INTRODUCTION
Endoscopic submucosal dissection (ESD) was developed in Japan in the late 1990s as an advanced, minimally invasive technique for endoscopic removal of early gastric cancers[1-5]. En-bloc resection with standard endoscopic mucosal resection (EMR) techniques is limited to lesions less than 2 cm in diameter, while ESD yields a higher complete resection regardless of size. EMR remains the typical approach in Western countries to treat dysplastic lesions and early cancers[6-9] while in Asia, ESD has become the preferred therapeutic modality of superficial tumors in both the upper and lower gastrointestinal tract[3]. It is even considered that it brought about a renaissance of therapeutic endoscopy[10] as it is able to offer organ-sparing cure in patient with early gastrointestinal (GI) cancers[11].
ESD has been a significant advancement in therapeutic endoscopy with its major advantages being the ability to achieve a higher en-bloc resection rate, accurate histological evaluation and lower cancer recurrence rates compared to EMR[3,12-15]. In addition, ESD enables en-bloc removal of previously unresectable lesions, such as large mucosal tumors, tumors with scars and submucosal fibrosis, or recurrent tumors after EMR[16,17]. Finally, as opposed to surgery, ESD preserves the structural integrity of the GI tract therefore protecting patient’s quality of life.
Despite its obvious advantages, ESD is one of the most complex endoscopic techniques, with several technical difficulties to overcome and potentially high complication rates, especially in the beginning of the learning curve[18-20]. The most frequent complications are bleeding and perforation. Bleeding during the procedure is very common but only rarely can be significant to the extent which requires the procedure to be stopped[21]. Compared to conventional EMR, the rate of perforation with ESD is higher, at about 1%-4% and it might require emergent surgical treatment but most of the time, perforations can be successfully managed conservatively[9,21,22].
In Japan, where there is a high incidence of the gastric cancer, a mass screening program with photofluorography, double-contrast radiography, chromoendoscopy, and endoscopy has been conducted since 1960[23-28] . Thus, a large proportion of Japanese gastric cancers are detected at an early stage, with a better overall survival rate[29,30]. ESD is routinely performed for resection of these early cancers in most centers in Japan including local branch hospitals. On the other hand, in the West ESD is still largely not available and is done only in a handful of centers by few advanced therapeutic endoscopy enthusiasts. Although ESD is largely not available in Europe and the United States, over the last 2-3 years there has been significant interest in ESD live demonstrations and hands-on seminars. There is a number of reasons for this slow dissemination of ESD in the West, including the complexity of the procedure, long procedure time, device availability, increased utilization of endoscopic resources, higher complication rates and, in the United States, lack of dedicated reimbursement code. However, the main obstacle for the wide availability of ESD in the West has been and remains the very flat learning curve and lack of training resources[31]. As ESD, with its advantages and challenges, has permeated deeper in the gastroenterology community, it became obvious that more endoscopists will be interested in acquiring this technique. It has been anticipated that the widespread adaptation of ESD for the treatment of pre- and early GI cancers will require major shifts in training and practice culture[32]. Therefore, we wanted to review the current state of training in ESD and emphasize the need for a structured training system in order to enhance trainee experience and, most importantly, to reduce the risks of procedural complications and inadequate treatment.
ESD LEARNING CURVE
It has been showed that when prior knowledge of advanced resection techniques is limited and no supervision by an expert in ESD is available, there is a learning curve in which not only the en-bloc resection rate and procedure duration improve with increasing experience but, more importantly, the perforation rate decreases too[33].
Learning curve for gastric ESD
Several reports have analyzed the learning curve for ESD in the stomach. Gotoda et al[34] found that experience of at least 30 cases is required for a beginner to gain early proficiency in this technique[34]. Choi et al[33] investigated the learning curve for ESD and reported an increase in the en-bloc resection rate from 45% to 85% after experience of 40 cases. They concluded that trainees need to perform 20-40 procedures to be able to use the technique effectively, although their method consisted of mucosal incision and snaring rather than standard ESD. From their data, which included 383 ESD procedures for gastric epithelial neoplasms performed over a 5-year period, Kakushima et al[11] estimated that a trainee could begin to treat lesions in the lower part of the stomach independently after performing about 30 supervised ESD procedures. In a more recent study, two of the three operators could not achieve a sufficient self-completion rate for submucosal dissection after 30 cases, which suggests that more extensive experience is required before the trainees can be considered proficient[35]. However, in this study, the trainees performed the ESD under the supervision of an experienced endoscopist and their training did not include hands-on training on ex-vivo animal models or living animals, which might have improved the learning curve. A study conducted by the same group in 2012 showed that the trainees required approximately 40 and 80 cases for successful removal of guideline-indication lesions and expanded-indication lesions by ESD. The procedural outcomes of ESD performed by preceptees who had experience in over 80 cases were similar to those by expert endoscopists. Thus, these findings suggest that the amount of training for achieving proficiency in ESD can be the performance of as many as 80 procedures[36]. Tsuji et al[37] concluded that the training system at their institution (which included training in animal models) enabled trainees to perform gastric ESD without decline in clinical outcomes, although 30 procedures were not enough for them to perform all gastric ESD independently without expert supervision, as expert assistance was still needed in a remaining 20% of ESDs. The keys to improving the learning curve were considered to be: good hemostasis technique and a sufficient level of submucosal dissection skill. Oda et al[38] used procedure time as an indicator of ESD proficiency and determined that 30 cases were necessary to acquire the basic technical skills for successfully performing ESD in the lower third of the stomach. In their estimation, performing at least 40 ESD would be the minimum learning curve point before starting to perform ESD in the middle and upper thirds of the stomach.
Learning curve for extra-gastric ESD
Recent studies showed that high cure rates are achievable using ESD for appropriate lesions in the esophagus and colorectum with no increase in complication rates, when the procedure is done by experienced endoscopist[39-42]. In a meta-analysis including 14 studies, Puli et al[43] concluded that ESD is the best minimally invasive endoscopic technique, and an important alternative to surgery, in the treatment of large (> 2 cm) sessile and flat polyps because it allows full pathological evaluation and cure in most patients. In a match-control study comparing ESD with EMR for treatment of early-stage colorectal tumors, Kobayashi et al[9] showed that colonic ESD achieved a high en-bloc resection rate and a low recurrence rate in short term. Most of the learning curve studies and training strategies have been developed for gastric ESD. However, the increased use of ESD in the colon and esophagus created a demand to further study and ESD skill acquisition in extra-gastric sites[9,44-51]. In Japan, endoscopists typically first experience ESD in the stomach because of the high incidence of gastric neoplasms and the relative safety of ESD in this location[36]. These conditions allow for opportunities to acquire sufficient experience in performing ESD. However, esophageal and colonic ESD presents the significant hurdle of technical difficulty and risk of severe complications even among Japanese endoscopists, who generally have greater experience in ESD than endoscopists in other countries.
Some experts consider that ESD in extra-gastric locations should not be attempted unless the endoscopist has experience in performing gastric ESD. Dinis-Ribeiro et al[52] suggested that only after performing 20-40 ESDs in the distal stomach, should lesions located in proximal sites in the stomach, esophagus, and colon be tried. Hotta et al[53] reported on the learning curve for colonic ESD, and they concluded that performance of approximately 40 procedures was sufficient to acquire the skill to avoid causing perforations during the ESD procedure, and approximately 80 procedures must be carried out to acquire adequate skill to successfully remove large colorectal tumors. Sakamoto et al[54] reported that trainees can perform colorectal ESD safely and independently after preparatory training and experience with more than 30 cases. In these two latter studies, the operators had performed 20 upper GI ESD before starting colorectal ESD.
A small number of analyses conducted in an earlier Japanese multicenter study indicated a higher complication rate during colorectal ESDs and that standardization of the colorectal ESD procedure would be difficult[55]. Despite greater risks of postoperative complications, particularly, more and more endoscopists are making an effort to study this new technique in terms of its capability of larger neoplasms resection, higher en-bloc resection rate and lower local recurrence rate of neoplasms in comparison with other endoscopic treatments. Ohata et al[56] proposed a 7-step training system for learning colorectal ESD, which is very similar to the training algorithms used for gastric ESD, but with the emphasis on technical differences imposed by performing the procedure in a narrower space with thinner wall. One of the mandatory enrolment criteria was performance of at least 30 gastric ESDs. The results suggested that trainees with relatively little prior experience with gastric ESD (i.e., 30 procedures) could reach a stable level of technical competency in colorectal ESD after an average of 30 cases of the latter procedure. The study found that, regardless of the gastric ESD experience, the mean procedure time of each trainee became less than 80 min after performing more than 30 cases. Trainees with experience in many (i.e., 200) gastric ESDs could perform colorectal ESD skillfully from the initial period of training onward[56].
What have we learned about ESD learning curves
Despite significant efforts to evaluate the learning curve of acquiring ESD skills no definitive conclusions can be reached due to the differences among studies as far as the type of lesions included, type of ESD devices used, degree of supervision, type of training system, trainee exposure to animal models, definition of outcomes and in the case of colonic ESD the degree of prior experience with gastric ESD. Therefore, in Japan, although ESD training varies among institutions, skills are still acquired in the traditional time honored apprenticeship model of training in endoscopy “see one, do one, teach one”. There has been a recognized need for structured training system for ESD in order to enhance trainee experience and, most importantly, to reduce the risks of procedural complications and inadequate treatment[11].
ESD TRAINING SYSTEMS
At present there is no universally accepted algorithm for training in ESD. Nevertheless, it appears that there is a consensus on some key points. Given the complexities of this technique, the training program must contain a solid cognitive-based preparation, and hands-on patient-based training. Also, the minimal requirements and final attainments for trainees at each level must be established prior to starting the training[37].
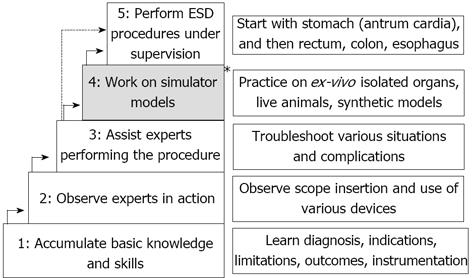
As expected, most well-implemented training programs/algorithms are in Japan. These algorithms typically include two major stages of training: pre-procedural, theoretic preparation and hands-on training[32,35-38]. The first stage has two phases: phase 1-accumulation of basic knowledge and phase 2-observe experts in action. The second stage includes phase 3-assist experts performing the procedure, phase 4-working on simulator models, such as ex-vivo and in-vivo animal model, or synthetic models of organ of interest, and phase 5-perform ESD procedures under supervision (Figure 1).
Figure 1 Japanese model for a structured endoscopic submucosal dissection training.
The * indicates the 4th step (practice on ex-vivo and live animal models) which is not employed in all Japanese training algorithm. ESD: Endoscopic submucosal dissection.
Recently, several training algorithms have been proposed. One of the earliest proposed training algorithms by Yamamoto et al[35] in 2009 puts emphasis on the initial pre-procedural phase of the training. Thus, the endoscopists who intend to learn ESD must attend pre- and post-treatment conferences, and take part in actual ESD procedures as an assistant for at least 1 year before beginning doing the procedure themselves. In addition to gastroenterologists, surgeons and pathologists are included in these conferences, and thus the trainee learns how to diagnose the extent and depth of the tumor, establish the optimum treatment strategy, and manage the patients appropriately according to the histopathological findings in resected specimens. By assisting experienced endoscopists, trainees acquire the skills needed to troubleshoot various situations. Moreover, obtaining expertise in hemostasis before starting ESD is highly recommended since most of the difficulties surrounding the procedure were related to uncontrollable hemorrhage[35]. The same group expanded the requirements of the pre-procedural training to master detailed preoperative examination by magnifying endoscopy with narrowband imaging, preoperative marking using ink and endo-clips, hemostasis of second-look endoscopy after ESD[36]. Similar approach is proposed by Kaltenbach et al[32], where the trainees are assisted in developing crucial diagnostic skills to select appropriate lesions and specific management strategy for ESD cases. The next step is for trainees to observe expert endoscopists in action as they perform various ESD procedures[38].
ESD is a technically demanding procedure requiring a high level of endoscopic skill. Consequently, in the second stage of the training, the trainees start by assisting experts in performing ESD procedure. Next, the trainees are exposed to animal models to enhance their technical skills. Hands-on experience with ESD in isolated pig stomach or live pigs facilitates familiarity with the tools and techniques of the procedure. Trainees can appreciate the differences in technique depending on lesion size and location. After gaining familiarity with the tools and technique, trainees typically start performing ESD in patients by removing small gastric lesions in the antrum or body under the close supervision of an experienced endoscopist, who both offers advice and can complete the procedure if necessary[32,38]. Yamamoto et al[35] propose a system where the trainees do not use animal models but start as assistants in live patient cases and then continue with performing ESD on patients under expert supervision. For this reason, they recommend that in this “supervision-only” training algorithm, one should start with small lesions in the lower third of the stomach. These lesions are relatively easy and less time-consuming to remove, so the trainees have the opportunity to learn the entire ESD procedure. After this, it is easier to move on to larger lesions, because the procedure for large lesions consists of repeating certain basic steps[35].
In summary, in Japan, a consensus exists on the following issues: (1) need of solid cognitive background regarding lesion evaluation, indications, contraindications and technical aspects of ESD; (2) need for observation of ESD as done by experts; (3) need to assist experts and operate the ESD devices; (4) need for hands-on training in humans under direct expert supervision; and (5) starting hands-on training with easier lesions and progressing to more difficult ones. Importantly, in Japan there is a number of areas where diverging opinions exist. These include: (1) need for simulation-based training; (2) need to use live animal models; (3) need to acquire a predetermined number of ESD cases in the stomach before moving to esophagus and colorectum; and (4) specific milestones for competency that the trainee has to meet before starting to practice ESD independently.
ESD TRAINING IN THE WEST
Unfortunately, the extensive Japanese experience in ESD training cannot be directly applied in the West due to a number of substantial differences. At present, in the West, there is only a handful of highly qualified experts in ESD. Therefore, doing ESD under direct expert supervision is not feasible in most cases. Importantly, the type of pathology seen in the West is different than the one in Japan. Specifically, there are very few cases of early gastric cancer and therefore no opportunity for the trainee to start their training in locations that are considered easier, such as the gastric antrum[35,57,58]. In addition, the choice of devices, endoscopes and ancillary equipment for ESD available in the West is different compared with the one available in Japan[59]. Likewise, the technical expertise and backgrounds of endoscopists embarking on ESD in the West differs significantly than their Eastern counterparts. At present, in Japan, the typical trainee learning ESD is a GI fellow. On the other hand, in the West, physicians embarking on ESD typically are more mature and otherwise well experienced therapeutic endoscopists. Furthermore, in Japan, physicians learning and performing ESD tend to focus their practice exclusively on ESD as opposed to the endoscopists in the West who tend to incorporate ESD into a developed advanced therapeutic endoscopy practice that typically includes endoscopic retrograde cholangiopancreatography (ERCP) and/or endoscopic ultrasound (EUS). In addition, even if ESD is considered more economical and less invasive, in the West laparoscopic surgery and transanal resection for colorectal lesions are more established techniques[59]. It has been well recognized that the specific circumstances in the West call for tailored approach in ESD training.
In the West, opportunities to pursue ESD training using the Japanese training algorithm have been limited by the low rates of early gastric cancer and thus the inability to enter the ESD learning curve at the relatively safest location[19,32]. To master the techniques of ESD, particularly in areas with a low incidence of early GI cancers, it was recommended to formulate a standardized protocol for training following the Japanese training model. The role of adequate training is, of course, to influence the spread of this technique, to set standards for training and certification, to promote quality management, and to limit complications inherent to early learning[31]. Several studies published good results after successful ESD procedures performed in humans in several Western countries[52,60-62].
In 2008, a panel of experts gathered in Rotterdam (“Experts meet experts,” Rotterdam, The Netherlands, 11-12 February 2008) to discuss indications, training, and the wider use of ESD. The minimum training requirements were also defined: knowledge in indications and instruments, exposure to experts (currently mostly in Japan), hands-on experience in a model of isolated pig stomach and in live pigs, and management of complications. The experts did not reach a consensus on a minimum case load, or whether the technique should be restricted to expert centers. Dr. Jelle Haringsma proposed a structured training algorithm with the following steps: (1) acquire basic knowledge, defined as knowledge about the types of disease treated with this approach, instrumentation, operation of the electrosurgical unit, and familiarity with indications, limitations, risks, and outcomes of ESD; (2) see experts at work, namely in Japan; (3) assist in procedures; (4) training on animal models-isolated pig stomach and live pigs. In animal models, a minimum of 30 resections reaching a resection speed of 30 min for a lesion with maximum diameter of 5 cm, and management of complications, were suggested as aims of training; (5) perform procedures on patients; and (6) continue training. Emphasis is also put on a training continuum with books, DVDs, journals, conferences, live demonstrations (master classes and courses), and visits to expert centers.
As outlined earlier, in Japan, one area of diverging opinions is the value of practice in explanted or live animal models. Kakushima et al[11] noticed that there does not seem to be any differences in the perforation rates when performing ESD between trainees and experts when the former are supervised by the latter. As a result, training on animal models is not routinely accepted practice in Japan. While training in animal models may not be needed in Japanese institutions where supervision by experts is easily available, these models can be a valuable resource when training in the West. Models could allow endoscopists to ascend the learning curve in a relatively short time, especially when training in low volume centers or/and without direct expert supervision[32,37,63,64]. Two prospective studies were aimed in determining the results, efficacy, and safety of ESD performed in pigs by an endoscopist at the beginning of the learning curve prior to its application in humans. The strategy proposed was to start training in ESD on animal models in the absence of experts to supervise the procedures and ensure the patients’ safety. The studies showed that training in pigs could be started without such previous learning, and may augment the acquisition of skills in low-volume centers. However, ESD involves maneuvers that traditionally have not been used during flexible endoscopy, which would be difficult to master by oneself[64,65].
The harvested porcine organs are ready-to-use and inexpensive means of becoming proficient in these techniques. Multiple large resections in the esophagus and stomach may be practiced before using a live porcine model. However, one of the main perceived disadvantages is that the ex-vivo animal models do not help in acquiring the skills of hemostasis and approaching a deep enough level of the submucosal layer, because bleeding does not occur[32].
The live pig model simulates a more realistic endoscopy setting and provides the opportunity to respond to and treat potential complications including bleeding and perforation[19]. However, some of the differences between pig and human stomach, such infrequent bleeding and lack of fibrosis in the pig stomach might make the procedure less challenging than in humans. Another potential disadvantage is that live animal models are expensive and not all institutions or hospitals are equipped for their usage.
Animal models could be used not only for training in gastric ESD but also for esophageal and colonic ESD[66-69]. Tanaka et al[67] developed an original training model for esophageal ESD using isolated pig esophagus and assessed this ex-vivo model in endoscopists with experience in gastric ESD. The operation time and number of muscularis propria layer injuries decreased gradually as endoscopists gained training experience, while the mean number of muscularis propria layer injuries significantly decreased for all of the endoscopists in the latter period compared with the former period.
While it has been demonstrated that certain skills can be acquired during self-guided animal model training, learning from experts appears crucial to achieve the ability to perform ESD safely in humans[64]. Therefore observing experts and performing ESD under expert supervision in addition to practicing on animal model appears a necessary step while training in the West[31,70-72]. Since, at this time most highly experienced endoscopists performing ESD are in Japan, a visit to a specialized center in Japan most likely will remain, for some time, an essential component of ESD training in the West. Other possible strategies would be to organize training courses (with animal and/or human training) under the supervision of experts, or to attempt ESD procedures supervised by means of a videoconference. However, the impact of these methods on ESD performance has yet to be determined[64]. Such, Western and Asian centers should collaborate closely in terms of training, exchange of data, and initiation of international multicenter trials[60].
We propose a training algorithm for Western physicians which integrates both hands-on training courses, animal model work as well as visit to expert centers (Figure 2). The initial step of the training can be accomplished through independent effort, using printed and video materials to learn about the procedure, indication and diagnosis. We believe that at this stage a dedicated effort to acquire detailed knowledge of the principles of electrosurgery is an essential step. Modern electrosurgical generators provide menus of predetermined settings for most routine procedures (e.g., polypectomy, sphincterotomy, etc.). On the other hand, no such preset menus exist for ESD. Settings can vary dramatically based on stage of the procedure, type of instrument and lesion location. In addition, multiple other variables can significantly contribute to the final tissue effect. These include the surface area of the device electrode in contact with the tissue, the speed of movement of the electrode, the pressure applied with the electrode, the presence of coagulated tissue debris sticking to the electrode and the target tissue itself (fibrotic versus high water content). Importantly, the most significant factor remains the endoscopist’s ESD technique. Therefore, a thorough understanding of the various modulated currents and their relation to ESD technique is essential to allow individualized choice of electrosurgical unit settings. Then, the endoscopists should attend live presentations and enroll in hands-on training courses to learn about the use of various devices and to practice on animal or synthetic models. After accumulation of this theoretical and practical fund of knowledge, we recommend a visit to an expert center. Most of these centers are currently located in Japan. However, with more endoscopists learning this technique, we anticipate that new training centers will open throughout the world. We are aware that not all endoscopists can spend long periods of time outside their practice, but we encourage at least 3 to 4 wk visit to a high volume ESD center in Japan. During this time, the trainees will assist experts in performing procedures, thus reaching the necessary diagnostic and therapeutic skill level. Upon return, the trainees should practice their newly acquired skills continuing training on simulator models. The next step is to start performing ESD on human patients. We advocate to start with lesions located in the distal stomach or rectum, as these are easier to remove and have a lower complication rate. During the initial human cases, expert supervision by means of videoconference is encouraged if direct supervision is not possible. Review of the endoscopy images prior to the ESD by an expert can provide the valuable opportunity to outline a specific procedure strategy which is an essential part of successful ESD. Then, gradually, the endoscopists can expand to cases of increasing difficulty such as treating larger lesions, or lesions located in the cardia, fundus, colon or esophagus. Finally, as in any other field, we recommend continuous training, with attending/presenting at conferences, re-visiting expert centers, reviewing literature and participating in courses and live demonstrations.
Figure 2 Proposed training algorithm for Western physicians, which integrates hands-on training courses, animal model work and visit to expert centers.
ESD: Endoscopic submucosal dissection.
TRAINING PROGRAMS FOR TRAINERS
This is a relatively new but important concept, as the training program for trainers is highly demanded for permeation of ESD worldwide and it is also necessary for trainers to be evaluated and rewarded. Endoscopists in Asian as well as Western countries are waiting for Japanese endoscopists to assist them more or less, in different ways according to the background of each country[73]. To assess the prerequisites for preceptorship, Goda et al[74] used a questionnaire survey to Japanese experts in representative teaching hospitals regarding their training method of gastric and esophageal ESD. This study indicated many requirements for the preceptor: having quite a high level of diagnostic ability, and proficient ESD techniques in the colorectum as well as the stomach and esophagus. It is also necessary that they are a regular staff with a certified qualification.
In a previous report, most Japanese experts set the level of expertise at 50-100 cases of gastric ESD in order to become proficient in gastric ESD. In a more recent study, Yamamoto et al[36] agreed with previous finding, showing that the minimal amount of training for achieving preceptorship in ESD is performance of at least 80 of the procedures.
Thus, so far, it appears that, to reach preceptorship level, the endoscopists need both a certain level of expertise, defined in number of procedures performed and a certification of their skills by an authorized body such as Gastroenterological or Endoscopy Societies. However, no specific preceptor training programs have been yet developed but there is a consensus that these programs are important for spreading ESD worldwide[73].
CONCLUSION
ESD represents an evolutionary step in therapeutic endoscopy. Using new skills, devices, and disposables, ESD achieves high rates of en-bloc curative resection rates for early GI cancers. However, the learning process for this advanced endoscopic procedure requires a lengthy training period and considerable experience to be proficient. A well-structured training program, safe, effective and easily reproducible is essential for the trainee, because the outcome of ESD is highly dependent on the experience of the endoscopist. It is also recommended that the training program should be tailored around needs based on culture and/or country since the incidence of disease and working environment may be different.
In Western countries, training in ESD is challenging given the lack of training in early gastric cancer lesions, assumed to be a relatively safer location to enter the learning curve. Currently, esophageal and colonic ESD are getting wider acceptance in the West where there is an effective screening process for Barrett’s and colon cancer with a large number of these lesions been detected in an early stage. We are proposing a training algorithm that will employ local resources to start the training in ESD and consolidate the knowledge and skill by learning from experts in Japanese centers.
Despite of all obstacles, ESD applications are continuing to grow in the West. Close collaboration between Western and Asian countries will be helpful to improve ESD technique for various sites and to benefit patients who are suffering from early gastric, esophageal or colorectal cancer.