Published online Aug 16, 2020. doi: 10.4253/wjge.v12.i8.231
Peer-review started: April 2, 2020
First decision: April 22, 2020
Revised: May 9, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: August 16, 2020
Pancreatic cancer (PC) mortality remains high despite advances in therapy. Combination chemoradiotherapy offers modest survival benefit over monotherapy with either. Fiducial markers serve as needed landmarks for image-guided radiotherapy (IGRT). Traditionally, these markers were placed surgically or percutaneously with limitations of each. Endoscopic ultrasound-guided placement overcomes these limitations.
To evaluate the safety, efficacy, and feasibility of endoscopic ultrasound (EUS)-guided fiducial placement for PC undergoing IGRT.
Articles were searched in MEDLINE, PubMed, and Ovid journals. Pooling was conducted by fixed and random effects models. Heterogeneity was assessed using Cochran’s Q test based upon inverse variance weights.
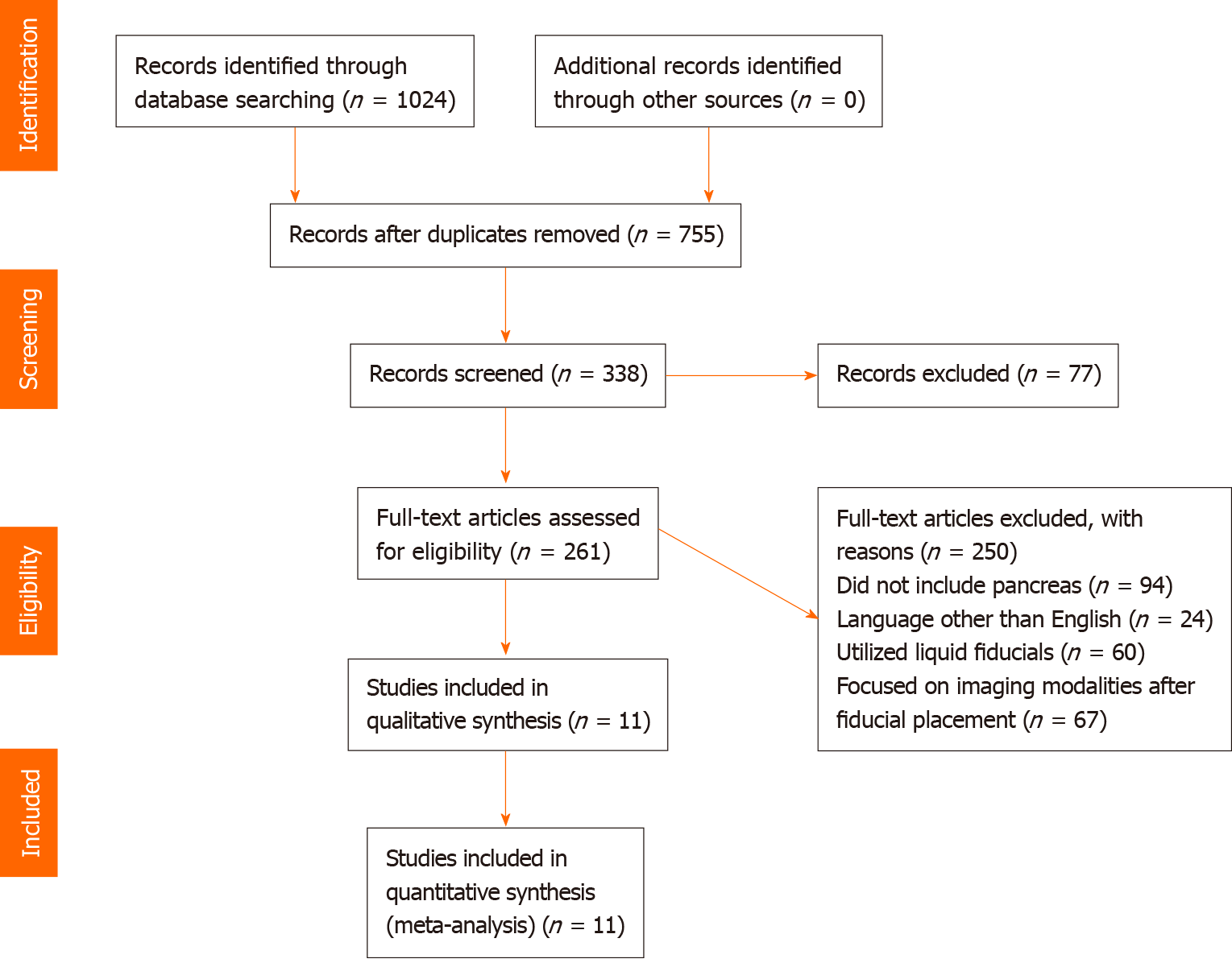
Initial search identified 1024 reference articles for EUS-guided fiducial placement in PC. Of these, 261 relevant articles were reviewed. Data was extracted from 11 studies (n = 820) meeting inclusion criteria. Pooled proportion of successful placement was 96.27% (95%CI: 95.35-97.81) with fiducial migration rates low at 4.33% (95%CI: 2.45-6.71). Adverse event rates remained low, with overall pooled proportion of 4.85% (95%CI: 3.04-7.03).
EUS-guided placement of fiducial markers for IGRT of PC is safe, feasible, and efficacious. The ability to target deep structures under direct visualization while remaining minimally invasive are added benefits. Moreover, the ability to perform fine needle aspiration or celiac plexus neurolysis add value and increase patient-care efficiency. Whether EUS-guided fiducial placement improves outcomes in IGRT or offers any mortality benefits over traditional placement remains unknown and future studies are needed.
Core tip: Historically, fiducial marker placement for pancreatic cancer has been performed surgically or percutaneously. The former is invasive and the latter is limited to superficial structures and lesions. Endoscopic ultrasound-guided fiducial placement for pancreatic cancer is a safe, efficacious, and feasible modality. It offers a minimally invasive approach that can target deep structures and lesions, and results in more efficient care delivery via the ability to perform additional procedures at the time of marker placement.
- Citation: Patel JB, Revanur V, Forcione DG, Bechtold ML, Puli SR. Endoscopic ultrasound-guided fiducial marker placement in pancreatic cancer: A systematic review and meta-analysis. World J Gastrointest Endosc 2020; 12(8): 231-240
- URL: https://www.wjgnet.com/1948-5190/full/v12/i8/231.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i8.231
Pancreatic cancer (PC) is the fourth most common cause of cancer-related mortality among both genders in the United States, with pancreatic adenocarcinoma comprising the bulk. Of the nearly 57000 patients diagnosed annually, the majority will succumb to their disease[1]. The poor prognosis of PC is attributed to its usually advanced stage at presentation, as well as local recurrence within 2 years in operable cases. Median survival among those undergoing surgical resection is 13 to 15 mo, and overall 5-year survival rates vary, but typically range from 3% to 25%[2-6]. Depending upon the extent and location of disease, treatment options include surgical resection, chemotherapy, and radiation therapy. Chemotherapy and radiation therapy have been shown to improve both survival and quality of life in patients with advanced stages of disease, with combination therapy offering a modest improvement in survival over monotherapy[7-11].
Image-guided radiotherapy (IGRT) allows for targeted application of radiation therapy using real-time imaging for precise delivery to affected tissue resulting in improved tumor control while sparing surrounding tissue[12,13]. Stereotactic body radiotherapy is a form of IGRT in which multiple beams of radiation therapy can safely and effectively target a precise location, enabling high-dose radiation to a selective location while minimizing radiation where unnecessary[14-16]. Given the soft tissue nature of the pancreas without reliable landmarks, the use of inert and implantable markers known as fiducials have served as landmarks allowing for tumor-tracking when placed in or near the tissue of interest. Placement of fiducials was previously limited to percutaneous placement by interventional radiology or operative placement by surgery[17,18]. However, deep placement of fiducials percutaneously by interventional radiology may be limited by intervening structures, and operative placement by surgery is invasive, making endoscopic ultrasound-guided fiducial placement an ideal potential modality. EUS-guided fiducial placement permits targeting of deep structures and remains minimally invasive thereby reducing risk of complications. Additionally, Doppler imaging during EUS reduces the risk of vascular penetration, and placement can be performed in close proximity under direct visualization. EUS-guided fiducial placement also offers the ability to perform other procedures during the same session. Patients presenting with imaging features suggestive of pancreatic malignancy can undergo fine needle aspiration (FNA) of the suspicious tissue for preliminary assessment or confirmation, followed by placement of fiducials thereby decreasing the interval between diagnosis and treatment[19,20]. Furthermore, patients have tolerated same-session FNA, celiac plexus block to achieve pain control, as well as fiducial placement[21].
Despite the relative safety of EUS-guided fiducial placement, minor potential complications are noted. A few studies have indicated a low rate of minor bleeding, abdominal pain, acute pancreatitis, elevated liver chemistries, and cholangitis[22-25].
We aim to evaluate the feasibility, safety, and efficacy of EUS-guided fiducial placement for IGRT for PC.
We solely included studies involving EUS-guided fiducial placement for intended IGRT for PC. We excluded abstracts without full text, studies involving purely extra-pancreatic fiducial marker placement, studies in languages other than English, and studies involving liquid fiducial markers.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement[26] was utilized as a guide to our study design. As this study was a systematic review and meta-analysis, ethical approval was unnecessary. Databases searched included MEDLINE (through PubMed, an electronic search engine for published articles and Ovid), Medline non-indexed citations, old Medline, PubMed, Ovid journals, OVID Healthstar, American College of Physicians journal club, Google Scholar, Database of abstracts of Reviews of effectiveness, Cumulative Index for Nursing & Allied Health Literature, International Pharmaceutical Abstracts, and Cochrane Central Register of Controlled Trials. Our search included articles with parameters from January 1, 2000 to December 31, 2019. Terms used for search were Endoscopic ultrasound, PC, fiducial marker, image-guided radiation, stereotactic body radiation therapy. If there was unascertainable data from reviewed publications, corresponding study authors were contacted. Three authors (Patel JP, Puli SP, Revanur R) independently extracted the data into an abstraction form. Any divergences were resolved by mutual agreement. Cohen’s κ[27] was used to quantify the agreement between the reviewers for the data.
Various criteria have been employed to assess the quality of a study with control and treatment arms (e.g., randomization, concealment of allocation, selection bias in the arms of the study, and blinding of outcome)[28,29]. There is no consensus on assessment of studies without a control arm. These criteria, therefore, do not apply to studies without a control arm[29]. Consequently, studies for this meta-analysis and systematic review were selected based on completeness of data and inclusion criteria. Completeness was defined as availability of data for pooled proportions with 95% confidence intervals.
Meta-analysis for the assessment and outcomes of EUS-guided fiducial marker placement in PC for anticipated IGRT was performed by calculating pooled estimates. Pooling was performed utilizing the Mantel-Haenszel method (fixed effects model) and DerSimonian Laird method (random effects model). Confidence intervals (CIs) were computed using the F distribution method[30]. Forrest plots were constructed to demonstrate the point estimates in each study, with respect to the summary pooled estimate. The width of the point estimates in the Forrest plots corresponded the assigned weight for that study. For any 0 values, 0.5 was added as described by Cox[31]. Based upon inverse variance weights, the heterogeneity of likelihood and diagnostic odds ratios were assessed utilizing Cochran’s Q test[32]. The Egger[33] and Begg-Mazumdar[34] bias indicators were utilized to evaluate the effect of publication and selection bias of the summary estimates. Funnel plots were generated for assessment of interobserver variability utilizing the standard error and diagnostic odds ratio[35,36].
Our initial search resulted in 1024 reference articles for endoscopic ultrasound-guided fiducial marker placement in PC for image-guided radiation therapy. Two hundred sixty one of these articles were reviewed, of which, 11 studies met inclusion criteria (Table 1) and underwent data extraction (n = 820). Of the 11 studies, nine included demographic information, with 524 males and 283 females with a mean age of 65.66 (SD: 4.15) years. A mean of 2.97 (SD: 1.06) fiducials were placed. The mean fiducial length and diameter were 6.55 (SD: 3.22) mm and 8.43 (SD: 0.24) mm. Pancreatic head and neck lesions were most frequently encountered (n = 157), followed by body and tail lesions (n = 76), and lastly uncinate process lesions (n = 14). The mean tumor size undergoing fiducial replacement was 34.6 (SD: 5.53) mm. Included studies were published as full texts. Our search results and methodology are outlined in the flow diagram labeled Figure 1.
| Ref. | n | Mean age | No. male | No. female | Type of study |
| Pishvaian et al[22], 2006 | 13 | 67.62 | 8 | 5 | Prospective |
| Sanders et al[23], 2010 | 51 | 73 | 29 | 22 | Prospective |
| DiMaio et al[24], 2010 | 30 | 63.2 | 19 | 11 | Retrospective |
| Park et al[25], 2010 | 57 | 67 | 29 | 28 | Prospective |
| Varadarajulu et al[21], 2010 | 9 | 57.67 | 4 | 5 | Prospective |
| Khashab et al[19], 2012 | 39 | 66.5 | 25 | 14 | Retrospective |
| Fajardo et al[38], 2013 | 23 | 63.13 | 13 | 10 | Prospective |
| Majumder et al[39], 2013 | 39 | 66.7 | 18 | 21 | Retrospective |
| Choi et al[20], 2014 | 32 | 66 | 21 | 11 | Prospective |
| Dhadham et al[40], 2016 | 188 | Retrospective |
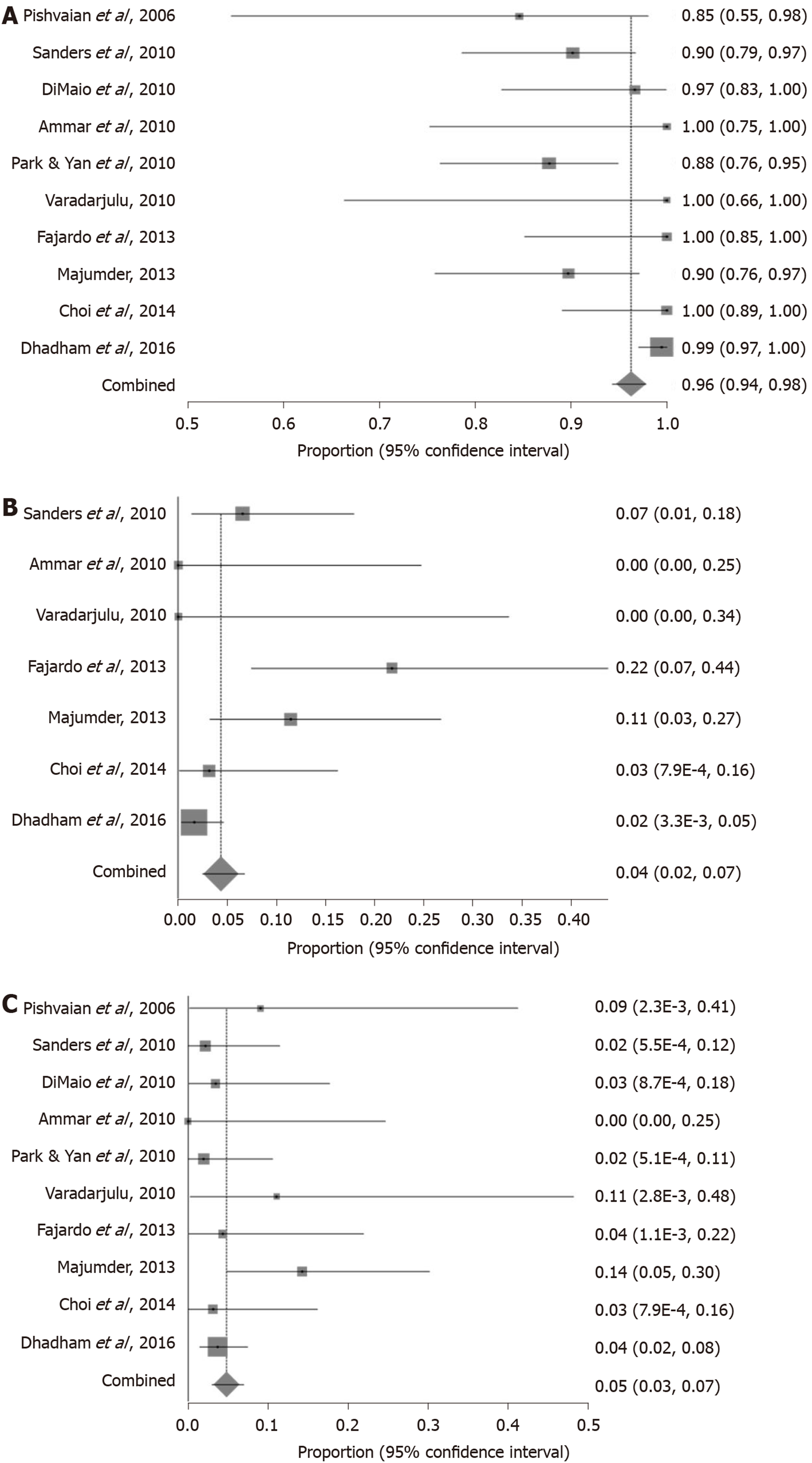
Successful fiducial marker placement under EUS-guidance gave a pooled proportion of 96.27% (95%CI: 95.35-97.81) as shown in Figure 2A. Begg-Mazumdar bias indicator gave a Kendall's tau = -0.42 (P = 0.07), and Egger bias gave a value of -1.05 [95%CI: -2.07-(-0.02), P = 0.05].
Pooled proportion of fiducial marker migration was 4.33% (95%CI: 2.45-6.71) as shown in Figure 2B. Begg-Mazumdar bias indicator gave a Kendall's tau = 0.43 (P = 0.24), and Egger bias gave a value of 1.01 (95%CI: -3.85-2.41, P = 0.12).
Pooled proportion of adverse events was 4.85% (95%CI: 3.04-7.03) as demonstrated in Figure 2C. Begg-Mazumdar bias indicator gave a Kendall's tau = 0.47 (P = 0.07), and Egger bias gave a value of 0.49 (95%CI: -0.42-1.39, P = 0.25).
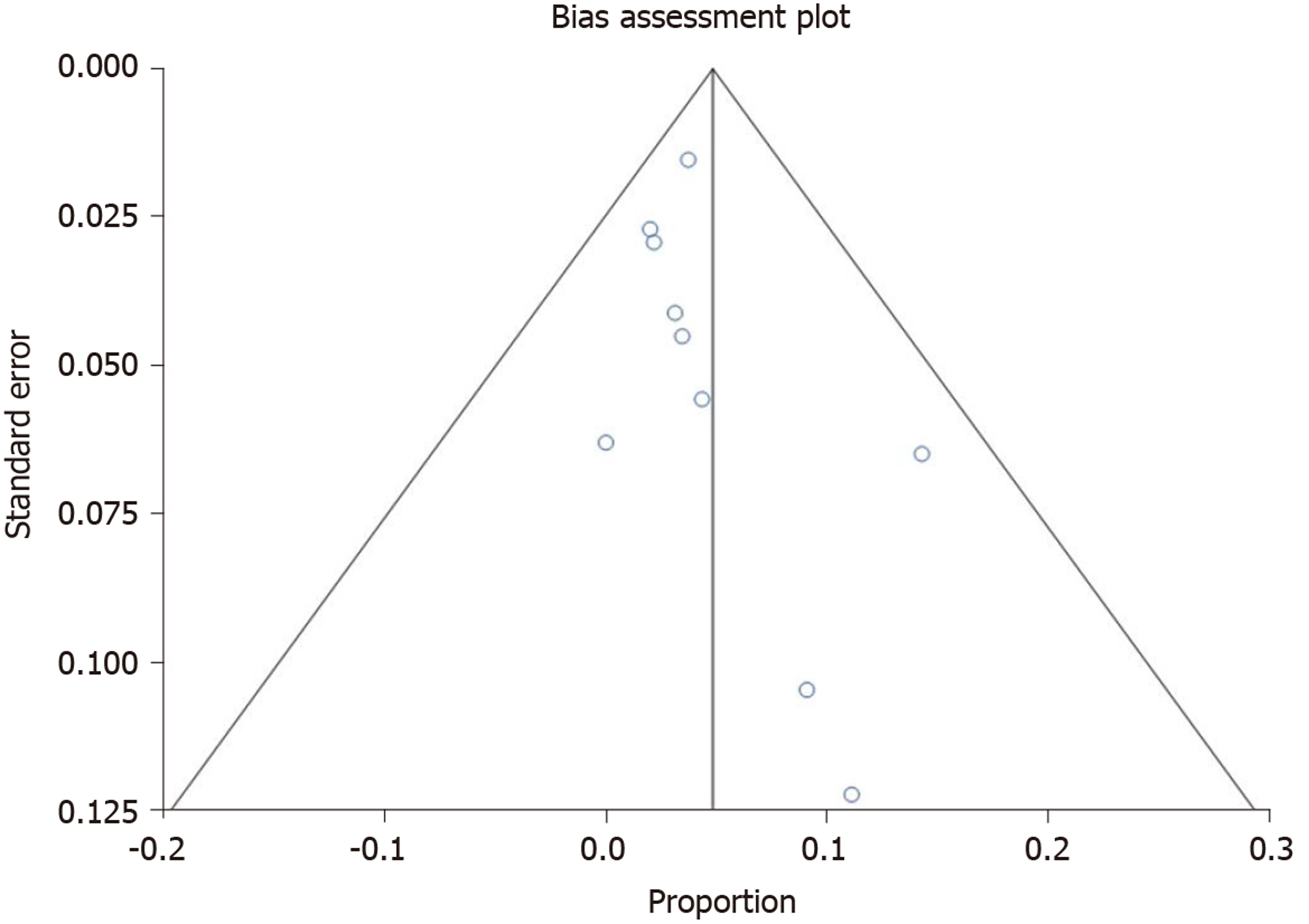
Figure 3 demonstrates funnel plot showing no significant publication bias. All pooled estimates calculated by fixed and random effects models yielded similar results. The change adjusted agreement analysis for data collected separately between reviewers gave a kappa value of 1.0.
EUS-guided fiducial placement offers benefit over surgical or percutaneous placement due to ability to access deep structures and a variety of tissues, provide real-time high-resolution visualization near tissue of interest, and potentially decrease risk of peritoneal seeding[37]. Although various factors can preclude successful placement, technical success rates are noted to range from 85% to 100%, with our meta-analysis revealing a pooled success rate of 96.2%. Novelty and lack of experience are two such factors that can impede successful placement. Park et al[25] noted an initial learning curve as a barrier as all technical failures occurred in their first 12 cases, with no further failures in their subsequent 45 patients. Pishvaian et al[22] and Sanders et al[23] noted a limitation to be a history of pancreaticoduodenectomy resulting in inability to visualize tumor within the surgical bed. Additional challenges include transduodenal placement and fiducial delivery via 19-gauage needle in pancreatic head and uncinate process lesions but may be able to be overcome with a 22-gauge needle as it produces less rigidity and therefore results in ability to obtain more optimal positioning[20,24,38].
Previously, fiducial placement was presumed to require specific placement and orientation with respect to the tissue of interest known as ideal fiducial geometry (IFG). Majumder et al[39] evaluated success rate of endoscopically placed versus surgically placed fiducials with respect to attaining IFG as well as whether IFG was necessary to successfully undergo IGRT. They noted that surgical placement resulted in higher rates of attaining IFG, however, fiducial tracking success rates were higher in the EUS-guided group over the surgically placed group. This study further concluded that attaining IFG during fiducial placement was unnecessary for successful delivery of radiation and tracking. Visibility of fiducials appears greater for traditional fiducials as compared to Visicoil fiducials[19].
Fiducial migration can impede IGRT due to imprecise targeting or nonvisualization. Our meta-analysis shows a low rate of migration of 4.3%. Factors associated with migration include prior use of neoadjuvant chemotherapy resulting in tissue changes such as regression, as well post-procedural migration from post-procedure inflammation or movement within the tumor. Additionally, fiducial marker placement itself may introduce air bubbles into the target lesion at the time of insertion obscuring visualization and resulting in difficulty confirming successful placement. To overcome this, withdrawal of the stylet approximately seven to eight millimeters while backloading the fiducial and sealing in place with bone wax appears to prevent introduction of air bubbles[20].
Adverse event rates were low with our meta-analysis demonstrating a rate of 4.8%. The most frequently encountered adverse event was mild procedural bleeding, and none required hospitalization or transfusion as a result. Mild pancreatitis was the next most commonly encountered adverse event, and all were treated with supportive care including fluid resuscitation, pain control, and pancreatic rest with subsequent discharge home within 48 h. As previously noted, and advantage of EUS-guided fiducial marker placement included the ability to perform multiple procedures under one session, though this theoretically may increase the likelihood of procedure related complications[20].
All studies included in our meta-analysis were of prospective or retrospective case series’. Per our search, there were no identified randomized controlled trials on the subject. Consistency among studies was noted as they each consisted of initial fiducial marker placement and inherently had follow up for evaluation of adverse events when patients presented for follow up imaging for confirmation of successful placement. Telephone follow ups were also included. Additionally, nearly all studies provided characteristics of fiducial markers and needles used for placement, approach undertaken for placement, mean number of fiducial markers placed, tumor location, success and adverse event rates, as baseline patient characteristics. They demonstrated significant congruency with respect to their study designs, methods, and outcomes.
Our meta-analysis has a few limitations that are noteworthy. Different types of fiducials of variable diameters and lengths were used in the studies included which may impact visualization of fiducials for successful IGRT as well as affect migration rates. For the purposes of our study, we assumed no differences amongst the different fiducials exist. One study[40] had a substantially larger sample size which can skew results. Additionally, retrospective studies were included in this meta-analysis which may result in selection bias. Furthermore, given the specific intent of our meta-analysis, there is some paucity with respect to subject volume as the total number of patients included in our study was 820. Lastly, there was variability amongst studies regarding inter-fiducial distance and tumor size which can affect successful placement, migration, and visibility.
Studies with statistically significant results are generally published and cited. Smaller studies may demonstrate larger treatment effects due to fewer differences than larger studies. This publication and selection bias may affect the summary estimates. This bias can be estimated by Egger bias indicators and Funnel plot construction. In this meta-analysis, both Egger bias and Begg-Mazumdar bias indicators were utilized and no statistically significant bias was shown. Additionally, no significant publication bias was demonstrated using Funnel plots.
In conclusion, survival rates for PC are abysmal and therapies that may help prolong survival are needed. IGRT offers a modest survival benefit over chemo or radiation therapy alone and is facilitated by fiducial markers allowing precise delivery of high dose radiation therapy. Our meta-analysis demonstrated that fiducial maker placement under EUS-guidance is safe, efficacious with lofty technical success rates, and associated with a low rate of adverse events. In addition, EUS-guided fiducial marker placement may offer higher rates of successful tumor tracking than surgically placed markers. The ability to obtain tissue for definitive diagnosis of PC and perform plexus block for pain control in the same session are added benefits not seen with other modes of fiducial marker delivery. Given the advantageous nature and favorable safety profile of EUS-guided fiducial marker placement, consideration should be given to this method of fiducial marker delivery for patients with PC who would benefit from radiotherapy as it may hasten diagnosis and improve quality of life. Further studies evaluating for improved outcomes in IGRT or for improved mortality rates are needed.
Fiducial marker placement for pancreatic cancer (PC) has demonstrated utility as a landmark to target radiotherapy with or without chemotherapy. Historically, these have been placed surgically or percutaneously, each with their own limitations. More recently, endoscopic ultrasound (EUS) guided placement has been undertaken.
PC remains a leading cause of cancer related mortality owing to its advanced stage at time of symptom development and subsequent inability to undergo surgery for definitive treatment. EUS has conferred diagnostic and therapeutic benefits with respect to tissue sampling and celiac plexus block. Given the inability to target deep structures with percutaneous fiducial marker placement and invasive nature of surgical fiducial marker placement, EUS has emerged as a potential marker placement modality that can overcome the aforementioned challenges.
We sought to evaluate the safety, efficacy, and feasibility of EUS-guided fiducial marker placement for PC patients anticipated to undergo radiotherapy via meta-analysis of available case series as no randomized clinical trials exist. The derived data has the potential to alter the clinical course of patients.
Articles were searched in Medline, PubMed, and Ovid journals and ultimately, 11 studies met inclusion criteria and underwent data extraction (n = 820). Data extracted from included studies then underwent analysis by performing pooled estimates by Mantel-Haenszel (fixed effects model) and DerSimonian Laird method (random effects model). Confidence intervals (CIs) were computed using the F distribution method. Forrest plots were constructed to demonstrate the point estimates in each study, with respect to the summary pooled estimate. Heterogeneity was assessed using Cochran’s Q test based upon inverse variance weights. The Egger and Begg-Mazumdar bias indicators were used to assess for publication and selection bias, and funnel plots were generated for assessment of interobserver variability.
Of the meta-analysis of 820 patients who underwent fiducial marker placement under EUS guidance, technical success of fiducial marker placement pooled proportion was 96.27% (95%CI: 95.35-97.81). EUS-guided placement was well tolerated with adverse event pooled proportion 4.85% (95%CI: 3.04-7.03). Given the need for the markers to serve as stationary landmarks to facilitate image-guided radiation therapy, post-procedural migration of fiducials is of significance. Pooled proportion of fiducial marker migration was 4.33% (95%CI: 2.45-6.71).
Our meta-analysis demonstrated high technical success rates of EUS-guided fiducial placement, low rates of complete fiducial marker migration, and low adverse event rates demonstrating its utility as a fiducial marker placement modality. Further studies evaluating for improved outcomes in image-guided radiotherapy or improved modality are needed.
EUS-guided fiducial placement is demonstrated to be a safe, efficacious, and feasible modality of marker placement. In addition, the ability to perform concomitant diagnostic procedures, such as fine needle biopsy, as well as therapeutic procedures, such as celiac plexus block, may hasten treatment and improve quality of life.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H S-Editor: Ma YJ L-Editor: A P-Editor: Liu JH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13300] [Cited by in F6Publishing: 14452] [Article Influence: 2890.4] [Reference Citation Analysis (2)] |
| 2. | Stephens J, Kuhn J, O'Brien J, Preskitt J, Derrick H, Fisher T, Fuller R, Lieberman Z. Surgical morbidity, mortality, and long-term survival in patients with peripancreatic cancer following pancreaticoduodenectomy. Am J Surg. 1997;174:600-3; discussion 603-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Varadarajulu S, Eloubeidi MA. The role of endoscopic ultrasonography in the evaluation of pancreatico-biliary cancer. Gastrointest Endosc Clin N Am. 2005;15:497-511, viii-viix. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 453] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 5. | Mosca F, Giulianotti PC, Balestracci T, Di Candio G, Pietrabissa A, Sbrana F, Rossi G. Long-term survival in pancreatic cancer: pylorus-preserving versus Whipple pancreatoduodenectomy. Surgery. 1997;122:553-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Allema JH, Reinders ME, van Gulik TM, Koelemay MJ, Van Leeuwen DJ, de Wit LT, Gouma DJ, Obertop H. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer. 1995;75:2069-2076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 7. | Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database Syst Rev. 2006;3:CD002093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Willett CG, Czito BG, Bendell JC, Ryan DP. Locally advanced pancreatic cancer. J Clin Oncol. 2005;23:4538-4544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Moertel CG, Frytak S, Hahn RG, O'Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, Lavin PT, Livstone E, Spiro H, Knowlton A, Kalser M, Barkin J, Lessner H, Mann-Kaplan R, Ramming K, Douglas HO, Thomas P, Nave H, Bateman J, Lokich J, Brooks J, Chaffey J, Corson JM, Zamcheck N, Novak JW. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705-1710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 10. | Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 533] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Miller RC, Iott MJ, Corsini MM. Review of adjuvant radiochemotherapy for resected pancreatic cancer and results from Mayo Clinic for the 5th JUCTS symposium. Int J Radiat Oncol Biol Phys. 2009;75:364-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, Ford J, Poen J, Gibbs IC, Mehta VK, Kee S, Trueblood W, Yang G, Bastidas JA. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 13. | Koong AC, Christofferson E, Le QT, Goodman KA, Ho A, Kuo T, Ford JM, Fisher GA, Greco R, Norton J, Yang GP. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 305] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 15. | Milano MT, Chmura SJ, Garofalo MC, Rash C, Roeske JC, Connell PP, Kwon OH, Jani AB, Heimann R. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Schellenberg D, Goodman KA, Lee F, Chang S, Kuo T, Ford JM, Fisher GA, Quon A, Desser TS, Norton J, Greco R, Yang GP, Koong AC. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 17. | Kothary N, Dieterich S, Louie JD, Chang DT, Hofmann LV, Sze DY. Percutaneous implantation of fiducial markers for imaging-guided radiation therapy. AJR Am J Roentgenol. 2009;192:1090-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Kothary N, Heit JJ, Louie JD, Kuo WT, Loo BW, Koong A, Chang DT, Hovsepian D, Sze DY, Hofmann LV. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Interv Radiol. 2009;20:235-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Khashab MA, Kim KJ, Tryggestad EJ, Wild AT, Roland T, Singh VK, Lennon AM, Shin EJ, Ziegler MA, Sharaiha RZ, Canto MI, Herman JM. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc. 2012;76:962-971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Choi JH, Seo DW, Park DH, Lee SK, Kim MH. Fiducial placement for stereotactic body radiation therapy under only endoscopic ultrasonography guidance in pancreatic and hepatic malignancy: practical feasibility and safety. Gut Liver. 2014;8:88-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Varadarajulu S, Trevino JM, Shen S, Jacob R. The use of endoscopic ultrasound-guided gold markers in image-guided radiation therapy of pancreatic cancers: a case series. Endoscopy. 2010;42:423-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, Burton S, McGrath K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | DiMaio CJ, Nagula S, Goodman KA, Ho AY, Markowitz AJ, Schattner MA, Gerdes H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos). Gastrointest Endosc. 2010;71:1204-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, Patalano C, Van Dam J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15040] [Cited by in F6Publishing: 13774] [Article Influence: 1530.4] [Reference Citation Analysis (1)] |
| 27. | Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ. 1992;304:1491-1494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 679] [Cited by in F6Publishing: 712] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 28. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12275] [Cited by in F6Publishing: 12446] [Article Influence: 444.5] [Reference Citation Analysis (0)] |
| 29. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14425] [Cited by in F6Publishing: 15643] [Article Influence: 651.8] [Reference Citation Analysis (0)] |
| 30. | Leemis LM, Trivedi KS. A comparison of approximate interval estimators for the Bernoulli parameter. Am Stat. 1996;50:63-68. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Cox DR, Snell EJ. Analysis of binary data. Monogr Stat Applied Probability. 1989;32. [Cited in This Article: ] |
| 32. | Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323:157-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 822] [Cited by in F6Publishing: 864] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 33. | Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443-3457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1463] [Cited by in F6Publishing: 1592] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 34. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10586] [Cited by in F6Publishing: 11210] [Article Influence: 386.6] [Reference Citation Analysis (0)] |
| 35. | Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1504] [Cited by in F6Publishing: 1445] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 36. | Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2183] [Cited by in F6Publishing: 2305] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 37. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 326] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 38. | Dávila Fajardo R, Lekkerkerker SJ, van der Horst A, Lens E, Bergman JJ, Fockens P, Bel A, van Hooft JE. EUS-guided fiducial markers placement with a 22-gauge needle for image-guided radiation therapy in pancreatic cancer. Gastrointest Endosc. 2014;79:851-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Majumder S, Berzin TM, Mahadevan A, Pawa R, Ellsmere J, Sepe PS, Larosa SA, Pleskow DK, Chuttani R, Sawhney MS. Endoscopic ultrasound-guided pancreatic fiducial placement: how important is ideal fiducial geometry? Pancreas. 2013;42:692-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Dhadham GC, Hoffe S, Harris CL, Klapman JB. Endoscopic ultrasound-guided fiducial marker placement for image-guided radiation therapy without fluoroscopy: safety and technical feasibility. Endosc Int Open. 2016;4:E378-E382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |