytokines, chemokines, extracellular matrix components, and proteases in an accelerated and aberrant fashion. Through this activated state, CAAs and CAFs remodel the TME, thereby driving all aspects of tumor progression, including tumor growth and survival, chemoresistance, tumor vascularization, tumor invasion, and tumor cell metastasis. Similarities in the tumor-promoting functions of CAAs and CAFs suggest that a multipronged therapeutic approach may be necessary to achieve maximal impact on disease. While CAAs and CAFs are thought to arise from tissues adjacent to the tumor, multiple alternative origins for CAAs and CAFs have recently been identified. Recent studies from our lab and others suggest that the hematopoietic stem cell, through the myeloid lineage, may serve as a progenitor for CAAs and CAFs. We hypothesize that the multiple origins of CAAs and CAFs may contribute to the heterogeneity seen in the TME. Thus, a better understanding of the origin of CAAs and CAFs, how this origin impacts their functions in the TME, and the temporal participation of uniquely originating TME cells may lead to novel or improved anti-tumor therapeutics.
Core tip: This review examines the roles of cancer-associated adipocytes (CAAs) and cancer-associated fibroblasts (CAFs) in remodeling of the tumor microenvironment (TME), presents evidence for a unique hematopoietic stem cell origin for both CAAs and CAFs, and discusses potential therapeutic implications of this novel origin. Studies highlighted herein emphasize the necessity of developing an understanding of the origins of cells in the TME and the importance of multipronged therapeutic targets directed at preventing both the incorporation and effects of stromal remodeling by the cells of the TME.
- Citation: Xiong Y, McDonald LT, Russell DL, Kelly RR, Wilson KR, Mehrotra M, Soloff AC, LaRue AC. Hematopoietic stem cell-derived adipocytes and fibroblasts in the tumor microenvironment. World J Stem Cells 2015; 7(2): 253-265
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/253.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.253
The “seed and soil” hypothesis suggests that an appropriate host microenvironment (“soil”) must be present for the optimal growth of tumor cells (“seed”)[1-3]. Although this paradigm was initially proposed by Stephen Paget in 1889, research efforts have predominantly focused on the epithelial component of solid tumors and tumor cell-intrinsic factors leading to tumorigenicity. However, in the last decade, Paget’s hypothesis has again come to focus and it has been recognized that the epithelial “seed” and stromal “soil” components co-evolve and interact during tumor progression[4]. Seminal works from Weinberg’s group have shown that this stromal compartment, often referred to as the reactive stroma or tumor microenvironment (TME), directly and indirectly supports tumor survival, growth, vascularization, escape from immune surveillance, drug resistance, and metastasis via extracellular matrix (ECM) remodeling and production of growth factors, cytokines, and chemokines (reviewed in[5-7]). The TME is comprised of a variety of cell types including endothelial cells, perivascular cells, immune cells, adipocytes, and fibroblasts/myofibroblasts. These cells interact with one another as well as with tumor cells to create an intricate network of cellular crosstalk and bidirectional regulation.
This crosstalk results in a heterogeneous population of tumor cells exhibiting varying degrees of differentiation, unregulated proliferation, the capacity to migrate and invade through surrounding tissue, and the ability to establish a dense irregular and leaky vascular network, all critical steps in metastatic tumor progression. Concomitantly, this crosstalk leads to changes in the local stromal populations, contributing to the heterogeneity of TME cells. The heterogeneity of the cells of the TME, the factors they contribute and their broad functional ability to promote all aspects of tumor progression make the “soil” a challenging and complex therapeutic target. Many factors contribute to the heterogeneity of these cell types, including exposure to the local tumor milieu, the plasticity between cells of the TME, and the multiple potential origins of each cellular population. Understanding the mechanisms behind this heterogeneity could lead to the identification of novel therapeutic targets for cancer. This review will focus on two stromal cell types, the cancer-associated adipocyte (CAA) and the cancer-associated fibroblast (CAF). The adipocyte is a stromal cell type that has recently been implicated in tumor initiation, growth, and metastasis (reviewed in[8]). Several epidemiologic studies have linked obesity with multiple types of cancer[9-11]. Recent clinical studies have reported a positive correlation between the presence of CAAs at the tumor margin and poor patient outcome, suggesting that CAAs contribute to the permissive pro-TME, particularly in adipocyte-rich tissues, such as the mammary gland[12,13] (and reviewed in[14]). CAFs, the most abundant cellular component of the TME in solid tumors, have a significant impact on tumor progression during multiple stages[5-7]. While more extensively studied than CAAs, the numerous roles of CAFs in tumor progression and metastasis are still under investigation. Like CAAs, CAFs have clinically been correlated with tumorigenesis and poor prognosis in many cancer types[15-18]. Similarities in the pro-tumorigenic functions of CAAs and CAFs suggest that these TME cell types may act in concert to promote tumor progression, indicating that therapeutic targeting of the TME may need to encompass both cell types. Herein, we will examine the phenotype and function of CAAs and CAFs in remodeling of the TME, present evidence for a unique hematopoietic stem cell origin for both CAAs and CAFs, and discuss potential therapeutic implications of this novel origin.
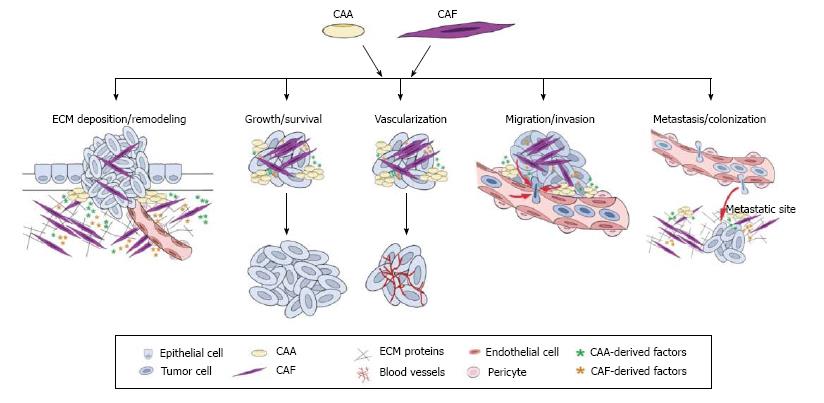
Cancer has been likened to a perpetual wound healing process[19] since both processes begin with the formation of a reactive stroma. During wound healing the reactive stroma resolves rapidly, but, during cancer progression, this actively remodeling, inflammatory state is perpetuated. CAAs and CAFs have been shown to play a role in a variety of tumor promoting processes including ECM deposition/degradation, inflammation and immune surveillance, tumor growth and survival, angiogenesis, invasion, and metastasis[5,6,20-24], suggesting similarities in the pro-tumorigenic functions of these cells. As summarized in Figure 1, this section will discuss the CAA and CAF phenotypes and their roles in generating and maintaining the reactive stroma associated with cancer progression and metastasis.
Adipocytes, surrounded by fibroblasts, preadipocytes, pluripotent stem cells, endothelial cells, and immune cells, are the major components of the adipose tissue. Apart from their traditional function in energy storage, adipocytes are also considered endocrine cells, producing hormones, growth factors, cytokines and adipokines, including leptin, adiponectin, resistin, vascular endothelial growth factor (VEGF), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6)[25]. During interaction with cancer cells, adipocytes acquire phenotypic changes and are reprogrammed to an activated state during which they are referred to as CAAs[26]. CAAs are generally located at the invasive front of tumors[21]. Several reports suggest that cancer cells can induce metabolic changes in adipocytes, resulting in enhanced lipolytic activity and an inability to properly store triglyceride[27]. Moreover, transforming growth factor-β (TGF-β), secreted by cancer cells or local stroma, is a potent inhibitor of adipocyte differentiation[28]. Thus, the associated morphological changes upon activation include loss of lipid content (delipidation) and acquisition of a fibroblast-like/preadipocyte phenotype (de-differentiation)[21]. Functional alterations in CAAs include loss of terminal adipocyte markers and products (adiponectin, resistin, fatty acid binding protein-4 (FABP4), hormone sensitive lipase (HSL), and CCAAT/enhancer binding protein-alpha (C/EBPα) and an increased production of pro-inflammatory cytokines IL-6, IL-1β, plasminogen activator inhibitor-1 (PAI-1)[21]. As detailed below, in this activated state, CAAs produce adipokines and inflammatory factors that have been shown to promote tumor progression in adipocyte-rich environments.
In non-malignant tissues, fibroblasts provide structure and ECM scaffolding for tissues. In a wound environment, these fibroblasts become activated, produce increasing amounts of ECM proteins and migrate to wound interfaces to cause wound contraction and closure. In both wound healing and the TME, fibroblast activation is marked by increased α-smooth muscle actin (αSMA) protein expression, along with increased expression of vimentin, desmin, fibroblast specific protein, platelet-derived growth factor receptor α and β (PDGFRα and β), fibroblast activation protein (FAP), or a combination of these markers. TGF-β is present in both the wound and TMEs and has been shown to both induce and suppress differentiation and tumorigenesis in a dose and context specific manner[29]. With respect to fibroblasts, TGF-β has been shown to upregulate αSMA expression[30,31], induce expression of FAP[32], and promote collagen synthesis[33], hallmarks of activated fibroblasts. Like CAAs, activated fibroblasts in the TME are also characterized by increased and altered production of inflammatory cytokines, chemokines, ECM components, and growth factors. The clinical importance of this activated phenotype is highlighted by molecular profiling studies of CAFs and matched normal fibroblasts. Studies in non-small cell lung cancer[16] and breast cancer[34] revealed that the cancer-associated gene signatures in CAFs correlated to disease outcome.
CAAs have been shown to play an important role in stromal remodeling during tumorigenesis. Type VI collagen, a soluble ECM protein, was reportedly up-regulated in peritumoral adipocytes during tumorigenesis[22] and was shown to promote early mammary tumor progression in vivo[23]. The α3 chain cleavage product of type VI collagen, endotrophin, augmented fibrosis, angiogenesis and inflammation through recruitment of macrophages and endothelial cells[23]. Rio and colleagues found that the native α3 chain of type VI collagen constituted a specific substrate for matrix metalloproteinase (MMP)-11, whose collagenolytic activity was functional in fat tissue ontogenesis as well as during cancer invasive steps[24]. Interestingly, they also reported that invasive breast cancer cells induced the expression of MMP-11 in the neighboring CAAs[20], suggesting that exposure to tumor cells promotes stromal remodeling abilities of CAAs.
CAFs function to generate and remodel ECM through production of collagens, fibronectin, and laminin[30,35], and proteases such as MMPs[36,37]. Collagens, fibronectin, and laminin contribute to the stiffness and density of the stroma, give structural support to the tumor cells, and provide important mechano-signals in the TME. Additionally, the expression of αSMA by activated fibroblasts was shown to promote matrix contraction[38-40], suggesting a direct effect on matrix stiffness. Like CAAs, CAFs also produce MMPs that degrade matrix collagens, fibronectins, and proteoglycans, profoundly contributing to structural remodeling of the TME. It has also been demonstrated in vitro that fibroblast overexpression of FAP, a serine protease selectively produced by CAFs, remodeled the ECM by increasing expression levels of αSMA, fibronectin, and collagen I[41]. These data indicate that, orchestrated by the tumor-stroma crosstalk, CAAs and CAFs actively remodel the ECM to favor local tumor progression.
In addition to elaboration and remodeling of matrix, CAAs and CAFs may also promote changes in the local stroma by contributing to the inflammatory state of the TME. As described above, CAAs have been shown to produce a variety of inflammatory cytokines including IL-6, IL-8, IL1-β and TNF-α[8,26]. Through production of factors such as IL-1β, IL-23, TGF-β, IL-6, and IL-8[42-44], CAFs exert considerable influence over the inflammatory state of the TME. CAF production of ECM components such as hyaluronic acid further drive the inflammatory state by recruiting tumor-associated macrophages[45] that promote tumor vascularization and proliferation. While the interactions between CAAs, CAFs and immune cells are only beginning to be explored, it is clear that cellular cross-talk modulates the inflammatory state of the TME, potentially influencing tumor-specific immunity.
Both CAAs and CAFs aid the tumor in meeting requirements for rapid growth by providing structural matrix as described above as well as directly promoting tumor cell proliferation and survival. Rapid, unchecked proliferation is characteristic of tumor cells, and as the cells of the TME remodel the reactive stroma, tumor cell proliferation is further accelerated. In addition to proteases and ECM constituents, CAAs provide their high energy content lipids to cancer cells resulting in accelerated tumor progression[46]. In support of this, morphologically, CAAs at the tumor invasive front are smaller than those observed at a distance, which implies lipolysis. In the case of ovarian cancer, the cancer cell-adipocyte interaction initiated HSL-mediated lipolysis in the adipocytes, releasing fatty acids, which were then taken up by the ovarian cancer cells for energy production through β-oxidation[46]. In a PC-3 model of prostate cancer, the translocation of lipid from adipocytes to prostate cancer cells was visualized by Fourier transform infrared spectroscopy[47]. Together these studies suggest that in multiple cancer types, CAAs supply the TME with energy rich lipids that may act to promote tumor growth by supplying tumor cells with essential metabolites.
Furthermore, adipocytes secrete adipokines into the TME, such as TNFα, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1/CCL2), and leptin which have been shown to enhance tumor growth locally and systemically in a variety of cancer types (reviewed in[8]) including prostate[48,49] and breast[14,21] cancers. Another aspect of adipocyte pro-tumorigenic activity is their contribution to chemo/radio-therapy resistance. The antitumor effect of vincristine, daunorubicin and dexamethasone to acute lymphoblastic leukemia (ALL) was impaired under the influence of adipocytes, which augmented ALL cell survival due to increased expressions of Bcl-2 and Pim-2[50]. Likewise, breast tumor cells co-cultivated with adipocytes or recombinant IL-6 exhibited radioresistance, an increase in the effector kinase Chk1, and a decrease in cell death[51]. Taking into account that CAAs secrete elevated levels of IL-6 in the TME, CAAs could promote chemoresistance via multiple mechanisms. These studies shed light on a new role of CAAs in fostering a chemo/radio-resistant phenotype in cancers and suggest targeting CAAs may increase effectiveness of conventional chemotherapy treatments.
CAFs also promote tumor growth through the production of factors that have been shown to be involved in proliferation of tumor cells, including TGF-α[52], TGF-β[53], hepatocyte growth factor (HGF)[54], and others (reviewed in[44,55]). Through production of MMPs, CAFs act to release stored growth factors from within the matrix, further contributing to an enriched host microenvironment and promoting proliferation of tumor cells (reviewed in[37]). Studies have identified a novel mechanism of cellular respiration, coined the “reverse Warburg effect” that involves an interplay and exchange between tumor cells and stromal cells whereby tumor cells take up energy-rich metabolites from CAAs[8] and CAFs[56] for use in the mitochondrial TCA cycle. This may contribute to the rapid proliferation of tumor cells by directing cellular energy towards cell division rather than cellular respiration. CAFs, like CAAs, have been implicated in promoting tumor chemoresistance. The expression and organization of collagen type I has been inversely correlated with intratumoral uptake of chemotherapeutic agents in vivo as it contributes to increased interstitial fluid pressure, forming a barrier to trans-capillary transport of agents[57]. DNA vaccine targeting of FAP on CAFs led to decreased deposition of a collagen I rich matrix and improved chemotherapeutic drug uptake in pre-clinical animal studies[58]. Direct targeting of CAF-derived FAP also suppressed primary tumor cell growth in a pre-clinical murine model of multi-drug resistant breast cancer[58]; however, this finding has not held in Phase II clinical trials. In vitro studies revealed that adherence of melanoma cells to fibroblast monolayers allowed for reduction of the cytotoxic effects of cisplatin, supporting a role for the CAF-induced ECM[59] in tumor cell survival. CAFs from melanoma and prostate cancer were found to be less sensitive to etoposide and vincristine due to expression of a non-mutated but functionally deficient form of p53[60]. Together, these findings suggest CAFs promote tumor growth and survival through multiple mechanisms and these effects may contribute to both the primary tumor and metastatic site.
Angiogenesis is a critical step in tumor progression, without which tumors cannot maintain growth beyond 1-2 mm3[61]. Many of the key factors required to initiate the angiogenic switch in solid tumors are produced by CAAs and CAFs. Adipocytes are known to produce multiple angiogenic factors [VEGF, fibroblast growth factor-2 (FGF2)], adipokines (leptin, adiponectin, resistin) and cytokines (IL-6), all of which stimulate angiogenesis and contribute to an overall pro-angiogenic microenvironment for tumor progression[62]. CAFs have been shown to produce angiogenic factors including stromal derived factor-1 (SDF-1)[63], TGF-β[64], IL-6[65] and VEGF[66,67], which support endothelial cell proliferation and tumor vascularization. In addition to their production of angiogenic cytokines, CAFs may play a more direct role in tumor vascularization through their ability to serve as vascular support cells. CAF and myofibroblast expression of αSMA closely links this cell type with the pericyte, a fibroblast-like cell, which plays a supportive role for endothelial cells in both normal and tumor systems. Thus, CAAs and CAFs are a source for critical angiogenic factors and may be involved in flipping the hypothetical switch to a vascularized tumor site, thereby acting to support an essential early step in tumorigenesis.
One of the initial steps in metastasis of solid tumor is the migration and invasion of the tumor cell through the ECM and through the basement membrane. This is followed by intravasation of tumor cells into a local blood vessel and the extravasation of the tumor cell to colonize and proliferate at a distant site. CAAs secrete similar levels of MMP-2 and MMP-9 compared to normal adipocytes[21], whereas MMP-11 is highly expressed by CAAs in the proximity of invading cancer cells, but not in normal resting adipocytes[24]. The role of MMP-11 (stomelysin-3) in tumor biology is still unclear (reviewed in[37,68]). However, MMP-11 has been shown to promote tumorigenesis and function through its proteolytic activity[69,70], but only weakly degrades matrix molecules. CAFs have been shown to produce a variety of matrix metalloproteinasese (MMP), including MMP-1, MMP-2, MMP-3, MMP-9, MMP-11, MMP-13, and MMP-14 (reviewed in[36,37]). Degradation of ECM allows tumor cells to cross the structural barrier of the basement membrane, a key step in tumor metastasis (reviewed in[37]). In an elegant imaging study, Gaggioli[35], demonstrated that CAFs are able to degrade matrix to form tracks through the ECM that allow invading tumor cells to efficiently follow behind.
CAAs and CAFs also directly affect the migratory and invasive abilities of tumor cells through production of adipokines, cytokines, and chemokines. Adipocyte/CAA-secreted IL-6 has been shown to play a key role in mediating adipocyte-dependent invasive activity of both breast cancer cells and melanoma cells[21,71]. FAP production by fibroblasts was linked to the increased invasion of pancreatic cancer cells in a β1-integrin/FAK mediated fashion[41]. In breast cancer, CAFs were shown to increase the invasive ability of DCIS epithelial cells and this was related to their production of MMP-9 and MMP-14[72,73]. In addition, these factors secreted by CAAs and CAFs may flood the circulation with signals for distant metastatic sites to initiate their own expression of chemokines that will aid the tumor cell in homing to the metastatic site and preparing the site for colonization once the tumor cell arrives[74]. Research conducted on human omental adipocytes indicates they secrete IL-6 and IL-8 and that antibody-mediated inhibition of either factor resulted in reduced homing of ovarian cancer cells in vitro and in vivo, although inhibition of IL-8 was more efficient at reducing homing of cancer cells in vivo[46]. Once ovarian tumors are established on the omentum, they may convert adjacent adipocytes into CAAs, which results in a positive feedback loop leading to increased IL-6 production and further recruitment of cancer cells to the omentum. It has also been suggested that tumor cells do not metastasize alone, rather, they “travel” with stromal cells. Studies from Duda et al[75] demonstrated by cannulating primary tumor bearing mice, that tumor cells are shed with stromal cells in heterotypic “clumps” from the primary tumor. The stromal component, which included fibroblasts, acted to support the viability of the tumor cells while traveling through the circulation to the metastatic site. Together, these studies suggest that CAAs and CAFs promote the migratory and invasive phenotype in a variety of solid tumors and highlight the importance of elucidating mechanisms to target both CAAs and CAFs.
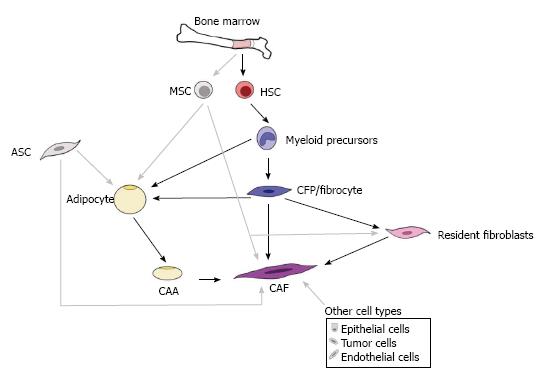
Both CAAs and CAFs are generally thought to arise from tissues adjacent to the tumor; however, recent studies have begun to demonstrate alternative sources, including other resident stromal cells, epithelial cells, and bone marrow. This complex and ever-growing understanding of the origins for CAAs and CAFs can be appreciated in Figure 2. It is possible that these multiple sources are reflected in the morphological, phenotypic, and functional heterogeneity described for adipocytes from different fat depots[76] and for CAFs[77,78]. Given that this heterogeneity is a significant hurdle in therapeutically targeting the TME populations, it will be essential to elucidate the multiple origins for these cells as well as to examine the impact these origins may have on cellular function.
The expansion of adipose tissue is achieved via increases in size (hypertrophy) and/or number (hyperplasia) of adipocytes. Mature adipocytes are postmitotic, therefore, adipocyte hyperplasia requires new adipocytes be produced from their adipogenic precursors. A long-standing paradigm of adipocyte generation is that all adipocytes are differentiated from mesenchymal progenitor cells resident in the vascular stroma, referred to as adipose stem cells (ASCs), where the regional fat depots eventually form[79,80]. However, these progenitor cells are found associated with adipose vessels[79], bringing up the possibility that circulating progenitors, such as those provided by bone marrow, home to adipose tissue through the bloodstream followed by extravasation across the endothelium of blood vessels, subsequently undergoing adipogenic conversion. To test this hypothesis, several groups transplanted GFP-labeled bone marrow into wild-type mice[81-83]. Two of these groups detected GFP-expressing adipocytes in the major adipose depots[81,82], while one failed to detect these cells[83], perhaps due to low marker expression or limited engraftment. When engrafted mice were treated with rosiglitazone or a high fat diet that stimulated adipogenesis, the number of GFP-expressing adipocytes was elevated, and cells were often found in clusters, suggesting clonal growth from bone marrow-derived progenitors[81]. While these studies support a bone marrow origin for CAAs, it is unclear which bone marrow stem cell serves as the CAA progenitor.
It is commonly held that the bone marrow contains two types of stem cells, the mesenchymal stromal cell (MSC) and the hematopoietic stem cell (HSC). MSCs are defined by their adherence to plastic and potential to differentiate into mesenchymal tissue cells such as bone, fat, muscle, cartilage, and fibroblasts[84-87]. HSCs are defined by their capability of hematopoietic reconstitution in vivo and have also been shown to give rise to other tissue cell types including mast cells and osteoclasts. Our laboratory has developed a method for transplantation of a clonal population from a single sorted HSC defined as an EGFP+Lin-Sca-1+c-Kit hiCD34- cell. Using this single cell transplantation model, we have demonstrated that HSCs give rise to a variety of mesenchymal cell types including adipocytes[88], osteocytes and chondrocytes[89], cardiac valve interstitial cells[90]; circulating fibroblast precursors[91], and fibroblasts and myofibroblasts in multiple tissues[92-94] (reviewed in[95]). Further use of our unique clonal cell transplantation model revealed the generation of adipocytes in vivo from clonally derived bone marrow HSCs[88]. Similar to findings from Crossno et al[81], we found that rosiglitazone stimulated adipogenesis from the HSC[88]. In vitro, clones giving rise to monocytes/macrophages under hematopoietic conditions were also able to generate adipocytes under adipogenic conditions, suggesting a differentiation pathway from HSCs-myeloid precursors-adipocytes[88]. Similar results were obtained from the Klemm group who confirmed the de novo generation of a subset of adipocytes from bone marrow myeloid progenitor cells using a non-transplant transgenic mouse model in which LacZ expression was restricted to the myeloid lineage[96]. Moreover, Sterieter and colleagues reported that circulating fibrocytes were capable of adipogenic differentiation both in vitro and in vivo[97]. Due to the dual hematopoietic/mesenchymal nature of fibrocytes, they may be considered as an intermediate for myeloid-derived adipocyte population. These studies demonstrate the ability of the HSC to give rise to adipocytes through the myeloid lineage. While studies have not yet directly examined the role of HSC-derived CAAs in tumor progression, findings from our laboratory suggest that they can enhance tumor growth and tumor cell motility in breast cancer and melanoma models (unpublished data).
Traditionally, CAFs are thought to arise from resident tissue fibroblasts[98]. However, recent studies have suggested alternative sources including other resident stromal cells, epithelial cells, epithelial-mesenchymal transition or endothelial-mesenchymal transdifferentiation (EndoMT), and adipose tissue. Several studies have also suggested a bone marrow origin for myofibroblasts and CAFs[99] (and reviewed in[100-102]). As described above, the bone marrow provides a rich source for both MSCs and HSCs. Using our model for transplantation of a clonal population from a single sorted HSC in conjunction with a variety of solid tumor models, we have demonstrated the presence of HSC-derived fibroblasts in tumor sections from mice transplanted with a clonal population of cells derived from a single, sorted HSC[91,103]. Analysis of sections from Lewis lung carcinoma (LLC) and melanoma (K1735-M2) tumors harvested from clonally engrafted animals showed the presence of HSC-derived CAFs[91,103]. These EGFP-expressing cells had a fibroblastic morphology and constituted 8%-28% of the tumor stromal cells[103]. Characterization of these HSC-derived cells indicated that they were activated fibroblasts, based on expression of αSMA and mRNA expression of collagen I[103]. Also prevalent in the specimens were EGFP+ pericyte-like perivascular cells, suggesting that HSCs contribute to tumor vasculature[103].
Work from our laboratory has also identified a population of circulating fibroblast precursors (CFPs) that express markers of both hematopoietic cells (CD34, CD45) and fibroblasts [collagen I (Col I), discoidin domain receptor-2 (DDR2; a collagen 1 recpetor)]([91] and unpublished data). The CD45+DDR2+ population was shown to differentiate along the monocyte/macrophage lineage, contain the CD34+Col I+ fibrocyte and rapidly differentiate to collagen 1+, αSMA+ cells with fibroblastic morphology. Using our clonal hematopoietic stem cell transplantation model, we conducted an in vitro examination of CFPs/fibrocytes derived from peripheral blood cells of clonally engrafted mice[92]. In these studies, nucleated blood cells were cultured and the appearance of EGFP+ (HSC-derived), spindle-shaped or polygonal cells was detected by the seventh day. Flow cytometric time course analysis of the cultured cells demonstrated decreasing CD45 expression and increasing DDR2 expression. Our studies have demonstrated that CFPs may be stimulated to express markers of activated fibroblasts including collagen, vimentin, and αSMA by exposure to tumor conditioned media. This demonstrates a possible differentiation pathway from the HSCs-myeloid precursors-CFP and with exposure to tumor, HSCs-myeloid precursors-CFP-CAF[91]. These findings are supported by our in vivo data demonstrating the activated fibroblast phenotype of HSC-derived cells recruited from the bone marrow to the tumor stroma[91].
As summarized in Figure 2, multiple origins for CAAs and CAFs have been proposed. Evidence also suggests that CAAs and CAFs may share a common origin. Data from our laboratory using clonal cell lineage tracing demonstrated a monocyte lineage origin for adipocytes, specifically the Mac1lo fraction of bone marrow[88]. Similarly, lineage and gene expression analyses demonstrated that adipocytes and adipocyte progenitors arise from the hematopoietic stem cell via the myeloid lineage[96]. Our studies of CAFs and their circulating precursors demonstrated that these cells originate in the Mac1hi population of peripheral blood and that their participation in tumor may be regulated by MCP-1[91]. Additional evidence suggests plasticity between preadipocytes and macrophages, with preadipocytes being a source for macrophages[104] and tissue macrophages being a source for preadipocytes[105]. Histological evidence of a high ratio of adipocytes to fibroblasts at the tumor invasive front and an extremely high fibroblast-like cell to adipocyte ratio observed at the tumor center, suggests that CAAs may transition and/or give rise to CAFs as tumor progresses[14]. Breast cancer cells were also shown to induce de-differentiation of adipocytes to a more fibroblastic phenotype[21]. Human adipose tissue derived stem cells (hASCs) were found to give rise to CAF-like cells when cultured with conditioned media from MDA-MB-231 or MCF-7 breast cancer cell lines[106]. Under tumor conditions, the hASC-derived CAF-like cells were shown to have a myofibroblastic phenotype, with increased expression of aSMA and tenascin C. This change in phenotype was found to be dependent upon TGFβ signaling in the hASCs. Studies have yet to directly demonstrate a CAF to CAA conversion, however, we have observed in vitro that non-adherent bone marrow cells, enriched for hematopoietic progenitors, cultured in the presence of M-CSF and mouse serum give rise to lipid laden cells with a fibroblast-like morphology (unpublished observation). Together, these studies support an HSC origin for both CAAs and CAFs, suggesting plasticity exists between adipocytes, CAAs, and CAFs. This plasticity may be one mechanism by which heterogeneity of the TME is generated.
Evidence suggests that CAAs and CAFs play a critical role in tumor progression as well as patient prognosis and survival. It is thought that the metabolic changes associated with obesity underlie the increased risk of cancer and cancer-related mortalities. It has been estimated that excess weight and obesity were responsible for 20% of all cancer deaths in women in the United States[107], consistent with poor outcome of cancer in overweight/obese patients (reviewed in[108]). While no direct comparisons of CAAs derived from distinct sources have been conducted, it is clear that HSC-derived adipocytes represent a subpopulation distinct from conventional white and brown adipocytes based on their low expression level of leptin, low mitochondrial/peroxisomal content and oxidative capacity, and elevated inflammatory cytokine production[96]. HSC-derived adipocytes also share numerous features with CAAs including low expression of terminal adipocyte markers and high expression levels of inflammatory cytokines, indicating that HSC-derived adipocytes may be considered “activated” contributors to the TME. Like CAAs, HSC-derived adipocytes were found to be smaller in size than “resident” adipocytes ([81] and our unpublished observation), but whether, as with CAAs, this is related to a higher rate of lipolysis in these cells requires further exploration. It has been noted that HSC-derived adipocytes preferentially accumulate in visceral adipose tissue (VAT) rather than subcutaneous adipose tissue (SAT)[96]. Excess adiposity in VAT is specifically linked to type 2 diabetes and certain forms of cancer[76]. As compared to adipocytes from SAT, VAT adipocytes exhibited higher rates of fatty acid turnover and lipolysis[109] and produced more IL-6 and less adiponectin and leptin[110]. These data could indicate that VAT and SAT adipocytes are generated from different progenitors, or functional changes in different depots are due to differential accumulation of adipocytes arising from distinct progenitors. Furthermore, the accumulation of HSC-derived adipocytes was increased in female mice over males, which may have important inference in human biology[96], as women generally possess a higher percentage of body fat and tend to disproportionally gain fat in VAT following menopause. Coupled with preferential accumulation of HSC-derived adipocytes in VAT, this pattern of adiposity represents a higher risk of adipose-related gynecological cancers for postmenopausal women and suggests HSC-derived CAAs may represent a novel target these patients.
Several studies suggest that CAFs and their unique phenotypes are associated with increased malignant potential. In the case of breast cancer, women with denser breast tissue have an increased tendency to develop cancer[15]. The presence of a fibrous stroma was found to be associated with poor prognosis in squamous cell carcinoma[18]. In non-small cell lung carcinoma, molecular analysis revealed a gene signature for CAFs that was associated with patient prognosis[16]. Interestingly, recent pre-clinical studies in pancreatic ductal adenocarcinoma have demonstrated a protective role for CAFs[111,112], suggesting that the role of these cells is tumor-type dependent. CAFs are a heterogeneous population of cells that can differ based on both location within the tumor and between tumor types and demonstrate different phenotypes, activation states, and/or functions throughout tumor progression. This diversity may, in part, be due to the multiple proposed origins of CAFs, which have led to CAFs being referred to as a “cell state” rather than a specific cell type[113]. A more in depth understanding of the origins of CAFs may shed light on the array of markers expressed by these cells, help to better define the “CAF”, and elucidate their roles based on origin and tumor type.
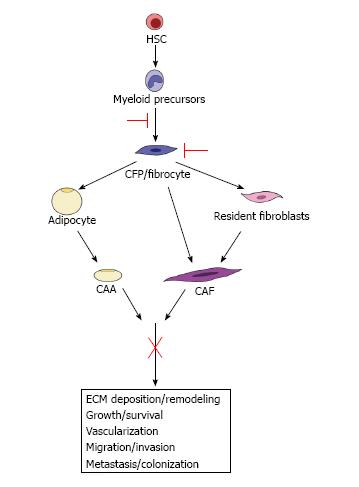
Given the essential roles of the TME in tumor development, progression and metastasis, it is clear that successful anti-tumor therapeutics should include those directed at the support cells of the TME. A key step towards this goal is the gaining knowledge of the role(s) of the different TME cell types (e.g., CAAs and CAFs), their origins, their temporal participation and mechanisms by which they influence tumor. However, the complexity of the crosstalk between the cells of the TME, the broad impact of CAAs/CAFs on the reactive stroma and their contribution to the evolving TME has made therapeutically targeting individual stromal cell derived factors difficult. For example, targeting fibroblast activation protein alpha (FAPα) produced by activated CAFs, while showing promise in preclinical studies[58,114,115], was demonstrated to have no beneficial response in Phase II clinical trials for metastatic colorectal cancer and soft-tissue sarcoma patients[116] (and reviewed in[117]). However, we propose that targeting CAAs, CAFs and their precursors based on their origin may lead to significant advances in treatment by directly targeting the cells before their incorporation into tumor rather than targeting their varied products and multiple effects (Figure 3). We and others have shown that HSCs give rise to adipocytes via the myeloid lineage[88,96]. Likewise, our studies demonstrate an HSC origin for CAFs via the same myeloid lineage[91]. Studies have also demonstrated a myeloid lineage origin for the fibrocyte[118] that also gives rise to adipocytes[97] and fibroblasts[92,119]. These HSC-derived CAAs (unpublished observation) and CAFs[91,103] (and unpublished observation) contribute to the TME and have a significant impact on tumor progression in mouse models. Identification of pathways from the HSC to the CAA or CAF provides a potential opportunity to target CAAs and CAFs both early in their differentiation and at multiple points in their maturation. For example, early inhibition of CFP/fibrocyte differentiation from the myeloid lineage would lead to fewer CAA and CAF precursors available for incorporation in the TME. Likewise, directly targeting CFPs/fibrocytes in circulation may prevent their incorporation into the local and metastatic TME as both CAAs and CAFs, essentially hitting two arms of pro-tumorigenic stromal cells. Given their ability to invade, circulate and extravasate, therapeutically targeting HSC-derived CFPs/fibrocytes may also directly affect the population of CAFs demonstrated to chaperone cancer cells to metastatic sites. Finally, the ability to isolate HSC-derived CAA/CAF progenitors in circulation, combined with their intrinsic ability to home to tumor[91], may provide a novel modality for drug delivery vehicles for chemotherapy. Thus, targeting the precursors of CAAs and CAFs may lead to a more inclusive and encompassing downstream inhibition of their multiple contributions to tumor progression and metastasis. Taken together, these studies highlight the necessity of developing an understanding of the differences and similarities between TME cell types of multiple origins as well as research directed at elucidating the differentiation pathway of these populations for the ultimate goal of TME-based anti-tumor therapy.
P- Reviewer: Fukuda S, Isidori A, Ramirez M, Schwarz H S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Paget J; Lectures on surgical pathology. Philadelphia: Lindsay & Blakinston 1860; . [Cited in This Article: ] |
| 2. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] [Cited in This Article: ] |
| 3. | Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3231] [Cited by in F6Publishing: 3179] [Article Influence: 151.4] [Reference Citation Analysis (0)] |
| 4. | Weinberg RA. Coevolution in the tumor microenvironment. Nat Genet. 2008;40:494-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [PubMed] [Cited in This Article: ] |
| 6. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39812] [Cited by in F6Publishing: 43025] [Article Influence: 3309.6] [Reference Citation Analysis (4)] |
| 7. | Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2921] [Cited by in F6Publishing: 3043] [Article Influence: 253.6] [Reference Citation Analysis (0)] |
| 8. | Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533-1541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 522] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 9. | McMichael AJ. Food, nutrition, physical activity and cancer prevention. Authoritative report from World Cancer Research Fund provides global update. Public Health Nutr. 2008;11:762-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1054] [Cited by in F6Publishing: 969] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 11. | Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3505] [Cited by in F6Publishing: 3431] [Article Influence: 214.4] [Reference Citation Analysis (0)] |
| 12. | Kimijima I, Ohtake T, Sagara H, Watanabe T, Takenoshita S. Scattered fat invasion: an indicator for poor prognosis in premenopausal, and for positive estrogen receptor in postmenopausal breast cancer patients. Oncology. 2000;59 Suppl 1:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Yamaguchi J, Ohtani H, Nakamura K, Shimokawa I, Kanematsu T. Prognostic impact of marginal adipose tissue invasion in ductal carcinoma of the breast. Am J Clin Pathol. 2008;130:382-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol. 2011;55:851-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1610] [Cited by in F6Publishing: 1533] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 16. | Navab R, Strumpf D, Bandarchi B, Zhu CQ, Pintilie M, Ramnarine VR, Ibrahimov E, Radulovich N, Leung L, Barczyk M. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci USA. 2011;108:7160-7165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 283] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 17. | Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Takahashi Y, Ishii G, Taira T, Fujii S, Yanagi S, Hishida T, Yoshida J, Nishimura M, Nomori H, Nagai K. Fibrous stroma is associated with poorer prognosis in lung squamous cell carcinoma patients. J Thorac Oncol. 2011;6:1460-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650-1659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3033] [Cited by in F6Publishing: 2973] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 20. | Andarawewa KL, Motrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C, Rio MC. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005;65:10862-10871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455-2465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 633] [Cited by in F6Publishing: 715] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 22. | Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C, Lisanti MP. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408-6423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115:1163-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 291] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Motrescu ER, Blaise S, Etique N, Messaddeq N, Chenard MP, Stoll I, Tomasetto C, Rio MC. Matrix metalloproteinase-11/stromelysin-3 exhibits collagenolytic function against collagen VI under normal and malignant conditions. Oncogene. 2008;27:6347-6355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765-3773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 836] [Cited by in F6Publishing: 819] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 26. | Dirat B, Bochet L, Escourrou G, Valet P, Muller C. Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev. 2010;19:45-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 664] [Cited by in F6Publishing: 634] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 28. | Choy L, Derynck R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem. 2003;278:9609-9619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1001] [Cited by in F6Publishing: 961] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 30. | Rønnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696-707. [PubMed] [Cited in This Article: ] |
| 31. | Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1625] [Cited by in F6Publishing: 1665] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 32. | Chen H, Yang WW, Wen QT, Xu L, Chen M. TGF-beta induces fibroblast activation protein expression; fibroblast activation protein expression increases the proliferation, adhesion, and migration of HO-8910PM [corrected]. Exp Mol Pathol. 2009;87:189-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167-4171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1935] [Cited by in F6Publishing: 1996] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 34. | Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1232] [Cited by in F6Publishing: 1249] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 35. | Gaggioli C. Collective invasion of carcinoma cells: when the fibroblasts take the lead. Cell Adh Migr. 2008;2:45-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3192] [Cited by in F6Publishing: 3489] [Article Influence: 249.2] [Reference Citation Analysis (0)] |
| 37. | Noël A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol. 2008;19:52-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Barker HE, Bird D, Lang G, Erler JT. Tumor-secreted LOXL2 activates fibroblasts through FAK signaling. Mol Cancer Res. 2013;11:1425-1436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Karvonen HM, Lehtonen ST, Sormunen RT, Lappi-Blanco E, Sköld CM, Kaarteenaho RL. Lung cancer-associated myofibroblasts reveal distinctive ultrastructure and function. J Thorac Oncol. 2014;9:664-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Horie M, Saito A, Mikami Y, Ohshima M, Morishita Y, Nakajima J, Kohyama T, Nagase T. Characterization of human lung cancer-associated fibroblasts in three-dimensional in vitro co-culture model. Biochem Biophys Res Commun. 2012;423:158-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 42. | Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4:e7965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 358] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 43. | Barnas JL, Simpson-Abelson MR, Yokota SJ, Kelleher RJ, Bankert RB. T cells and stromal fibroblasts in human tumor microenvironments represent potential therapeutic targets. Cancer Microenviron. 2010;3:29-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 45. | Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M, Sano K, Amano J, Isogai Z, Niida S. Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res. 2010;70:7073-7083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 46. | Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1310] [Cited by in F6Publishing: 1545] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 47. | Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J Lipid Res. 2007;48:1846-1856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 48. | Kaneko A, Satoh Y, Tokuda Y, Fujiyama C, Udo K, Uozumi J. Effects of adipocytes on the proliferation and differentiation of prostate cancer cells in a 3-D culture model. Int J Urol. 2010;17:369-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem. 2003;278:42660-42667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Behan JW, Yun JP, Proektor MP, Ehsanipour EA, Arutyunyan A, Moses AS, Avramis VI, Louie SG, Butturini A, Heisterkamp N. Adipocytes impair leukemia treatment in mice. Cancer Res. 2009;69:7867-7874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 51. | Bochet L, Meulle A, Imbert S, Salles B, Valet P, Muller C. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun. 2011;411:102-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Gao MQ, Kim BG, Kang S, Choi YP, Yoon JH, Cho NH. Human breast cancer-associated fibroblasts enhance cancer cell proliferation through increased TGF-α cleavage by ADAM17. Cancer Lett. 2013;336:240-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2007;1775:21-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 54. | Jia CC, Wang TT, Liu W, Fu BS, Hua X, Wang GY, Li TJ, Li X, Wu XY, Tai Y. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS One. 2013;8:e63243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 55. | Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482-497. [PubMed] [Cited in This Article: ] |
| 56. | Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984-4001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 869] [Cited by in F6Publishing: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 57. | Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1526] [Cited by in F6Publishing: 1457] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 58. | Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955-1962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 492] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 59. | Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819-3825. [PubMed] [Cited in This Article: ] |
| 60. | Dudley AC, Shih SC, Cliffe AR, Hida K, Klagsbrun M. Attenuated p53 activation in tumour-associated stromal cells accompanies decreased sensitivity to etoposide and vincristine. Br J Cancer. 2008;99:118-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5115] [Cited by in F6Publishing: 5647] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 62. | Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 63. | Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2625] [Cited by in F6Publishing: 2751] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 64. | Xu LN, Xu BN, Cai J, Yang JB, Lin N. Tumor-associated fibroblast-conditioned medium promotes tumor cell proliferation and angiogenesis. Genet Mol Res. 2013;12:5863-5871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 66. | Al-Ansari MM, Hendrayani SF, Tulbah A, Al-Tweigeri T, Shehata AI, Aboussekhra A. p16INK4A represses breast stromal fibroblasts migration/invasion and their VEGF-A-dependent promotion of angiogenesis through Akt inhibition. Neoplasia. 2012;14:1269-1277. [PubMed] [Cited in This Article: ] |
| 67. | Pinto MP, Badtke MM, Dudevoir ML, Harrell JC, Jacobsen BM, Horwitz KB. Vascular endothelial growth factor secreted by activated stroma enhances angiogenesis and hormone-independent growth of estrogen receptor-positive breast cancer. Cancer Res. 2010;70:2655-2664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Rio MC. From a unique cell to metastasis is a long way to go: clues to stromelysin-3 participation. Biochimie. 2005;87:299-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Noël A, Boulay A, Kebers F, Kannan R, Hajitou A, Calberg-Bacq CM, Basset P, Rio MC, Foidart JM. Demonstration in vivo that stromelysin-3 functions through its proteolytic activity. Oncogene. 2000;19:1605-1612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Nöel AC, Lefebvre O, Maquoi E, VanHoorde L, Chenard MP, Mareel M, Foidart JM, Basset P, Rio MC. Stromelysin-3 expression promotes tumor take in nude mice. J Clin Invest. 1996;97:1924-1930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Kushiro K, Chu RA, Verma A, Núñez NP. Adipocytes Promote B16BL6 Melanoma Cell Invasion and the Epithelial-to-Mesenchymal Transition. Cancer Microenviron. 2012;5:73-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci USA. 2009;106:3372-3377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 73. | Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 74. | Rafii S, Lyden D. S100 chemokines mediate bookmarking of premetastatic niches. Nat Cell Biol. 2006;8:1321-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, Jain RK. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA. 2010;107:21677-21682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 76. | Majka SM, Barak Y, Klemm DJ. Concise review: adipocyte origins: weighing the possibilities. Stem Cells. 2011;29:1034-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 551] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 78. | Orimo A, Weinberg RA. Heterogeneity of stromal fibroblasts in tumors. Cancer Biol Ther. 2007;6:618-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 79. | Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 814] [Cited by in F6Publishing: 818] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 80. | Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 694] [Cited by in F6Publishing: 707] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 81. | Crossno JT, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220-3228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 82. | Tomiyama K, Murase N, Stolz DB, Toyokawa H, O’Donnell DR, Smith DM, Dudas JR, Rubin JP, Marra KG. Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem Cells. 2008;26:330-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684-3695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 84. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15372] [Cited by in F6Publishing: 14773] [Article Influence: 590.9] [Reference Citation Analysis (0)] |
| 85. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1391] [Cited by in F6Publishing: 1364] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 86. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11055] [Cited by in F6Publishing: 11776] [Article Influence: 692.7] [Reference Citation Analysis (1)] |
| 87. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1210] [Cited by in F6Publishing: 1083] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 88. | Sera Y, LaRue AC, Moussa O, Mehrotra M, Duncan JD, Williams CR, Nishimoto E, Schulte BA, Watson PM, Watson DK. Hematopoietic stem cell origin of adipocytes. Exp Hematol. 2009;37:1108-1120, 1120.e1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 89. | Mehrotra M, Williams CR, Ogawa M, LaRue AC. Hematopoietic stem cells give rise to osteo-chondrogenic cells. Blood Cells Mol Dis. 2013;50:41-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98:690-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 91. | Abangan RS, Williams CR, Mehrotra M, Duncan JD, Larue AC. MCP1 directs trafficking of hematopoietic stem cell-derived fibroblast precursors in solid tumor. Am J Pathol. 2010;176:1914-1926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Ebihara Y, Masuya M, Larue AC, Fleming PA, Visconti RP, Minamiguchi H, Drake CJ, Ogawa M. Hematopoietic origins of fibroblasts: II. In vitro studies of fibroblasts, CFU-F, and fibrocytes. Exp Hematol. 2006;34:219-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 93. | Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108:2893-2896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 94. | Shirai K, Sera Y, Bulkeley W, Mehrotra M, Moussa O, LaRue AC, Watson DK, Stuart RK, Lazarchick J, Ogawa M. Hematopoietic stem cell origin of human fibroblasts: cell culture studies of female recipients of gender-mismatched stem cell transplantation and patients with chronic myelogenous leukemia. Exp Hematol. 2009;37:1464-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Ogawa M, LaRue AC, Mehrotra M. Hematopoietic stem cells are pluripotent and not just “hematopoietic”. Blood Cells Mol Dis. 2013;51:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 96. | Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, Janssen RC, Friedman JE, Woessner BT, Shade TR. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc Natl Acad Sci USA. 2010;107:14781-14786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 97. | Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029-2031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 98. | Dunphy JE. The fibroblast-a ubiquitous ally for the surgeon. NEJM. 1963;268:1367-1377. [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 734] [Cited by in F6Publishing: 806] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 100. | Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed). 2010;15:166-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 495] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 101. | Gonda TA, Varro A, Wang TC, Tycko B. Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy? Semin Cell Dev Biol. 2010;21:2-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 102. | McDonald LT, LaRue AC. Hematopoietic stem cell derived carcinoma-associated fibroblasts: a novel origin. Int J Clin Exp Pathol. 2012;5:863-873. [PubMed] [Cited in This Article: ] |
| 103. | LaRue AC, Masuya M, Ebihara Y, Fleming PA, Visconti RP, Minamiguchi H, Ogawa M, Drake CJ. Hematopoietic origins of fibroblasts: I. In vivo studies of fibroblasts associated with solid tumors. Exp Hematol. 2006;34:208-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 104. | Charrière G, Cousin B, Arnaud E, André M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850-9855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 347] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 105. | Chazenbalk G, Bertolotto C, Heneidi S, Jumabay M, Trivax B, Aronowitz J, Yoshimura K, Simmons CF, Dumesic DA, Azziz R. Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity. PLoS One. 2011;6:e17834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 106. | Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song YH. Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cell Oncol (Dordr). 2011;34:55-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 107. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5430] [Cited by in F6Publishing: 5054] [Article Influence: 240.7] [Reference Citation Analysis (0)] |
| 108. | van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569-2578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 483] [Cited by in F6Publishing: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 109. | Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl. 1988;19:26-29. [PubMed] [Cited in This Article: ] |
| 110. | Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273-2282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1025] [Cited by in F6Publishing: 973] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 111. | Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1359] [Cited by in F6Publishing: 1470] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 112. | Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1517] [Cited by in F6Publishing: 1673] [Article Influence: 167.3] [Reference Citation Analysis (0)] |
| 113. | Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’--more than meets the eye. Trends Mol Med. 2013;19:447-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 230] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 114. | Brennen WN, Rosen DM, Wang H, Isaacs JT, Denmeade SR. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst. 2012;104:1320-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 115. | Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11:257-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 116. | Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jäger D, Renner C, Tanswell P, Kunz U, Amelsberg A, Kuthan H. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26:44-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 117. | Liu R, Li H, Liu L, Yu J, Ren X. Fibroblast activation protein: A potential therapeutic target in cancer. Cancer Biol Ther. 2012;13:123-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 118. | Pilling DP, Gomer RH. Regulatory pathways for fibrocyte differentiation. Fibrocytes: New insights into tissue repair and systemic fibroses. Yale University, USA: World Scientific 2007; 37-60. [Cited in This Article: ] |