Published online Oct 15, 2009. doi: 10.4253/wjge.v1.i1.21
Revised: September 5, 2009
Accepted: September 12, 2009
Published online: October 15, 2009
Compared with endoscopic submucosal dissection (ESD), endoscopic mucosal resection (EMR) is easier to perform and requires less time for treatment. However, EMR has been replaced by ESD, because achieving en bloc resection of specimens > 20 mm in diameter is difficult with EMR. The technique of ESD was introduced to resect large specimens of early gastric cancer in a single piece. ESD can provide precise histological diagnosis and can also reduce the rate of recurrence, but has a high level of technical difficulty, and is consequently associated with a high rate of complications, a need for advanced endoscopic techniques, and a lengthy procedure time. To overcome disadvantages in both EMR and ESD, various advances have been made in submucosal injections, knives, other accessories, and in electrocoagulation systems.
- Citation: Kume K. Endoscopic mucosal resection and endoscopic submucosal dissection for early gastric cancer: Current and original devices. World J Gastrointest Endosc 2009; 1(1): 21-31
- URL: https://www.wjgnet.com/1948-5190/full/v1/i1/21.htm
- DOI: https://dx.doi.org/10.4253/wjge.v1.i1.21
Early gastric cancer confined to the mucosa can be treated successfully with endoscopic resection alone. Endoscopic resection of early gastric cancer originated with the development of a polypectomy technique using high-frequency current for gastric polyps in 1968[1], and has become popular as endoscopic mucosal resection (EMR) since the birth of the strip biopsy method in 1984[2]. Endoscopic submucosal dissection (ESD) is a new endoscopic technique using cutting devices that developed from one of the EMR techniques, namely endoscopic resection after local injection of a solution of hypertonic saline-epinephrine[3]. EMR has recently been replaced by ESD, because en bloc resection of specimens > 20 mm in diameter is difficult to achieve with EMR, and piecemeal resection is associated with increased rates of local recurrence to about 15%[4,5]. The technique of ESD was introduced to resect large specimens of early gastric cancer in a single piece. However, the question remains as to whether ESD is superior to EMR in all regards.
This review provides an overview of the techniques and devices of EMR and ESD.
Various devices and techniques of EMR have been described. EMR is divided into techniques without an aspiration cap and techniques with an aspiration cap. Strip biopsy methods using a single-channel scope or double-channel scope are regarded as techniques without an aspiration cap. Cap-assisted endoscopic mucosal resection (EMRC), endoscopic aspiration mucosectomy (EAM), endoscopic mucosal resection with ligation (EMRL), and others are regarded as techniques with an aspiration cap.
The lesion is raised off the muscularis propia by the creation of a submucosal bleb, strangulated by a snare, and resected using an electrosurgical snare[2,6].
Submucosal injection is performed in standard fashion. Both the snare and grasping forceps are advanced through the channels. In preparation for EMR, the snare is opened to capture the forceps, then closed snugly. The lesion is grasped by the forceps and pulled gently into the now-opened snare. The snare is then closed and the lesion is resected[6-8].
EMRC (standard): EMRC is a simpler and easier refinement of EMR methods[9]. The technique requires a specialized transparent plastic cap that is fitted to the tip of the endoscope. Different-sized caps are available, according to the diameter of the endoscope (Olympus, Japan). In addition, a soft 18-mm diameter cap designed for en bloc resection of larger lesions is available. Matsuzaki et al[10] used this soft cap for resection of gastric lesions 1.4-times larger than specimens that could be removed by the conventional cap.
After marking the periphery of the lesion, submucosal solution (saline, glucose, Glycerol®, etc.) is injected into the submucosa. The crescent-shaped snare (SD-221L-25 or SD-7P-1; Olympus) is then prelooped into the groove of the rim of the cap. The endoscopist performs this pre-looping by lightly pressing against and suctioning normal mucosa to seal the cap outlet. The snare is opened and forced to rest along the inside groove of the rim of the cap to form the loop. Suction is released and the cap is then used to suck the lesion with medium to high vacuum into the cap. The endoscopist then strangulates the lesion by closing the snare and the suction is again released. Once the lesion looks similar to a snared polypoid lesion, blend electrosurgical current is typically used to resect the lesion.
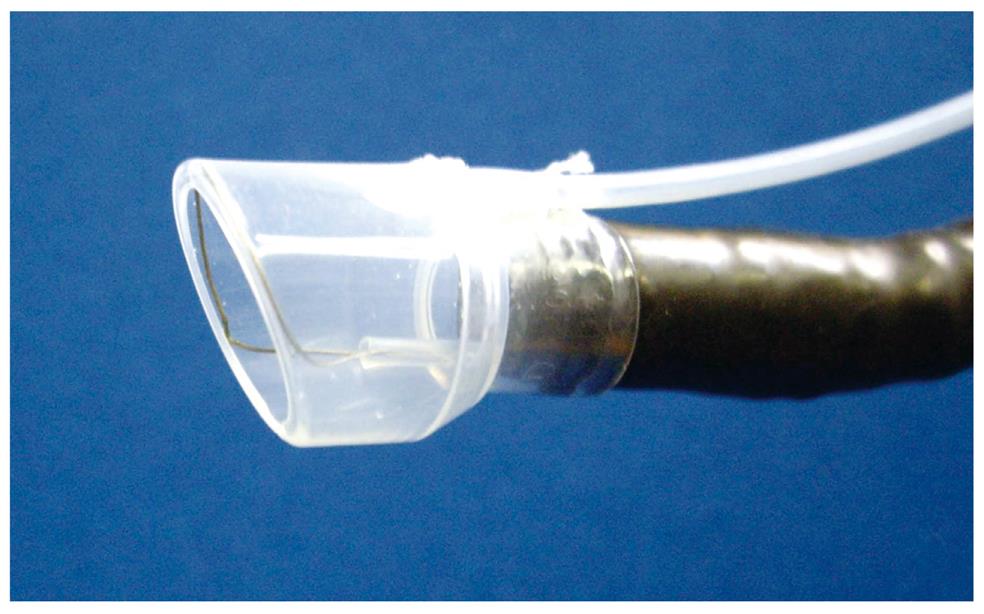
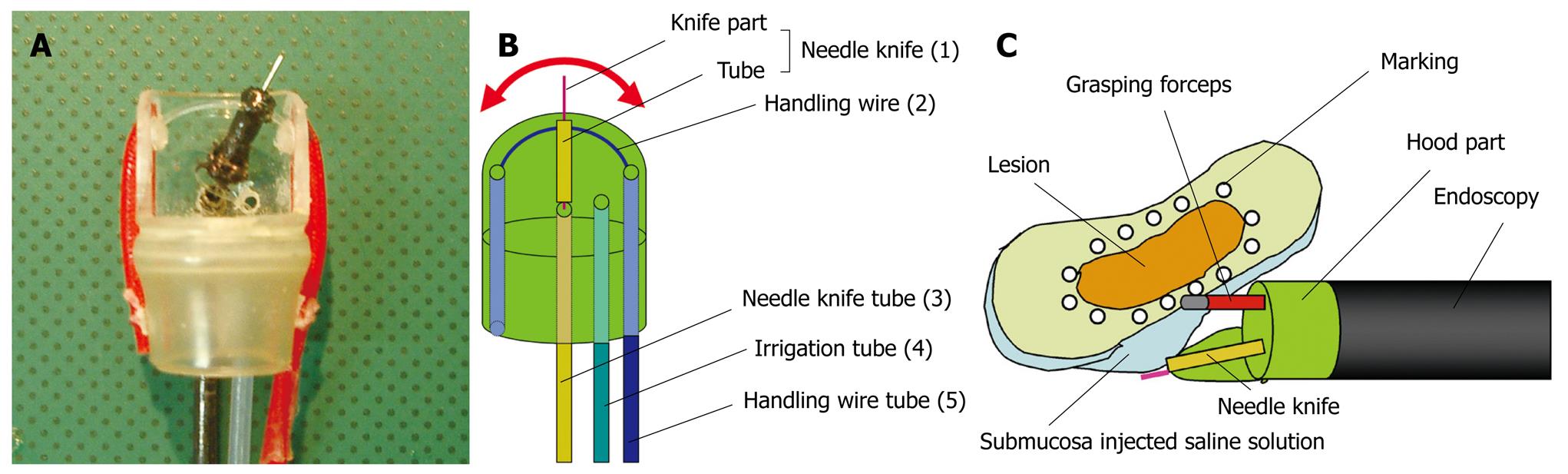
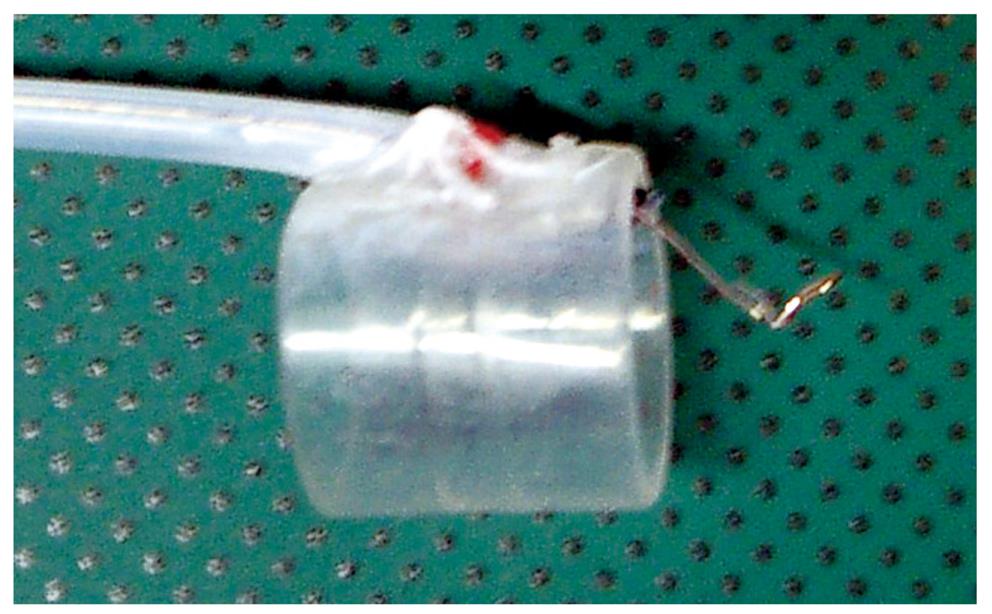
EMRC-UI (EMRC under irrigation): One problem with the EMRC method is that the lesion cannot always be kept in the center of the cap, because the procedure is performed in a blind manner after aspiration. The usefulness of a novel end-hood that facilitates endoscopic hemostatic procedures while simultaneously allowing irrigation of the bleeding site was improved by the author, who developed a soft, prelooped cap with attached irrigation tube (Figure 1)[11-14].
The aspiration method of EMRC-UI method is similar to EMRC. Aspiration is applied repeatedly until the lesion is stabilized in the center of the hood. If the field of view is compromised because of the presence of mucus and/or blood, the site is irrigated. After strangulating the lesion by closing the snare, the negative aspiration pressure is released.
EMR-UI was performed in 15 patients with a median time required of 19 min. Mean diameter of specimens was 24.5 mm (interquartile range, 15-35 mm). The proportion of en bloc-resected lesions was 86.7% (13/15).
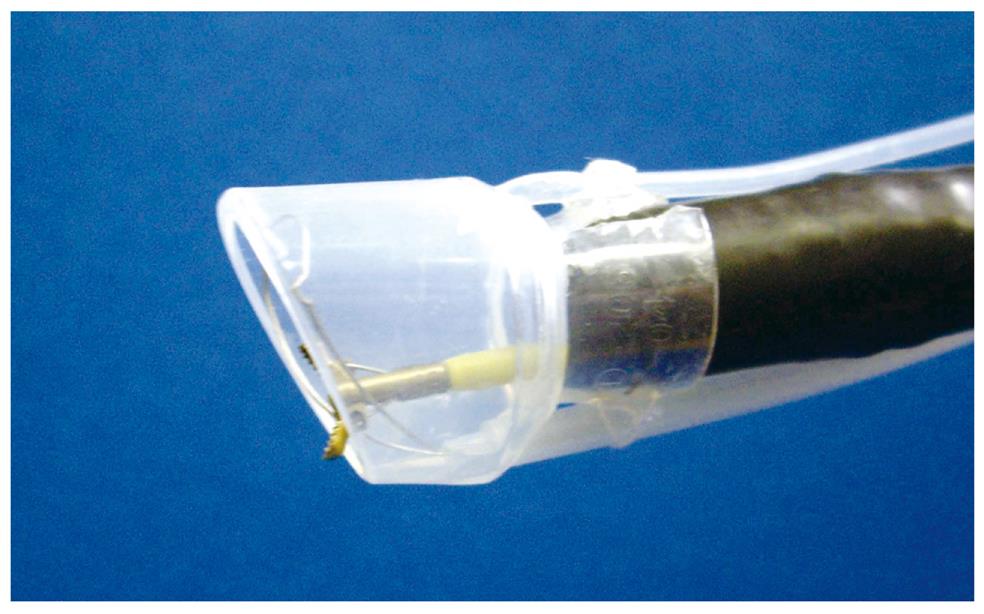
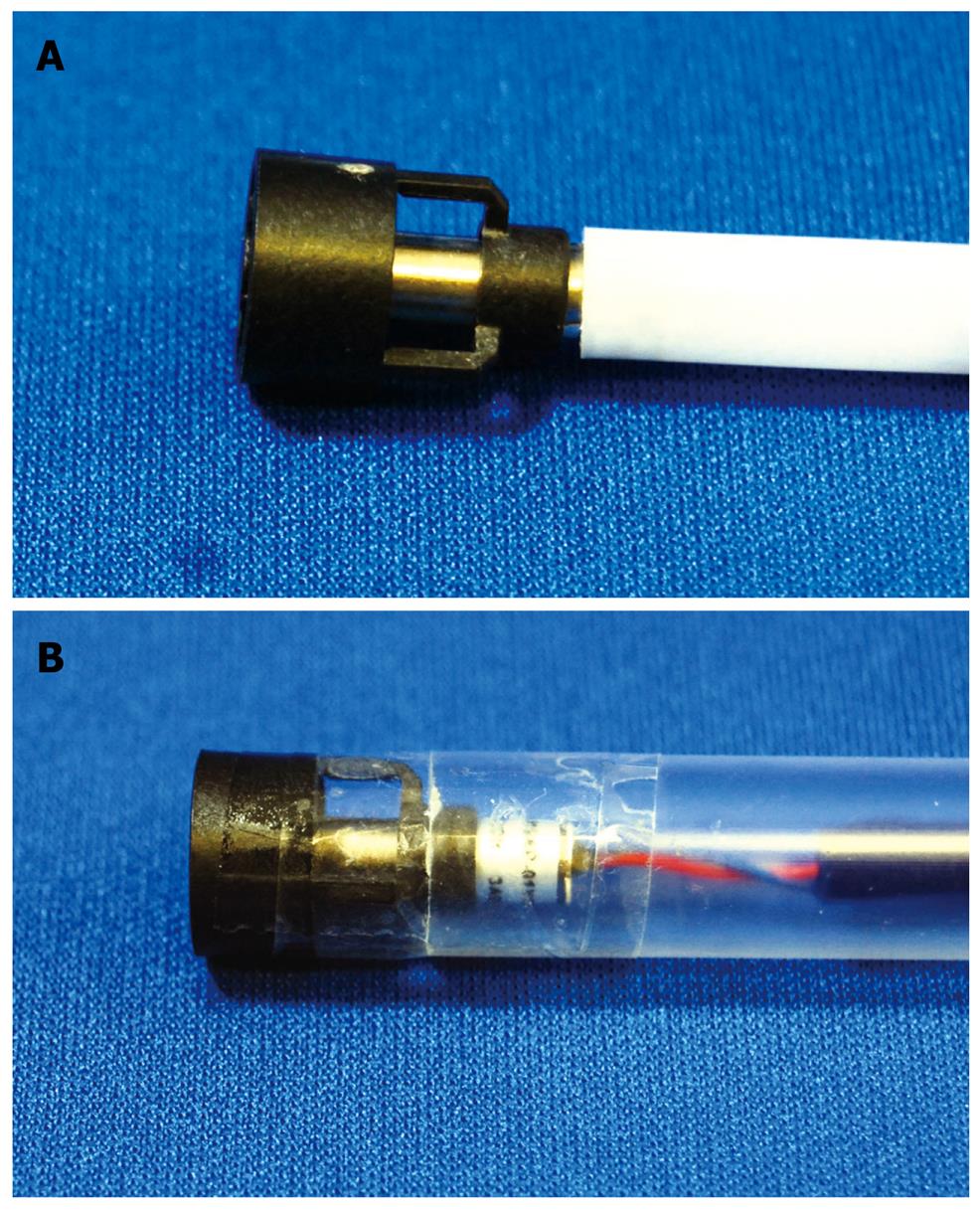
Grasping forceps-assisted EMRC using a 2-channel prelooped cap: The author has also improved the EMRC-UI cap. Two side holes were fabricated by drilling in the hood portion of a conventional soft prelooped cap, and then the irrigation tube and the accessory channel tube were glued to the exterior surface of the holes. The author developed a 2-channel prelooped cap that facilitates EMRC while simultaneously allowing both grip of the central position of the lesion and irrigation of the aspiration site (Figure 2)[15].
The aspiration method of grasping forceps-assisted EMRC using a 2-channel prelooped cap method is similar to EMRC. The endoscopist releases the negative aspiration pressure while slowly pulling the regular biopsy forceps gripping the center of the lesion. Until the lesion is stabilized in the center of the hood, the endoscopist repeatedly performs grasp and aspiration of the lesion. If the field of view at the aspiration site is poor as a result of contamination by mucus and blood, the endoscopist repeatedly performs irrigation of the site. After strangulating the lesion by closing the snare, the endoscopist again releases the aspiration.
Grasping forceps-assisted EMRC using a 2-channel prelooped cap was performed in 12 patients with a median time required of 19 min. Mean diameter of specimens was 22.3 mm (interquartile range, 15-31 mm). The rate of en bloc resection was 91.7% (11/12).
EMRC using IRS (internally retained snare) cap: In EMRC, the crescent-shaped snare needs to be prelooped into the groove on the rim of the cap during the procedure itself. As this pre-looping can be initially difficult, the author has avoided this step by developing a new type of prelooped cap, the “internally retained snare” (IRS) cap that makes pre-looping unnecessary (Figure 3)[16].
After fitting the IRS cap to the tip of the endoscope, EMRC using the IRS cap method is similar to EMRC. The endoscopist releases the negative aspiration pressure and the cap is then positioned to aspirate the lesion with medium to high vacuum into the hood. The endoscopist again releases the aspiration, after strangulating the lesion by closing the snare.
EMRC using an IRS cap was performed in 27 patients with a median time required of 16 min. Mean diameter of specimens was 27.6 mm (interquartile range, 15-38 mm). The rate of en bloc resection was 88.9% (24/27).
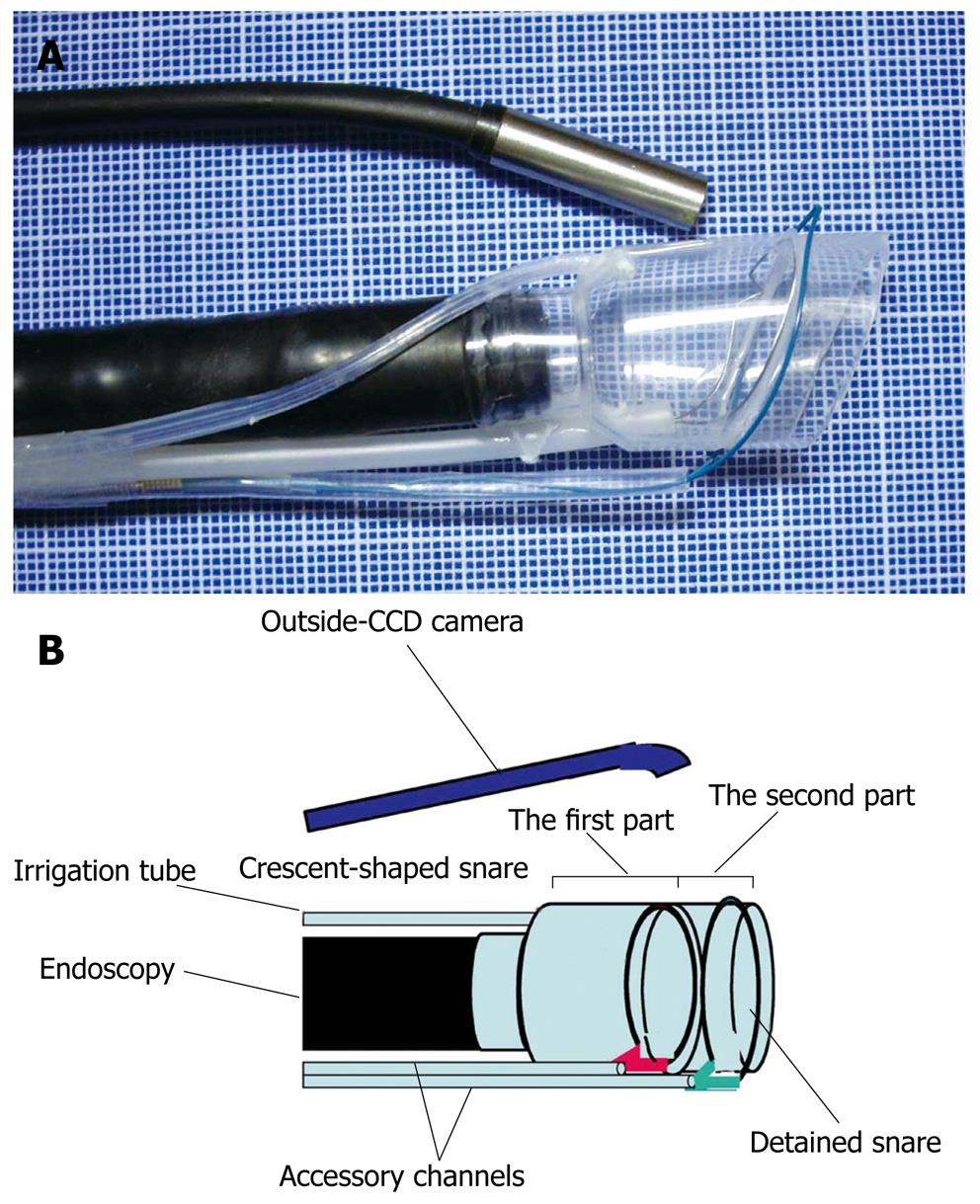
EMRC-C (EMRC and closure): Delayed bleeding may occur from a gastric ulcer after EMRC. Solving this problem may allow surgery on an outpatient basis. The author therefore developed a novel EMRC and closure (EMRC-C) cap that facilitates the EAM procedure whilst simultaneously allowing endoscopic closure (Figure 4)[17]. The EMRC-C hood was produced by attaching an additional hood of short length and another accessory channel to the top of the 2-channel prelooped cap. Two types of snares are then set. The crescent-shaped snare (SD-221L-25; Olympus) is inserted through the accessory channel tube of the first part of the hood, and prelooped into the groove of the rim of the hood. The detained snare (HX-20L-1; Olympus) is passed through and tightened around the outer circumference of the second part of the hood.
The endoscopist places the EMRC-C hood at the tip of the endoscope. Aspiration is released and the hood is then used to aspirate the lesion by high-power vacuum into the hood. The endoscopist confirms aspiration of the lesion with the outside CCD camera then snares the lesion using the detained and crescent-shaped snares. The endoscopist uses the former to tightly strangle the lesion, and resected the lesion using blend electrosurgical current and closing the latter snare.
Two specimens were resected in an animal model (pigs). Mean diameter of the resected specimens was 15 mm. This device has not yet been used in human patients.
EAM (standard): EAM uses a conventional hood (Create Medic, Yokohama, Japan, and TOP, Tokyo, Japan). In this device, a snare is passed through an outside channel and tightened around the outer circumference of the hood[16,18-20]. Pre-looping during the EAM procedure is thus unnecessary.
In an adaptation of EAM with a snare on the tip of the endoscope, the EAM hood method is similar to EMRC. The endoscopist releases the negative aspiration pressure and the hood is then placed to aspirate the lesion with medium to high vacuum into the hood. The snare is pushed over the tumor while the lesion is aspirated. In addition, the loop is pulled tightly around the specimen. The endoscopist again releases the aspiration, after strangulating the lesion by closing the snare.
EAM hood was performed in 27 patients. Mean diameter of specimens was 27.1 mm (interquartile range, 20-43 mm). The rate of en bloc resection was 85.2% (23/27).
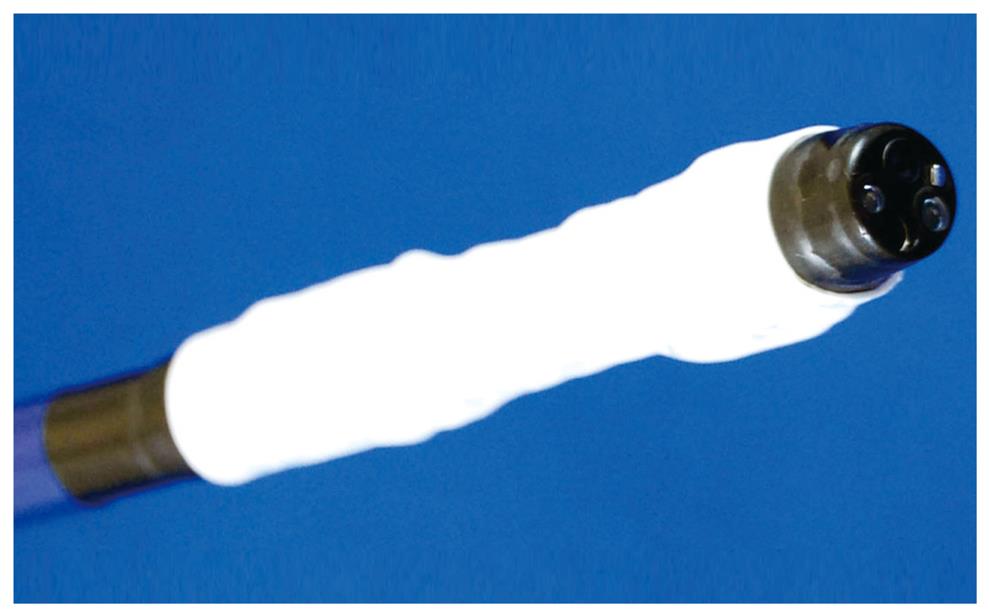
EAM-V (EAM with vibration): EAM carries a risk of aspirating and perforating the full thickness of the gastric wall. A novel vibration hood to reduce such risks was therefore developed (Figure 5)[21]. This novel hood enables strangulation and resection of only the mucosal and submucosal layers by vibrating the snare during strangulation to shake off the muscle layer and serous membrane.
Investigations were conducted separately with and without vibration at 10 000 rpm applied at the time of strangulation and resection. Eighteen specimens were resected in an animal model (pigs). Perforation rates were lower in the vibration group (0/9: 0%) than in the group without vibration (2/9: 22.2%). This device has not yet been used in human patients.
The technique of EMR with ligation (EMRL) uses a standard endoscopic variceal ligation device fitted to a single-channel endoscope[22]. The maximum lesion size for en bloc resection is 1.5 cm. Larger lesions may require piecemeal resection. This technique has been reported with or without prior submucosal injection. The lesion is snared by standard snare polypectomy after it has been ligated at its base with an endoscopic variceal ligation device.
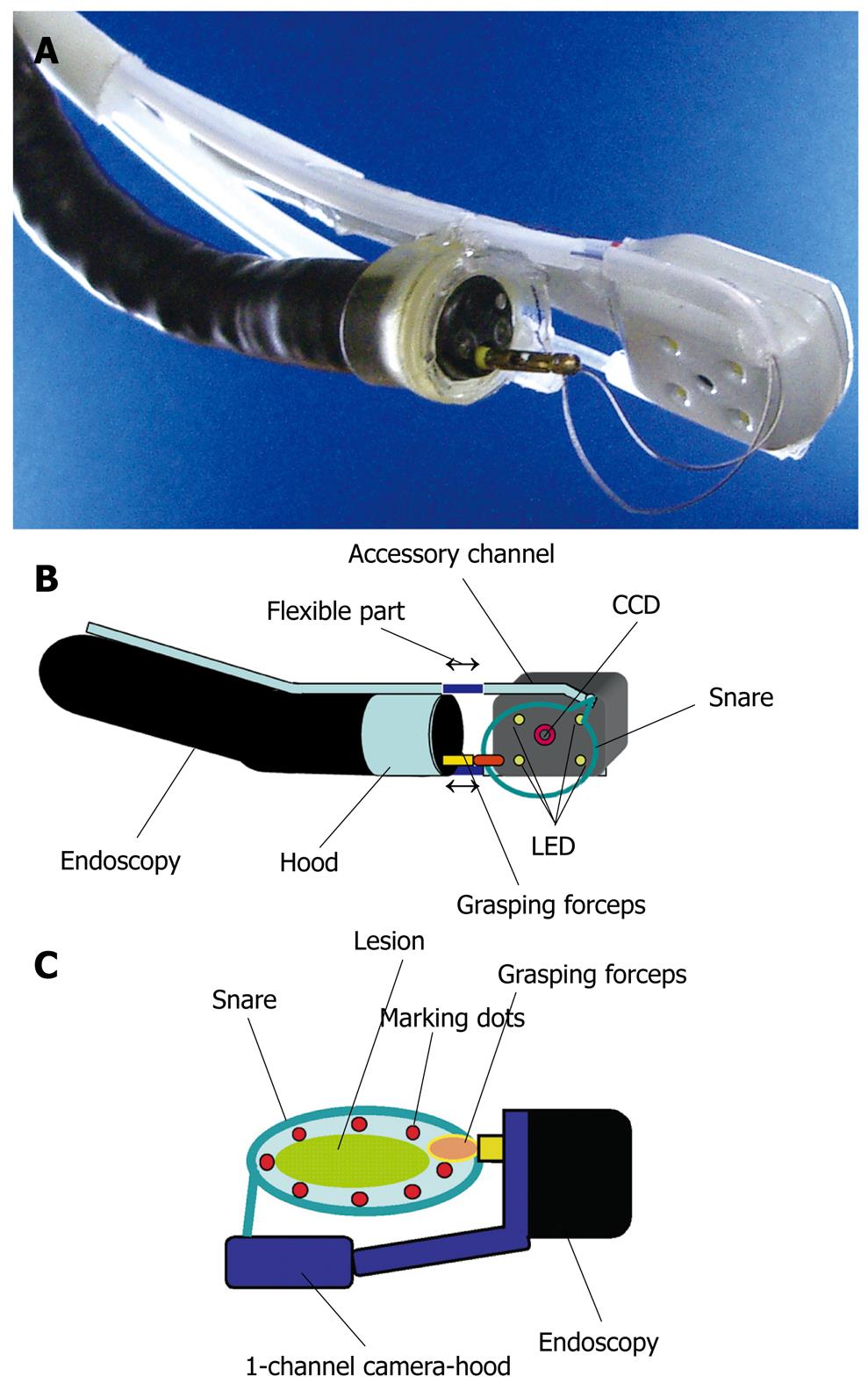
Precise snaring during EMR is important to achieve en bloc resection. However, this can be difficult to achieve in practice, because snaring cannot be performed under complete observation. Although we can easily observe the proximal side of the lifting lesion, the distal side is hard to see after injection of saline solution into the submucosa. The author therefore developed a novel 1-channel camera-hood that allows observation of the distal side of the lesion during snaring in the EMR procedure (Figure 6)[23]. The 1-channel camera-hood was fabricated by cutting a “U-shape” in the cap portion of the hood and then attaching a machined camera, originally developed for dental use, that consisted of a charge-coupled device (CCD) camera and 4 light-emitting diodes (LEDs) (“Miharu-kun”; RF System Lab, Japan) through two tubes. The length of the two tubes is variable and one is an accessory channel (Figure 6A and B).
EMR using the 1-channel camera-hood was performed as follows (Figure 6C). After injection of saline solution into the submucosa, the endoscope was removed and the 1-channel camera-hood was placed on the tip and fixed with tape. A snare was passed through the accessory channel of the hood, and grasping forceps were passed through the accessory channel of the endoscope. The grasping forceps were used to catch hold of the snare. The lesion was then strangulated by precisely closing the snare under adequate observation by both the CCD camera of the 1-channel camera-hood and the endoscope. Blend electrosurgical current was used to resect the lesion. This device has not yet been used in human patients.
The technique of ESD was introduced to resect large specimens of early gastric cancer in a single piece. ESD can provide precise histological diagnosis and can also reduce the recurrence rate[4]. The drawback of ESD lies in the technical difficulty, and this technique is therefore associated with a high rate of complications, the need for advanced endoscopic techniques, and a lengthy procedure time[5,24].
Standard ESD requires special cutting knives, such as a needle knife[3], an insulation-tipped electrosurgical (IT) knife[4,5,24-28], a hook knife[29,30], a flex knife[31], a flush knife[32], a triangle-tip (TT) knife[33], a Fork knife[34], or a mucosectomy[35].
Standard ESD is performed with a standard single accessory-channel endoscope. Typical sequences are the following: marking; incision; submucosal dissection with simultaneous hemostasis. After marking with several dots outside the lesion, various submucosal solutions are injected, including the normal saline solution and epinephrine mixture, glycerol mixture, and hyaluronic acid. A circumferential incision into the mucosa is made using one of the special cutting knives. Direct dissection of the submucosal layer is performed with one of the specified knives until complete removal is achieved. During ESD, the endoscopist performs endoscopic hemostasis with either the knife itself or hemostatic forceps whenever active bleeding is noticed. After ESD, the endoscopist performs preventive endoscopic hemostasis for any oozing or exposed vessels. High-frequency generators (Erbotom ICC200 or VIO 300D; ERBE, Tübingen, Germany) were used for marking, incision of the gastric mucosa, gastric submucosal dissection, and endoscopic hemostasis.
IT knife: The IT knife consists of a small ceramic ball attached to the tip of a high-frequency needle knife[4,5,24-28]. The ceramic ball functions as an insulator for the tip of the needle knife, so that incision and dissection of the mucosa and submucosa can be performed safely. The insulator helps to prevent perforation due to accidental cutting of the muscularis propria. A specialized feature of the IT knife is that the portion between the insulator tip and sheath is used for incision, sweeping off the tissue with the blade portion of the knife instead of the tip. This feature makes a pull-cut, whereas the direction of incision is limited, and straight-forward incision is difficult while looking directly at the incision line or submucosa.
Hook knife: The top of the hook-type knife is right-angled, 1 mm in size[29,30]. Compared to the use of a needle knife, safety is improved because the submucosal tissue is hooked and pulled before incision. This knife has a rotating function so that the operator can select the optimal direction of the hook.
Flex knife: The point of the flex knife is rounded with a twisted wire, like a snare[31]. The sheath is soft and flexible. This knife is less likely to cause perforation when reaching the muscular layer, as the tip is round and the entire knife is soft and flexible. As the tip of the sheath is thick and functions as a stopper, operators can easily control the depth of incision.
Flush knife (Water jet short needle knife): The Flush knife is a characteristic knife with a needle 0.4 mm in a diameter and five projecting parts of 1, 1.5, 2, 2.5, and 3 mm in length[32]. A knife clamp at the tip of the sheath is ceramic for heat insulation. The outer sheath is 2.6 mm in diameter and water emission is possible through the lumen of the sheath by connecting a water pump. The water jet is swiftly activated by pressing a foot pedal on the conduction pump. The conductor of the sheath lumen is insulated to prevent electric current dispersion.
TT knife: The TT knife evolved from the process of ESD, which began with the IT knife[33]. The triangular tip of the knife can be used for either cutting or coagulating, and has been designed to operate in any direction.
Fork knife: The Fork knife has two interchangeable knives; a fixed flexible snare and a forked knife, all of which form a single working unit. The Fork knife also has an inlet for material injection or saline irrigation during the procedure[34]. Such knives can be changed during a procedure by using two switches, the fork knob and core knob, located on the center of the body.
The first of the two knives that constitute the Fork knife is the fixed flexible snare, which is operated by sliding the core knob switch forward. The blade is shaped into an elongated loop much like the Flex knife. Endoscopists mainly use the fixed flexible snare for marking and making incisions around lesions.
The second knife is a forked knife, which has a double-tipped blade (longer tip length, 2 mm; shorter tip length, 1.5 mm) with a forked shape. The fork knife is located on the opposite side from the fixed snare knife and is operated by sliding the fork knob switch forward. This form of needle knife has an M-shape that maximizes the power applied to contacted surfaces and is advantageous for dissection and coagulation. The longer tip of the forked knife can be used as an injection needle for making a submucosal cushion, or for injecting agents. The opening of the needle is located in the center of the knife, between both tips, so that the mucosa must be injected deeply and at an oblique angle for maximum injection into the submucosa. Endoscopists use the forked knife mainly for submucosal dissection performed in the proximal to distal direction of the endoscope, under direct visualization of the dissection area.
Mucosectome: The mucosectome is composed of a flexible plastic shaft and cutting wire[35]. The handle-operated top of this device turns freely, assisting the cutting wire to face the proper direction. The plastic shaft moves the muscular layer aside. The cutting wire moves the mucosal layer aside from the submucosa during ESD, and then the procedure itself can be performed safely.
A transparent hood is helpful for better visualization of the operating field. In particular, good visualization of the submucosal tissue with the aid of a small-caliber-tip transparent hood makes cutting procedures easy and safe[36]. EMR using a small-caliber-tip transparent hood is a peeling-off method using a needle-knife for mucosal and submucosal incisions.
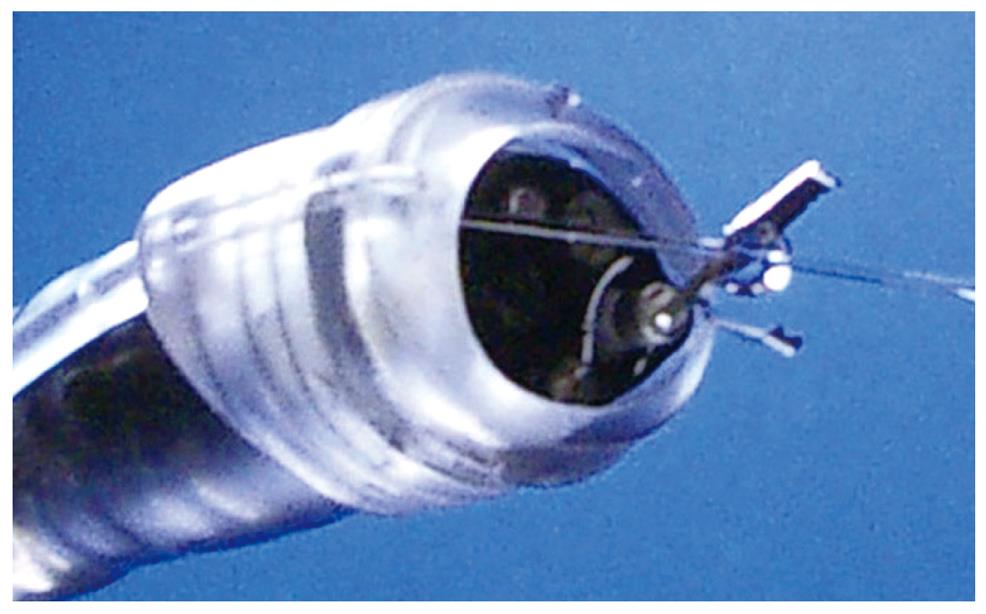
Cap knife: The author developed a novel one-third partially transparent hood that facilitates endoscopic hemostatic procedures while simultaneously allowing the irrigation of bleeding (Figure 7)[12]. The one-third partial hood is easily placed on the tip of the endoscope, although it must be fitted to the right side of the endoscope. The hood-knife was fabricated by drilling another side hole in addition to the hole for the irrigation tube in the cap portion of a transparent end hood[37]. A snare forceps was glued to the exterior surface over the hole and attached using short tubes on the inside of the cap. Based on this prototype, the irrigation cap-knife (cap-knife attachment (Type KUME) with a fixed snare) was developed as shown in Figure 8 (Create Medic, Yokohama, Japan)[38].
The ESD procedure using the cap-knife is performed as follows. After the tumor is separated from surrounding normal mucosa by complete incision around the lesion using the IT knife, the endoscope is then removed, and the cap-knife is placed on the tip and fixed with tape. Grasping forceps are passed through the accessory channel and push the lesion away from the muscle layer. Submucosal exfoliation was achieved simply by sliding the cap-knife onto the muscle layer and applying a coagulation current.
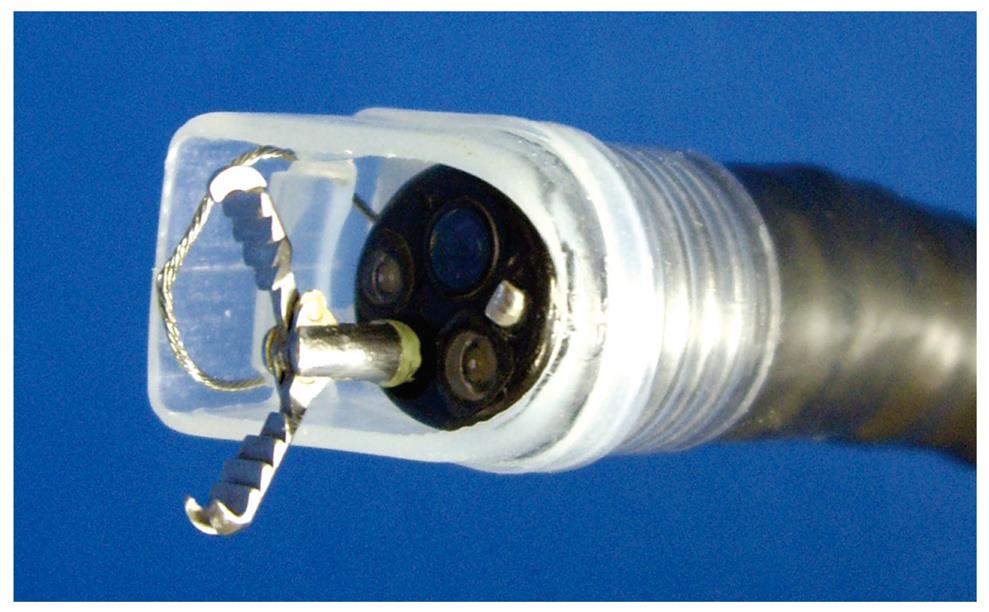
Wiper-knife: The wiper-knife was fabricated by installing a needle-knife in exchange for a snare forceps (Figure 9A and B)[39]. A handling wire intersects the needle-knife and fixes it. The handling wire is passed through a hole opening at either end of the hood. A novel wiper-knife was fabricated such that ESD could be performed by movements similar to a windshield wiper.
ESD using the wiper-knife was performed as follows (Figure 9C). A grasping forceps was passed through accessory channel and pushed the lesion away from the muscle layer. The wiper-knife moved like a windshield wiper, applying a coagulation current on the muscle layer to separate submucosal exfoliation from the muscle layer. This device has not yet been used in human patients.
B-cap: B-cap is a device in which the snare of the cap knife has been replaced with a bipolar knife[40]. The direction for use of the B-knife is the same as the cap knife.
Multi-bending scope: Some tumor locations make it difficult to carry out EMR using a conventional scope. There include the lesser curvature or posterior wall of the gastric body, and the cardia. To facilitate EMR of tumors at these locations, a two-channel scope with two independently curving segments, that is, a Multi-bending scope (the ‘M-scope’) was developed[41]. The M-scope consists of a distal flexible segment that can bend in any of the four major directions and a proximal flexible segment that can bend in two directions. Combined operation of the segments allows the operator to obtain a variety of visual fields, to randomly approach or recede from the lesions, and to obtain an en face view.
Multi-bending double-channel therapeutic endoscope: The multi-bending double-channel therapeutic endoscope (the ‘R-scope’) has been designed for lifting lesions and for improved dissection by the incorporation of two movable channels[42,43]. The R-scope has two movable instrument channels; one moving vertically and the other horizontally. The two instruments can be manipulated during the operation with a knob and a lever that surrounds the angulation control knobs of the R-scope.
Vibration endoscope: The author attempted to increase the efficiency of endoscopic treatment techniques by vibrating the endoscope itself. The vibration used must be at a frequency that ensures safety when applied to the body. Examples of inventions that are made effective by adding safe vibration to the body are vibrating oral care devices developed to clean between the teeth and manual multiple-blade shavers with vibration added to increase cutting efficiency. The latter is a commercial product in which vibration successfully raises cutting efficiency without harming the skin, even though the blades cut whiskers in direct contact with the face (M3 Power; Gillette, Japan; Figure 1). An endoscope with an incorporated eccentric motor was therefore developed and used in conducting ESD. The vibration endoscope comprised a modified commercial endoscope (GIF-Q200; Olympus). First, the covering plastic of the tip and the metal mesh were stripped off. After exposing the interior, a vibration motor (J71; Shicoh, Japan) fitted within a cylinder was attached and this section was covered using heat-shrinkable tubing (Figure 10)[44].
Mean procedure durations for circumferential incisions, submucosal dissection or a combination of both were significantly shorter when vibration at 10 000 rpm was applied, compared to procedures without vibration. When performing peripheral incisions and submucosal dissection with a knife in ESD, the time for the procedure can also be reduced by adding vibration. This device has not yet been used in human patients.
Magnetic anchor system: The magnetic anchor (Pentax, Tokyo, Japan) consists of 3 parts: a hand-made magnetic weight, made of magnetic stainless steel; microforceps; a connecting thread. A weight is designed to facilitate gastric ESD by use of an extracorporeal hands-free electromagnet, whereby magnetic forces allow a suitable counter-traction for submucosal dissection[45].
Percutaneous traction: A small snare is introduced into the gastric lumen through a percutaneous gastric port (2-mm diameter) to grasp and pull the lesion away from the muscularis propria to facilitate resection[46].
External grasping forceps: In ESD using an external grasping forceps, oral traction applied with the external forceps can elevate the lesion and make the submucosal layer on the aboral side wider and more visible, thereby facilitating submucosal dissection under direct vision[47].
Water jet endoscope: By washing the bleeding field with the water jet, the bleeding source can be immediately identified and coagulated, although in a small number of cases of erupting venous bleeding, identifying the bleeding source can be difficult.
Irrigation hood: The author developed an end hood that facilitates endoscopic hemostatic procedures while simultaneously allowing irrigation of the hemorrhage site. The end hood piece was fabricated by drilling a side hole in the cap portion of a conventional transparent hood, then the irrigation tube was glued to the exterior surface of the hole[11,12]. The fabricated transparent hood was placed at the tip of the endoscope. Based on this prototype, the irrigation hood (irrigation cap; Type KUME) was developed as shown in Figure 11 (Create Medic, Yokohama, Japan).
Coagula-irrigation hood (CI hood): The author developed a new type of hood, the “coagula-irrigation hood” (CI hood), which could simultaneously perform both coagulation and irrigation[48]. The CI hood was fabricated by installing a machined papillotomy knife in exchange for an irrigation tube on the irrigation hood (Figure 12)[11,12]. The tip of papillotomy knife was cut off and the tip of a wire was bent into a hoop. A CI hood was fabricated such that ESD and endoscopic hemostasis could be performed while simultaneously applying adequate coagulation and irrigation.
During resection, incision, and detachment using an endoscope, smoke is produced due to electrocautery. Accumulation of this smoke in the gastrointestinal (GI) tract impairs the visual field and makes continuation of the procedure difficult. The author therefore developed two types of new fan device that improve the visual field by circulating air without changing the air volume[49]. Both devices were created using a super-micro fan motor (Shiko, Japan). The first works by blowing air (Figure 13A), while the second uses ventilation (Figure 13B). This device has not yet been used in human patients.
Injection solutions for elevation: Two types of solution are used for submucosal injection: isotonic solution (normal saline, hyaluronic acid); and hypertonic solution (hypertonic saline, glucose, Glycerol®)[36,50-53]. The advantages of hypertonic solution are better mucosal elevation and better hemostatic effect than normal saline. However, hypertonic solution is more likely to damage tissue in a resection sample, post-resection ulcer, or the surrounding mucosa compared with isotonic solution..
Hyaluronic acid solution makes a better long-lasting submucosal cushion than other available solutions without causing tissue damage[36,50].
Injection solution for submucosal dissection: The author reported a new method of ESD by submucosal injection of a jelly, which obviates the need for submucosal incision with a knife[54]. As the jelly is thick and viscous, the mucosal layer can be dissected from the muscular layer when it is injected into the submucosal layer.
Sodium carboxymethylcellulose (SCMC) is a water-soluble polymer derived from cellulose. When dissolved in water, it becomes very viscous, like a jelly. We used SCMC for ESD in porcine stomachs[55]. The mucosal layer was dissected from the muscular layer with submucosal injection of 2.5% SCMC.
A Japanese multicentre collaborative prospective study of endoscopic treatment for early gastric cancer found that if the diagnosis of intramucosal cancer [≤20 mm, UL (-)] from the specimen resected at initial EMR was histologically correct, then local cure could be achieved with EMR, including cases of recurrence, with appropriate follow-up and use of concomitant techniques such as piecemeal resection and coagulation therapy[56].
EMRC and EAM represent simpler and easier refinements of EMR methods[9]. The main advantage of these techniques is that a standard endoscope can be used and only one endoscopy assistant is required to close the snare. However, two potential disadvantages of the EMR technique have been identified[57-59]. First, aspiration of the centre of the lesion into the cap may be difficult to ensure as it is often tethered down by a submucosal desmoplastic reaction. The mucosa which readily moves into the cap on aspiration may represent normal surrounding mucosa, while the centre of the lesion can be more difficult to aspirate. Second, ensuring that the lesion has been completely removed with an adequate clear margin of 1 cm remains the responsibility of the endoscopist. Reconstituting lesions removed piecemeal by this approach may be impossible.
On the other hand, the author reduced the disadvantages of both EMRC and EAM[13-17,21,23]. In fact, a high rate of en bloc resections of lesions < 20 mm in diameter has been achieved with the EMRC and EAM[13-16].
Compared with ESD, EMR is easier to perform and requires less time for treatment. For example, mean procedure time using the author’s device is < 20 min[13-16]. The longer the procedure time, the greater the potential disadvantages for high-risk patients (greater age or poor cardiopulmonary status). Moreover, the feasibility and safety of ESD by less-experienced endoscopists have not been evaluated. Under these conditions, a good alternative method to ESD is needed.
In conclusion, intramucosal cancer less than 20 mm in size and with no ulceration is considered appropriate for EMR.
ESD enables the treatment of even large ulcerative lesions and lesions with scarring, and a high en bloc resection rate (> 90%) has been reported in several Japanese studies[5,28,60-62]. A recent trend has been seen with the selection of ESD as an effective and safe endoscopic treatment method for all GI neoplasms. With accurate pathological evaluation by en bloc resection through ESD, the endoscopist can reach a decision about complete resection or curative resection as a complete therapeutic option for early gastric neoplasms. However, this highly technical procedure needs a high level of expertise and experience to correctly perform the submucosal dissection and to promptly control any procedure-related complications.
ESD procedure time is one important drawback. A long procedure can be followed by unwanted clinical complications related to premedication or heavy stress for patients. To reduce this time, various advances have been made in submucosal injections, knives, other accessories, and well-equipped electrocoagulation systems[24-55]. ESD procedure time is increased in cases with ulceration, scarring, a large lesion, or location in the upper portion of the stomach[63]. The large upper portion of the stomach region has a large vascular network, resulting in technical difficulty in the approach to dissection or control of bleeding, all of which increases the procedure time. Multi-bending endoscopy can be used to minimize technical difficulty[41]. In cases of recurrent lesion or a lesion with an accompanying scar, the endoscopist needs to dissect very carefully, as a thin submucosal cushion and hard fibrotic tissue both make dissection difficult to perform without perforation.
The comparison of outcomes between conventional EMR and ESD would obviously be better evaluated in a prospective randomized trial. Such a study, however, is difficult to conduct outside of Japan, as the incidence of early gastric cancer is extremely low in other parts of the world.
Peer reviewers: Paulina Salminen, MD, PhD, Department of Surgery Turku University Hospital, Kiinamyllynkatu 4-8FI-20520 Turku, Finland; Dimitrios Kapetanos, MD, Gastroenterology Department, George Papanikolaou Hospital, Exohi, Thessaloniki 57010, Greece
S- Editor Zhang HN L- Editor Hughes D E- Editor Ma WH
| 1. | Niwa H. Improvement of fibrogastroscope for biopsy and application of color television and high frequent currents for endoscopic biopsy (in Japanese). Gastroenterol Endosc. 1968;10:31. [Cited in This Article: ] |
| 2. | Tada M, Shimada M, Murakami F, Shimada M, Mizumachi M, Arima T, Yanai H, Oka S, Shigeeda M, Ogino M. Development of the strip-off biopsy (in Japanese with English abstract). Gastroenterol Endosc. 1984;26:833-839. [Cited in This Article: ] |
| 3. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [Cited in This Article: ] |
| 4. | Muto M, Miyamoto S, Hosokawa A, Doi T, Ohtsu A, Yoshida S, Endo Y, Hosokawa K, Saito D, Shim CS. Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy. 2005;37:178-182. [Cited in This Article: ] |
| 5. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [Cited in This Article: ] |
| 6. | Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445-450. [Cited in This Article: ] |
| 7. | Takekoshi T, Baba Y, Ota H, Kato Y, Yanagisawa A, Takagi K, Noguchi Y. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352-358. [Cited in This Article: ] |
| 8. | Karita M, Tada M, Okita K. The successive strip biopsy partial resection technique for large early gastric and colon cancers. Gastrointest Endosc. 1992;38:174-178. [Cited in This Article: ] |
| 9. | Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58-62. [Cited in This Article: ] |
| 10. | Matsuzaki K, Nagao S, Kawaguchi A, Miyazaki J, Yoshida Y, Kitagawa Y, Nakajima H, Kato S, Hokari R, Tsuzuki Y. Newly designed soft prelooped cap for endoscopic mucosal resection of gastric lesions. Gastrointest Endosc. 2003;57:242-246. [Cited in This Article: ] |
| 11. | Kume K, Yoshikawa I, Otsuki M. Endoscopic treatment of upper GI hemorrhage with a novel irrigating hood attached to the endoscope. Gastrointest Endosc. 2003;57:732-735. [Cited in This Article: ] |
| 12. | Kume K, Yamasaki M, Yamasaki T, Yoshikawa I, Otsuki M. Endoscopic hemostatic treatment under irrigation for upper-GI hemorrhage: a comparison of one third and total circumference transparent end hoods. Gastrointest Endosc. 2004;59:712-716. [Cited in This Article: ] |
| 13. | Kume K, Yamasaki M, Kanda K, Yoshikawa I, Otsuki M. Endoscopic procedure under irrigation. Dig Endosc. 2005;17:241-245. [Cited in This Article: ] |
| 14. | Kume K, Yamasaki M, Kubo K, Mitsuoka H, Oto T, Matsuhashi T, Yamasaki T, Yoshikawa I, Otsuki M. EMR of upper GI lesions when using a novel soft, irrigation, prelooped hood. Gastrointest Endosc. 2004;60:124-128. [Cited in This Article: ] |
| 15. | Kume K, Yamasaki M, Kanda K, Hirakoba M, Matsuhashi T, Santo N, Syukuwa K, Yoshikawa I, Otsuki M. Grasping forceps-assisted endoscopic mucosal resection of early gastric cancer with a novel 2-channel prelooped hood. Gastrointest Endosc. 2006;64:108-112. [Cited in This Article: ] |
| 16. | Kume K, Yamasaki M, Tashiro M, Santo N, Syukuwa K, Maekawa S, Aritome G, Matsuoka H, Murase T, Yoshikawa I. Endoscopic mucosal resection for early gastric cancer: comparison of two modifications of the cap method. Endoscopy. 2008;40:280-283. [Cited in This Article: ] |
| 17. | Kume K, Yamasaki M, Yoshikawa I, Otsuki M. Endoscopic aspiration mucosectomy and closure assisted by outside CCD camera. Endoscopy. 2007;39 Suppl 1:E214-E215. [Cited in This Article: ] |
| 18. | Katayama O, Honda H, Koike T, Uchida Y, Takahata T, Matsumoto T. Usefulness of oblique hood-fitted panendoscope: mucosal cut and aspiration method (in Japanese with English abstract). Endosc Digest. 2006;18: 1125-1130. [Cited in This Article: ] |
| 19. | Torii A, Sakai M, Kajiyama T, Kishimoto H, Kin G, Inoue K, Koizumi T, Ueda S, Okuma M. Endoscopic aspiration mucosectomy as curative endoscopic surgery; analysis of 24 cases of early gastric cancer. Gastrointest Endosc. 1995;42:475-479. [Cited in This Article: ] |
| 20. | Torii A. EAM (endoscopic aspiration mucosectomy) (in Japanese with English abstract). Gastroenterol Endosc. 2006;48:2528-2537. [Cited in This Article: ] |
| 21. | Kume K. Endoscopic aspiration mucosectomy using a novel vibration hood. Endoscopy. 2009;48:In press. [Cited in This Article: ] |
| 22. | Suzuki Y, Hiraishi H, Kanke K, Watanabe H, Ueno N, Ishida M, Masuyama H, Terano A. Treatment of gastric tumors by endoscopic mucosal resection with a ligating device. Gastrointest Endosc. 1999;49:192-199. [Cited in This Article: ] |
| 23. | Kume K, Yamasaki M, Yoshikawa I, Otsuki M. Multi-camera system of the endoscopy: endoscopic mucosal resection for large gastric lesion using a novel 1-channel camera-hood. Endoscopy. 2007;39 Suppl 1:E186-E187. [Cited in This Article: ] |
| 24. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [Cited in This Article: ] |
| 25. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [Cited in This Article: ] |
| 26. | Miyamoto S, Muto M, Hamamoto Y, Boku N, Ohtsu A, Baba S, Yoshida M, Ohkuwa M, Hosokawa K, Tajiri H. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 2002;55:576-581. [Cited in This Article: ] |
| 27. | Rosch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy. 2004;36:788-801. [Cited in This Article: ] |
| 28. | Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71-S73. [Cited in This Article: ] |
| 29. | Oyama T, Kikuchi Y. Aggressive endoscopic mucosal resection in the upper GI tract-hook knife EMR method. Minim Invasive Ther Allied Technol. 2002;11:291-295. [Cited in This Article: ] |
| 30. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [Cited in This Article: ] |
| 31. | Yahagi N, Fujishiro M, Kakushima N, Kobayashi K, Hashimoto T, Oka M, Iguchi M, Enomoto S, Ichinose M, Niwa H. Endoscopic submucosal dissection for early gastric cancer using the tip of an electro-surgical snare (thin type). Dig Endosc. 2004;16:34-38. [Cited in This Article: ] |
| 32. | Toyonaga T, Nishino E, Dozaiku T, Ueda C, Hirooka T. Management to prevent bleeding during endoscopic submucosal dissection using the flush knife for gastric tumors. Dig Endosc. 2007;19:S14-S18. [Cited in This Article: ] |
| 33. | Inoue H, Sato Y, Kazawa T, Sugaya S, Usui S, Satodate H, Kudo S. Endoscopic submucosal dissection-using a triangle-tipped knife (in Japanese). Sto Int. 2004;39: 53-56. [Cited in This Article: ] |
| 34. | Kim HG, Cho JY, Bok GH, Cho WY, Kim WJ, Hong SJ, Ko BM, Kim JO, Lee JS, Lee MS. A novel device for endoscopic submucosal dissection, the Fork knife. World J Gastroenterol. 2008;14:6726-6732. [Cited in This Article: ] |
| 35. | Kawahara Y, Takenaka R, Okada H. Risk management to prevent perforation during endoscopic submucosal dissection. Dig Endosc. 2007;19:S9-S13. [Cited in This Article: ] |
| 36. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [Cited in This Article: ] |
| 37. | Kume K, Yamasaki M, Kanda K, Yoshikawa I, Otsuki M. Endoscopic submucosal dissection using a novel irrigation hood-knife. Endoscopy. 2005;37:1030-1031. [Cited in This Article: ] |
| 38. | Kume K, Yamasaki M, Yoshikawa I, Otsuki M. Grasping-forceps-assisted endoscopic submucosal dissection using a novel irrigation cap-knife for large superficial early gastric cancer. Endoscopy. 2007;39:566-569. [Cited in This Article: ] |
| 39. | Kume K, Yamasaki M, Kanda K, Yoshikawa I, Otsuki M. Endoscopic submucosal dissection using a novel irrigation wiper-knife. Endoscopy. 2007;39 Suppl 1:E144. [Cited in This Article: ] |
| 40. | Miyamoto S, Aoi T, Morita S, Nitta T, Nishio A, Chiba T. Endoscopic submucosal dissection using the B-Cap (in Japanese). Clin Gastroroenterol. 2007;22:1263-1265. [Cited in This Article: ] |
| 41. | Isshi K, Tajiri H, Fujisaki J, Mochizuki K, Matsuda K, Nakamura Y, Saito N, Narimiya N. The effectiveness of a new multibending scope for endoscopic mucosal resection. Endoscopy. 2004;36:294-297. [Cited in This Article: ] |
| 42. | Yonezawa J, Kaise M, Sumiyama K, Goda K, Arakawa H, Tajiri H. A novel double-channel therapeutic endoscope ("R-scope") facilitates endoscopic submucosal dissection of superficial gastric neoplasms. Endoscopy. 2006;38:1011-1015. [Cited in This Article: ] |
| 43. | Neuhaus H, Costamagna G, Deviere J, Fockens P, Ponchon T, Rosch T. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the "R-scope"). Endoscopy. 2006;38:1016-1023. [Cited in This Article: ] |
| 44. | Kume K. Endoscopic submucosal dissection using a novel vibration endoscopy. Hepato Gastroenterol. 2009;38:In press. [Cited in This Article: ] |
| 45. | Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos). Gastrointest Endosc. 2009;69:10-15. [Cited in This Article: ] |
| 46. | Kondo H, Gotoda T, Ono H, Oda I, Kozu T, Fujishiro M, Saito D, Yoshida S. Percutaneous traction-assisted EMR by using an insulation-tipped electrosurgical knife for early stage gastric cancer. Gastrointest Endosc. 2004;59:284-288. [Cited in This Article: ] |
| 47. | Imaeda H, Iwao Y, Ogata H, Ichikawa H, Mori M, Hosoe N, Masaoka T, Nakashita M, Suzuki H, Inoue N. A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. Endoscopy. 2006;38:1007-1010. [Cited in This Article: ] |
| 48. | Kume K, Yamasaki M, Yoshikawa I, Otsuki M. New device to perform coagulation and irrigation simultaneously during endoscopic submucosal dissection using an insulation-tipped electrosurgical knife. Dig Endosc. 2006;18: 218-220. [Cited in This Article: ] |
| 49. | Kume K. Endoscopic therapy using novel fan devices. Endoscopy. 2009;41 Suppl 2:E236-E237. [Cited in This Article: ] |
| 50. | Yamamoto H, Yube T, Isoda N, Sato Y, Sekine Y, Higashizawa T, Ido K, Kimura K, Kanai N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251-256. [Cited in This Article: ] |
| 51. | Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243-249. [Cited in This Article: ] |
| 52. | Uraoka T, Fujii T, Saito Y, Sumiyoshi T, Emura F, Bhandari P, Matsuda T, Fu KI, Saito D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736-740. [Cited in This Article: ] |
| 53. | Akahoshi K, Yoshinaga S, Fujimaru T, Kondoh A, Higuchi N, Furuno T, Oya M. Endoscopic resection with hypertonic saline-solution-epinephrine injection plus band ligation for large pedunculated or semipedunculated gastric polyp. Gastrointest Endosc. 2006;63:312-316. [Cited in This Article: ] |
| 54. | Yamasaki M, Kume K, Kanda K, Yoshikawa I, Otsuki M. A new method of endoscopic submucosal dissection using submucosal injection of jelly. Endoscopy. 2005;37:1156-1157. [Cited in This Article: ] |
| 55. | Yamasaki M, Kume K, Yoshikawa I, Otsuki M. A novel method of endoscopic submucosal dissection with blunt abrasion by submucosal injection of sodium carboxymethylcellulose: an animal preliminary study. Gastrointest Endosc. 2006;64:958-965. [Cited in This Article: ] |
| 56. | Ida K, Nakazawa S, Yoshino J, Hiki Y, Akamatsu T, Asaki S, Kurihara H, Shimao H, Tada M, Misumi A. Multicentre collaborative prospective study of endoscopic treatment of early gastric cancer. Dig Endosc. 2004;16:295-302. [Cited in This Article: ] |
| 57. | Rembacken BJ, Gotoda T, Fujii T, Axon AT. Endoscopic mucosal resection. Endoscopy. 2001;33:709-718. [Cited in This Article: ] |
| 58. | Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567-579. [Cited in This Article: ] |
| 59. | Tani M, Sakai P, Kondo H. Endoscopic mucosal resection of superficial cancer in the stomach using the cap technique. Endoscopy. 2003;35:348-355. [Cited in This Article: ] |
| 60. | Oda I, Goyoda T, Hamanaka H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58. [Cited in This Article: ] |
| 61. | Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980-986. [Cited in This Article: ] |
| 62. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. [Cited in This Article: ] |
| 63. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [Cited in This Article: ] |