Published online Mar 16, 2014. doi: 10.4253/wjge.v6.i3.88
Revised: February 16, 2014
Accepted: March 3, 2014
Published online: March 16, 2014
AIM: To clarify the usefulness of postsurgical capsule endoscopy (CE) in the diagnosis of recurrent small bowel lesions of Crohn’s disease (CD).
METHODS: This prospective study included 19 patients who underwent ileocolectomy or partial ileal resection for CD. CE was performed 2-3 wk after surgery to check for the presence/absence and severity of lesions remaining in the small bowel, and for any recurrence at the anastomosed area. CE was repeated 6-8 mo after surgery and the findings were compared with those obtained shortly after surgery. The Lewis score (LS) was used to evaluate any inflammatory changes of the small bowel.
RESULTS: One patient was excluded from analysis because of insufficient endoscopy data at the initial CE. The total LS shortly after surgery was 428.3 on average (median, 174; range, 8-4264), and was ≥ 135 (active stage) in 78% (14 of 18) of the patients. When the remaining unresected small bowel was divided into 3 equal portions according to the transition time (proximal, middle, and distal tertiles), the mean LS was 286.6, 83.0, and 146.7, respectively, without any significant difference. Ulcerous lesions in the anastomosed area were observed in 83% of all patients. In 38% of the 13 patients who could undergo CE again after 6-8 mo, the total LS was higher by ≥ 100 than that recorded shortly after surgery, thus indicating a diagnosis of endoscopic progressive recurrence.
CONCLUSION: Our pilot study suggests that CE can be used to objectively evaluate the postoperative recurrence of small bowel lesions after surgery for CD.
Core tip: The usefulness of capsule endoscopy (CE) in diagnosing recurrent small bowel lesions after surgery for Crohn’s disease (CD) has not yet been sufficiently established. This study revealed that many inflammatory lesions were already present throughout the residual small bowel shortly after surgery for CD, thus indicating an active stage of the disease on the basis of the total Lewis score in 77.8% of the patients. We concluded that the CE findings shortly after surgery can be used as a baseline for comparison against the findings from additional CE sessions over time and that this method can be used to objectively evaluate the postoperative recurrence of small bowel lesions after surgery for CD.
- Citation: Kono T, Hida N, Nogami K, Iimuro M, Ohda Y, Yokoyama Y, Kamikozuru K, Tozawa K, Kawai M, Ogawa T, Hori K, Ikeuchi H, Miwa H, Nakamura S, Matsumoto T. Prospective postsurgical capsule endoscopy in patients with Crohn’s disease. World J Gastrointest Endosc 2014; 6(3): 88-98
- URL: https://www.wjgnet.com/1948-5190/full/v6/i3/88.htm
- DOI: https://dx.doi.org/10.4253/wjge.v6.i3.88
Crohn’s disease (CD) is a chronic progressive idiopathic inflammatory bowel disease that is characterized by lesions that can potentially affect the entire digestive tract. CD is likely to be complicated by stenosis, fistula, abscess, and other intestinal abnormalities, requiring surgical resection of the intestine in approximately 75% of all patients[1-3]. Although the pathophysiology of CD is increasingly understood and treatments with biological agents have advanced remarkably, the rate of small bowel resection has remained unchanged from that recorded 10 years ago[4].
One of the important issues in the management of CD is that the surgical resection of lesions is not compatible with radical treatment, and postoperative recurrence is observed in a high percentage of patients. Endoscopic recurrence primarily occurs at the site of the ileocolonic anastomosis and the neoterminal ileum, and is detected before clinical recurrence occurs. The incidence of endoscopic recurrence is 70%-73% at 3 mo, 72%-93% at 1 year, and 79%-100% at 3 years after surgery[5-7]. Clinical recurrence occurs in 20% at 1 year, 17%-55% at 5 years, and approximately 32%-76% at 10 years after surgery, requiring reoperation in approximately 50% of all patients during the 20-year period after surgery[3,5].
To prevent recurrence after surgery for CD, various prophylactic treatments have been attempted, such as the use of 5-aminosalicylates, immunomodulators, or antimicrobial agents. Recently, close attention has been given to anti-tumor necrosis factor (TNF) α antibody as an agent for preventing postoperative recurrence[8-10]. In evaluating the efficacy of these prophylactic therapies, it is essential to establish a diagnostic technique that enables the precise judgment of the presence of postoperative recurrence.
The current gold standard for the evaluation of postoperative endoscopic recurrence is to perform ileocolonoscopy to assess the severity of the lesions at the site of the ileocolonic anastomosis and the neoterminal ileum according to the Rutgeerts score[5]. However, this method, poses several problems. Ileocolonoscopy is an invasive procedure that often requires sedation and analgesia. Therefore, the tolerability of the procedure is an important problem if such an examination is repeatedly performed over time in patients under postoperative clinical remission. Furthermore, the insertion of the endoscope to the ileocolonic anastomosis is sometimes difficult because of adhesions and may not always be successful. Even in cases where the endoscope has reached the anastomosed site, only a limited area of the distal part of the small bowel is observable. Furthermore, we cannot rule out the possibility that inflammatory lesions detected by ileocolonoscopy in the neoterminal ileum within 1 year after surgery represents lesions that were left unresected during the operation (rather than new lesions that developed after surgery). In patients who underwent operative procedures other than ileocecal resection, e.g., partial small bowel resection or ileostomy, it is difficult to evaluate the postoperative recurrence affecting the residual small bowel using colonoscopy.
Capsule endoscopy (CE), a diagnostic imaging tool for the small bowel, is characterized by its less invasive nature and its capability to observe the entire small bowel. CE has been shown to be superior over other radiologic and endoscopic modalities, such as small bowel follow-through, computerized tomographic enterography, ileocolonoscopy, and push enteroscopy, in terms of the effectiveness in diagnosing small bowel lesions associated with the non-stricturing type of CD[11]. However, the usefulness of CE in diagnosing recurrent small bowel lesions after surgery for CD has not yet been sufficiently established[12-14].
The present study, had the following objectives: (1) to use CE to prospectively evaluate the presence/absence, location, and severity of inflammatory lesions in the entire residual small bowel of CD patients shortly (within 1 mo) after the surgical resection of macroscopic lesions; and (2) to perform CE again approximately 6 mo after surgery to compare the findings with those obtained shortly after surgery, with the goal of examining whether it is possible to judge the presence of progressive recurrence in the small bowel and the efficacy of postoperative prophylactic treatments.
The protocol for this study was prepared in compliance with the Declaration of Helsinki and the Ethical Guidelines on Clinical Studies, and was approved by the Ethics Committee of our university.
This study was carried out as a single-center prospective study at the Hyogo College of Medicine (Hyogo, Japan) between November 2009 and December 2011. The inclusion criteria were as follows: (1) patients aged ≥ 16 years with a definitive diagnosis of ileal-type or ileocolonic-type CD; (2) patients who had undergone ileocolonic or ileal resection for the treatment of CD-related lesions within the previous 1 mo; (3) patients in whom the lesions detected via preoperative evaluations or surgical inspection of the serosal surface had been completely resected, leaving no evident residual lesion; and (4) patients who fulfilled the above 3 criteria regardless of a history of colonic resection or the presence/absence of ileostomy. The exclusion criteria were as follows: (1) patients who had undergone stricture-plasty without the resection of narrowed small bowel lesions; (2) patients in whom macroscopically evident small bowel lesions were left unresected during surgery; (3) patients in whom the evaluation of the residual small bowel was difficult intraoperatively because of adhesion and other issues; and (4) patients judged by the attending physician as being inappropriate for the study. Informed consent was obtained from each eligible patient.
CE was performed shortly after surgery on 19 patients with CD who satisfied the criteria. Table 1 summarizes the clinical characteristics of these 19 patients. The operative procedure was ileocolonic resection (including previous anastomosis) in 16 patients and partial ileal resection in 3 patients. Of the patients who underwent ileocolonic resection, 7 required ileostomy. Anastomosis had been performed on 12 patients (8 ileocolonic anastomosis, 2 ileo-ileal anastomosis, and 2 ileocolonic anastomosis + ileo-ileal anastomosis). The mean length of the residual small bowel, measured intraoperatively, was 455 ± 152 cm. The CRP level at the time of CE was 0.26 ± 0.54 mg/dL. In 10 patients, excluding those who underwent ileostomy, the CD activity index (CDAI) was 161.66 ± 55.36.
| Characteristics | Statistics |
| Age (yr) | 37.6 ± 10.2 |
| Gender (male/female) | 14/5 |
| Duration of CD (yr) | 13.5 ± 10.6 |
| Smoking (yes/no) | 2/17 |
| Disease location | |
| Ileum only | 4 |
| Ileum and colon | 15 |
| Disease behavior (B1/B2/B3) | 2/6/11 |
| Surgical resection (times) | |
| 1 | 6 |
| ≥ 2 | 13 |
| Type of surgery | |
| Ileocolonic resection | 16 |
| Partial ileal resection | 3 |
| Ileostomy | 7 |
| Length of the residual small intestine (cm) | 455 ± 52 |
| Preoperative medications | |
| Mesalamine | 15 |
| Elemental diet | 12 |
| Corticosteroids | 6 |
| Immunomodulators | 1 |
| Anti-TNFα antibody | 4 |
CE was carried out with a Given PillCam SB system (Given Imaging Limited, Yoqneam, Israel). Because patients with evident residual stenosis found during open abdominal surgery were excluded from this study, no evaluation to verify the absence of stenosis was made prior to CE. All patients had undergone surgery within the previous 1 mo, and all of them received CE during the postoperative hospital stay.
Each patient fasted for 12 h or more, before the examination. A capsule endoscope was taken orally with 100-200 mL water. To improve the image quality and to ensure the visualization of the entire small bowel, an isotonic magnesium citrate solution (500 mL) combined with 2 mL dimethicone was orally administered in 5 divided doses at intervals of 30 min after swallowing the capsule[15]. The ingestion of clear liquids was permitted after finishing the intake of the isotonic magnesium citrate solution. During CE, symptoms such as discomfort reported by the patient, and the presence of adverse events (e.g., capsule retention) were recorded. The data were collected approximately 8 h later for image analysis.
The images were analyzed using the RAPID® 6.5 ACCESS software (Given Imaging Limited). In cases where the ileocolonic anastomosis was confirmed or capsule excretion into the ileostomy was definitely confirmed, a judgment of “observation of the entire residual small bowel possible” was made. Each CE image was evaluated by an experienced gastroenterologist (T.K.) familiar with the examination of inflammatory bowel disease and with CE evaluation. For each patient, the CE images were evaluated in detail over a period of 2 h on average.
The Lewis score (LS) was used as the scoring index to quantify the inflammatory small bowel lesions detected via CE[16]. The area from the duodenal bulb to the site of the ileocolonic anastomosis or ileostomy was divided into 3 equal portions (proximal, middle, and distal tertiles) according to the transition time, and the score for each tertile was determined. In cases where a lesion was found at the site of the ileocolonic anastomosis, it was reflected into the score for the distal tertile. The total LS was defined as the edema and ulcer score of the most severe tertile plus the stenosis score. Activity was rated according to the criteria given in the original paper on total LS: no disease (score, < 135), mild disease (135-790), or moderate to severe disease (> 790). In our study, aphthae or erosion was defined as small mucosal breaks of approximately ≤ 3 mm, and was evaluated separately from ulcers. The number of aphthae or erosions and the distribution of these lesions were measured in each patient.
CE was performed again approximately 6-8 mo after surgery to evaluate the changes in the small bowel lesions through a comparison with the CE findings recorded shortly after surgery.
Because the use of a patency capsule had not been approved in Japan during the study period, conventional small bowel enteroclysis was carried out in advance in all patients to exclude those with small bowel stenosis or proximal bowel dilatation from the study.
The follow-up CE was carried out in a similar way to the CE conducted shortly after surgery. An evaluation was made in terms of the LS and the number of aphthae or erosions. The difference between the total LS shortly after and 6 mo after surgery was calculated as ∆LS. The ratings used a 3-category scale: progressive recurrence (total LS higher by 100 or more than the score recorded shortly after surgery), improved (total LS lower by 100 or more than the score recorded shortly after surgery), or unchanged (total score changed by -99 to +99 compared with that recorded shortly after surgery).
Ileocolonoscopy was also performed on patients who underwent ileocolonic resection within 14 d following the follow-up CE procedure. For each patient, a gastroenterologist who was blind to the findings of CE performed ileocolonoscopy. Postoperative endoscopic recurrence in the neo-terminal ileum and the ileocolonic anastomosis was evaluated using the Rutgeerts score (RS)[5]. The details of RS are as follows: i0 (no lesions), i1 (< 5 aphthous lesions), i2 [diffuse aphthous lesions with normal mucosa between lesions, or skip areas of larger lesions confined to the ileocolonic anastomosis (< 1 cm in length)], i3 (diffuse aphthous ileitis with diffusely inflamed mucosa), or i4 (diffuse inflammation with large ulcers, nodules, and/or narrowing). A RS of 2 or more was defined as endoscopic recurrence.
Continuous variables were expressed as the mean and standard deviation, or mean and median plus range. Mann-Whitney’s U test was used to test the differences between the unpaired groups. The Wilcoxon sign-rank test was used to test the differences between the paired groups. Categorical data were analyzed with Fisher’s exact test. The statistical analysis was carried out using Dr. SPSS II for Windows version 11.0 (SPSS Japan Inc., Tokyo, Japan). A P value of < 0.05 was considered statistically significant.
Of the 19 patients with CD who satisfied the inclusion criteria, CE was performed 17.3 ± 5.6 d after surgery on average. No CE-related adverse event was recorded in any patient, and the examination was well-tolerated.
The observation of the entire residual small bowel was possible in 18 patients (95%). In the remaining 1 patient, CE did not reach beyond the middle segment of the ileum during the 8 h of recording; thus, this patient was excluded from analysis. No adverse events (e.g., delayed retention) developed later in this patient. CE was also carried out without any problems in patients who underwent ileostomy. Among the remaining 18 patients, the mean small bowel transition time was 245 ± 210.7 min.
In all 18 patients in whom the observation of the entire residual small bowel was possible, the CE procedure detected some abnormalities: edema was detected in 13 patients (72.2%), ulcers in 12 patients (66.7%), stenosis in 1 patient (5.6%), and aphthae or erosion in 16 patients (88.9%).
The total LS (the edema and ulcer score of the most severe tertile plus the stenosis score) averaged 428.3, and its median was 174 (range, 8-4264). Disease activity as rated from the total LS was as follows: no disease in 4 (22.2%) patients, mild disease in 13 (72.2%) patients, and moderate to severe disease in 1 (5.6%) patient. Thus, although the examination was conducted shortly after surgery, 14 (77.8%) patients were rated as having active disease.
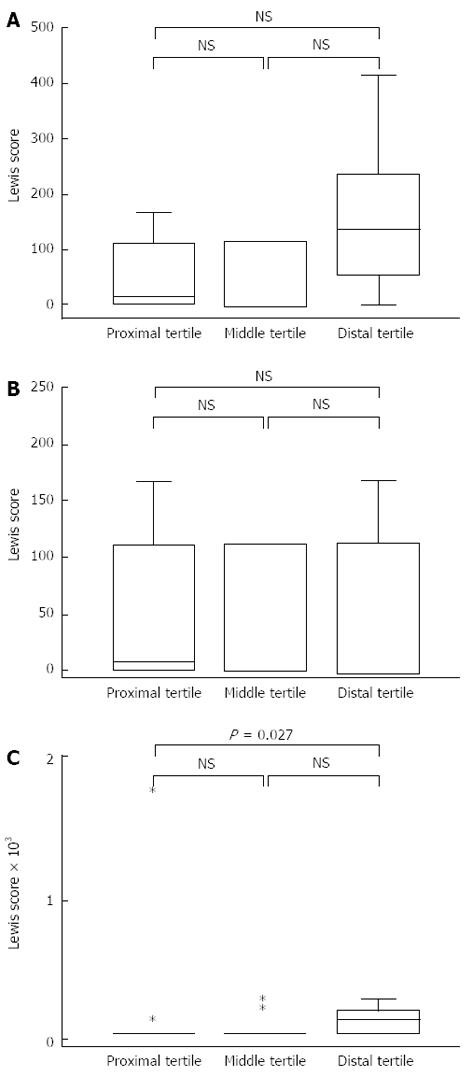
An analysis of the LS for each tertile (edema + ulcer + stenosis score for each of the 3 equal portions of the residual small bowel) showed that the proximal tertile had a mean score of 286.6 and a median score of 8 (range, 0-4264), the middle tertile had a mean score of 83.0 and a median score of 0 (range, 0-337), and the distal tertile had a mean score of 146.7 and a median score of 135 (range, 0-458) (Figure 1A). Lesions were found in the entire segments of the residual small bowel, including the proximal segment, without a significant difference between any 2 tertiles.
Figure 1B and C illustrates the edema and ulcer LS for each tertile. The edema LS did not differ significantly between any 2 tertiles. The ulcer LS for the distal tertile (mean, 114.7; median, 135; range, 0-459) was significantly higher than that for the proximal tertile (mean, 107.5; median, 0; range, 0-1800) (P = 0.027). Stenosis was detected in 1 patient who underwent partial ileal resection. The site of stenosis in this patient was the ileo-ileal anastomosis. The capsule passed this area after a 110-min retention in the proximal side of this area.
Among 12 patients who underwent ileocolonic or ileo-ileal anastomosis (14 sites in total), 10 patients (83.3%) developed ulcers in the anastomosed area. Of the 10 sites of the ileocolonic anastomosis, 7 (70%) sites developed ulcers, 1 site was free of ulcers, and 2 sites could not be evaluated because of the presence of residues. Ulcerous lesions were noted at all 4 sites of ileo-ileal anastomosis. Excluding 1 site of ileo-ileal anastomosis, all 13 anastomosis sites were in the distal tertile.
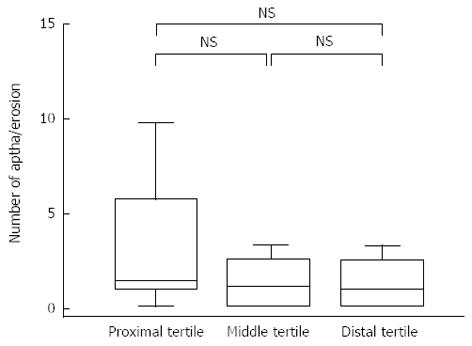
When the ulcer LS was calculated for each tertile, (excluding the anastomotic ulcers), the mean score was 57.5, 41.7, and 32.5 for the proximal, middle, and distal tertiles, respectively, without a significant difference between any 2 tertiles. The total LS, excluding ulcers and stenosis of the anastomosed site, averaged 176.4, and its median was 112 (range, 0-1012). Disease activity as rated on the basis of the total LS, excluding ulcers and stenosis of the anastomosed site, was as follows: no disease in 10 (55.6%) patients, mild disease in 7 (38.9%) patients, and moderate to severe disease in 1 (5.6%) patient. Eight (44.4%) patients had a high activity score, corresponding to mild or more severe disease. The mean number of aphthae or erosions observed was 3.2 ± 3.9, 2.1 ± 2.5, and 1.6 ± 1.6 for the proximal, middle, and distal tertiles, respectively (Figure 2).
When analyzed in relation to clinical background variables (presence/absence of preoperative anti-TNFα antibody therapy, penetrating/nonpenetrating type, history of surgery, and presence/absence of ileostomy), none of the background variables were associated with significant differences in the total LS, edema/ulcer/stenosis LS, or the number of erosions.
No restrictions were imposed on the postoperative treatment, which was decided through a discussion between the attending physician and each patient based on the clinical background, preoperative treatment, and early CE findings.
All 4 patients rated as having no disease based on the CE findings shortly after surgery desired to continue the 5-aminosalicylate therapy and elemental diet that had been started preoperatively. Of the 13 patients rated as having mild disease, 3 desired to continue the same therapy (5-aminosalicylates and elemental diet) and 10 desired to receive anti-TNFα antibody treatment. One patient rated as having moderate to severe disease desired to receive anti-TNFα antibody treatment.
Altogether, 8 (44.4%) of the 18 patients requested a change in their prophylactic treatment, upon being informed of the findings from the CE shortly after surgery.
The follow-up CE at 6-8 mo after surgery was possible in 13 of the 18 patients: 1 patient was excluded from follow-up because of the finding of a stenosed lesion in the CE shortly after surgery, and another 4 patients were excluded because they refused follow-up CE or were transferred to other facilities. The CRP level at the time of follow-up CE was 0.32 ± 0.18 mg/dL. In 7 patients, excluding those who underwent ileostomy, the CD activity index (CDAI) at the time of follow-up CE was 109.3 ± 28.34 (range, 68-139). No sign of clinical recurrence, as judged by the physician, was noted in any of these patients. Prestenosis dilatation was not detected via conventional small bowel enteroclysis prior to CE in any of the patients.
For the 13 patients in whom follow-up CE was possible, the total LS determined from CE findings shortly after surgery was 215.0 on average (median, 168; range, 8-562). The postoperative treatment given for these 13 patients was anti-TNFα antibody in 9 patients and 5-aminosalicylates + elemental diet in 4 patients.
The follow-up CE was performed 216.9 ± 23.6 d after surgery on average. In all patients, the follow-up CE enabled the observation of the entire residual small bowel. CE was well-tolerated, causing no adverse events such as capsule retention. The mean small bowel transition time was 132 ± 64.2 min. Edema was detected in 10 (76.9%) patients, ulcers in 9 (69.2%), and stenosis in none of the patients. Aphthae or erosion was detected in all 13 patients.
Table 2 compares the findings from CE performed shortly after surgery with those from the follow-up CE in the 13 patients. The total LS at the follow-up CE was 196.1 on average (median, 143; range, 8-450), without a significant difference from the LS recorded shortly after surgery. Furthermore, in terms of the edema and ulcer LS for each tertile, there were no significant differences between the data from shortly after surgery and those from follow-up CE. The number of aphthae or erosions in the middle and distal tertiles was significantly larger at the time of follow-up than that recorded shortly after surgery (P = 0.036 and P = 0.015, respectively).
| CE shortly after surgery | CE 6-8 mo later | P value | |
| Total LS | 215:168 (8-562) | 196:143 (8-450) | NS |
| Activity | NS | ||
| No disease: 2 | No disease: 4 | ||
| Mild disease: 11 | Mild disease: 9 | ||
| Edema LS | |||
| Proximal | 40.6:8 (0-168) | 36.3:8 (0-112) | NS |
| Mid | 48:0 (0-280) | 9.8:0 (0-112) | NS |
| Distal | 31.4:0 (0-168) | 27.1:0 (0-112) | NS |
| Ulcer LS | |||
| Proximal | 10.4:0 (0-135) | 41.5:0 (0-135) | NS |
| Mid | 57.7:0 (0-300) | 38.1:0 (0-225) | NS |
| Distal | 133.8:135 (0-450) | 118.8:0 (0-450) | NS |
| Aphthae/erosion (number) | |||
| Proximal | 3.2:1 (0-12) | 4.9:2 (0-17) | NS |
| Mid | 2.1:2 (0-9) | 10.5:3 (0-40) | 0.036 |
| Distal | 1.6:1 (0-4) | 8.1:5 (0-18) | 0.015 |
A total of 9 anastomosed sites were observed in 8 patients (ileo-ileal anastomosis at 2 sites and ileocolonic anastomosis at 7 sites). During the follow-up CE, observation was possible at all anastomosed sites, revealing active ulcers at 6 sites (66.7%), and scarring of the ulcers at 2 sites.
Of the 4 patients who underwent ileostomy, 3 had a total LS of ≥ 135 (corresponding to the active stage of the disease) according to the CE findings shortly after surgery. Of these 3 patients, only 1 was rated as having active disease at the time of follow-up.
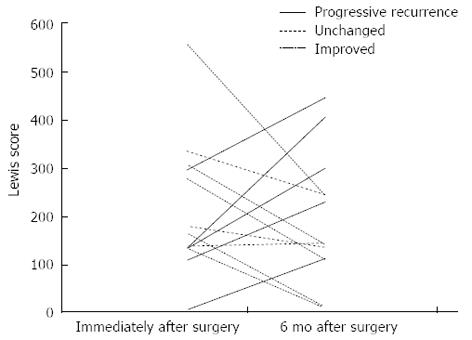
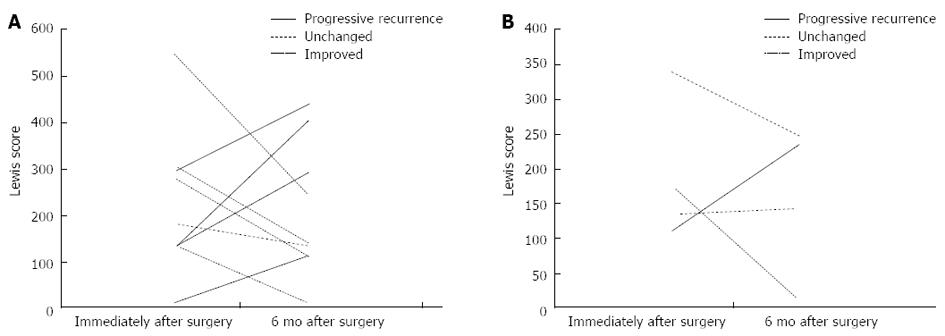
When the total LS at the time of follow-up was compared with the total LS shortly after surgery (ΔLS) in each patient, there were 5 patients in whom the score had increased by 100 or more (rated as progressive recurrence), 5 patients in whom the score decreased by 100 or more (rated as improved), and 3 patients in whom the score changed by -99 to +99 (rated as unchanged) (Figure 3). Figure 4 is a graphical comparison of the changes in total LS between the 9 patients who received postoperative anti-TNFα antibody treatment and the 4 patients who were not given anti-TNFα antibody. In the anti-TNFα antibody treatment group, the change was rated as progressive recurrence in 4 patients, improved in 4 patients, and unchanged in 1 patient. In the group without anti-TNFα antibody treatment, the change was rated as progressive recurrence in 1 patient, improved in 1 patient, and unchanged in 2 patients. Thus, in this study, the change in LS did not differ according to the presence or absence of the postoperative use of anti-TNFα antibody.
Ileocolonoscopy was also performed on all 6 patients who underwent ileocolonic resection. According to the RS, 3 patients (50%) showed endoscopic recurrence in the neo-terminal ileum and the ileocolonic anastomosis (Table 3). The mean ulcer LS of the follow-up CE in the distal tertile were 0, 217 ± 116.7, and 295 ± 157.6 in patients with Rutgeerts scores (RS) of 0, 1, and 2, respectively. The assessment results for the presence or absence of endoscopic recurrence based on RS were consistent with those based on ∆LS in 4 (67%) of 6 patients. In Case 3, (1 of the 2 patients with inconsistent assessment results), recurrence was determined via ∆LS, whereas no recurrence was determined by via RS because only a limited area of the neo-terminal ileum was observable by using ileocolonoscopy. In Case 4, the total LS was high shortly after surgery and showed improvement 6 mo later. However, recurrence was determined via RS because some lesions remained in the neoterminal ileum.
| Cases | Ileocolonoscopy | Capsule endoscopy | |||
| Rutgeerts score | Ulcer LS: 6-8 mo later (distal tertile) | A: Total LS shortly after surgery | B: Total LS: 6-8 mo later | ΔLS (B-A) | |
| 1 | 0 | 0 | 135 | 8 | -127 |
| 2 | 1 | 135 | 278 | 135 | -243 |
| 3 | 1 | 300 | 135 | 412 | 277 (recurrence) |
| 4 | 2 (recurrence) | 135 | 308 | 142 | -166 |
| 5 | 2 (recurrence) | 300 | 135 | 300 | 165 (recurrence) |
| 6 | 2 (recurrence) | 450 | 300 | 450 | 150 (recurrence) |
To our knowledge, this study was the first to attempt to evaluate small bowel lesions by using CE in patients shortly after surgery for CD. We believe that the evaluation of the presence/absence and severity of lesions in the entire residual small bowel shortly after surgery for CD could be an important step in postoperative endoscopic surveillance.
In patients with CD, intestinal complications such as obstruction, stenosis, abscess, fistula, and perforation, often serve as surgical indications[17]. These factors also make it difficult to perform a sufficient preoperative evaluation of the distribution and severity of small bowel lesions. As a result, surgeons are sometimes forced to check for the presence of lesions macroscopically during open abdominal surgery. In a previous study where intraoperative endoscopy was used to observe the entire small bowel in a retrograde manner from the distal end of the dissected intestine, lesions such as edema, redness, and ulcers were observed in 65% of all cases, and more than half of these lesions were not detected using preoperative radiography or surgical inspection of the serosal surface[18]. Thus, it has been reported that even when the surgical resection was considered radical or curative, the possibility of the presence of residual lesions was not low[19].
Bearing this in mind, we performed CE shortly after surgery (17 d after surgery on average) in patients with CD who had been intraoperatively judged to have no evident residual lesions in the small bowel. This evaluation revealed that lesions such as edema, ulcers, stenosis, aphthae, and erosions were already present in the entire residual small bowel shortly after surgery, enabling a judgment of an active stage of the disease based on the total LS in 77.8% of all patients.
Among others, the ulcer LS was particularly high in the distal tertile (corresponding to the neoterminal ileum, which is most likely to develop postoperative recurrence) shortly after surgery. This result appears to reflect the influence of ulcerous lesions left unresected during surgery and the presence of ulcers that developed in the anastomosed site. CE shortly after surgery revealed ulcers at anastomosed sites in 83.3% of the patients who underwent anastomosis. In the past, ulcerous lesions at the anastomosed sites detected using ileocolonoscopy at 6-12 mo after surgery were considered to represent a recurrence of CD[5]. However, our findings from CE shortly after surgery suggested that the ulcers at the anastomosed sites do not represent lesions recurring at a certain time after the surgery but are formed at a very early period after the surgery, due to factors such as responses of the anastomosed site to suturing or a disturbed blood flow[7,20], which could, in some cases, persist for an extended duration without complete healing. Furthermore, in using CE shortly after surgery, we found ulcers that formed at the site of the ileo-ileal anastomosis after partial small bowel resection (a site that is difficult to evaluate via ileocolonoscopy), indicating that CE is also useful in evaluating such lesions.
Even when ulcers and stenosis at the anastomosed sites were excluded, the total LS in 44.4% of all patients corresponded to the active stage. Although we cannot completely rule out that these small bowel lesions represent new lesions that developed shortly after surgery, it appears likely that many of these lesions are residual lesions that were left unresected during the operation. According to many of the recently published reports, the judgment about the presence of endoscopic recurrence after surgical treatment of CD is based on the evaluation of the neoterminal ileum via ileocolonoscopy at 6-12 mo after surgery. However, the term “recurrence” can be used only in cases where the lesions had been completely resected during surgery and remission was endoscopically demonstrated. Although checking for the absence of lesions at the ileal end using intraoperative endoscopy has also been proposed[7], it is not practically feasible to resect all active lesions detected via intraoperative endoscopy, due to the prevailing principle for the surgical treatment of CD (i.e., the extent of resection should be minimized by focusing on the major lesions). Therefore, it could be hypothesized that many lesions are left unresected during surgery, as shown by the results of the present study.
The ulcerous lesions detected via CE shortly after surgery were also often observed at the follow-up CE performed 6-8 mo later, regardless of the postoperative treatment used to prevent recurrence. The ulcer LS in the distal tertile showed no significant changes at the follow-up from the score recorded shortly after surgery. In view of the reports published to date, it is beyond doubt that the anastomosed site and the neoterminal ileum are the primary sites for postoperative recurrence. However, the possibility remains that the lesions detected at these sites could include diverse lesions not confined to true recurrent lesions that developed after surgery, for example, residual lesions that were left unresected during surgery (which either became aggravated or remained unchanged after surgery) or anastomotic ulcers that formed shortly after surgery and persisted.
The inflammatory lesions detected via CE shortly after surgery were observed in both the proximal and distal parts of the small bowel at a similar frequency. In the past, the frequency of proximal small bowel lesions in patients with CD was reported to be approximately 5%[21]. However, this frequency was based on radiographic diagnosis, and the use of CE has resulted in the detection of lesions in the proximal small bowel at a frequency of approximately 50%[22]. Furthermore, in a study involving CE in patients with CD at 3-6 mo after ileocolonic resection, a similar frequency (50%-56%) of proximal small bowel lesions was reported[12], in accordance with the results from the present study. According to a previous report, lesions found in the proximal small bowel are unlikely to be associated with clinical symptoms[22]. However, CD cases in which lesions developed in the proximal small bowel postoperatively have been reported, although the frequency of these lesions is not certain[23]. In the future, a long-term prospective survey with the use of CE may reveal the clinical significance and the natural history of the lesions found in the proximal small bowel after surgery for CD.
To date, only 3 reports have been published on the CE evaluation of recurrent small bowel lesions during the early postoperative period in CD patients[12-14]. In all 3 reports, the evaluation of endoscopic recurrence within 1 year after ileocolonic resection was performed by comparing the findings from ileocolonoscopy and CE; however, no consensus was reached regarding the usefulness of CE. In these studies, CE evaluation was done at only 1 time point after surgery. For an accurate evaluation of recurrent lesions after the surgical treatment of CD, it is necessary to repeat the test at multiple time points and, beginning shortly after surgery. With this in mind, we carried out CE at 2 time points (soon after surgery and half a year after surgery) and evaluated recurrence on the basis of changes in CE findings from the baseline to the second CE session. Patients showing a marked increase (by 100 or more) in the total LS during this period were rated as having “progressive recurrence” (the development of new lesions or aggravation of residual lesions). With this evaluation method, endoscopic progressive recurrence was noted in 5 of the 13 patients (38.5%), including 4 patients who received postoperative treatment with anti-TNFα antibody, a therapy previously reported to be highly effective in suppressing postoperative recurrence. This discrepancy in the results between the present study and the previously reported studies may be due to the following factors: (1) CE can be used to evaluate not only the neoterminal ileum but also the lesions remaining throughout the entire small bowel; (2) CE can be used to detect edema as a lesion, but edema is not covered by the Rutgeerts score; (3) the present study included patients who had used anti-TNFα antibody before surgery; and (4) the present study included patients who underwent ileostomy.
Our study had a limited number of patients in whom direct comparisons could be performed between the endoscopic recurrence results determined using RS based on ileocolonoscopy and ∆LS based on CE. However, it was suggested that CE performed at multiple time points could enable a more objective diagnosis of postoperative endoscopic recurrence of CD than ileocolonoscopy, especially in patients with several residual inflammatory lesions shortly after surgery or those with lesions located outside the ileocolonoscopy. A similar study needs to be conducted in a larger number of patients.
In the present study, CE enabled the evaluation of small bowel lesions not only in patients who underwent ileocolonic resection but also in those who underwent partial ileal resection or ileostomy. Moreover, a previous study reported that in patients who underwent ileostomy, ileoscopy through the stoma revealed endoscopic recurrence in 70% and clinical recurrence in approximately one-third of all patients[24,25]. Therefore, CE appears to be a useful tool for the diagnosis of endoscopic postoperative recurrence in patients who underwent these operative procedures.
This study has several limitations. First, we used the LS as a scoring index for the CE evaluation of inflammatory small bowel lesions. The LS has been reported to have a higher correlation with fecal calprotectin (a marker of intestinal inflammation) than with the capsule endoscopy CD activity index[26]. However, it is unknown, whether the LS is the optimal index for the evaluation of the postoperative recurrence of CD. The LS system involves the assessment of 3 factors: edema, ulcer, and stenosis. However, this scoring index assumes a high score in cases where edema is intense. Because edema could be a nonspecific sign of an inflammation that is not associated with CD, it remains controversial whether patients presenting with edema as a major symptom could be judged as having residual or recurrent lesions. Furthermore, aphthae, a factor evaluated in the Rutgeerts scoring system, is not evaluated in the LS system. In our evaluation, the number of aphthae and erosions detected at the second CE session (6 mo after surgery) was higher than that recorded shortly after surgery. This issue needs to be addressed further, as does the necessity of developing a new and more specific index for CE evaluation after surgery for CD.
In the present study, no adverse events, such as capsule retention, occurred. However, according to a report by Pons et al[13], passage failure of the patency capsule was seen observed in 8% of the stenosed cases where the test was conducted at 6-12 mo after surgery. Such events need to be carefully monitored. In the present study, it was not possible to use the patency capsule because it has not been approved in Japan. In the future, it will be necessary to establish a safer testing procedure that, includes the use of a patency capsule prior to CE.
CD patients who require surgery despite a previous strict medical treatment may be viewed as having a high disease activity. It is therefore essential to identify patients who require postoperative treatment aimed at preventing recurrence, and to provide therapeutic intervention with appropriate timing. In cases where ileocolonoscopy reveals recurrence within 1 year after surgery, the risk for subsequently developing clinical recurrence is high[5], and such patients would likely be indicated for prophylactic treatment with biological agents[10,27]. The question remains as to which postoperative treatment should be selected for patients in whom the total LS indicates a high disease activity, as evaluated via CE shortly after surgery.
It is currently unknown whether the severity of inflammatory small bowel lesions detected using CE shortly after surgery is associated with a risk of subsequent clinical recurrence. However, lesions that remain in the small bowel after surgery can be viewed as being resistant to preoperative medical treatment even when the lesions may not be severe enough to require surgery. Therefore, continuing the preoperative treatment in such patients after surgery will not result in mucosal healing of the residual lesions. When dealing with patients with high disease activity detected via CE shortly after surgery, it may be advised to immediately apply active treatment with anti-TNFα antibody and other medications aimed to prevent clinical recurrence. The clinical observation of CE findings over a longer duration is required to evaluate the relationship between CE findings and the postoperative clinical course of CD.
In a conclusion, CE enabled the evaluation of residual lesions throughout the entire small bowel shortly after surgery, regardless of the operative procedure applied. Comparing the CE findings collected at multiple time points after surgery with the CE findings at baseline (shortly after surgery) may enable an objective evaluation of true endoscopic recurrence, the natural history of CD after surgery, and the efficacy of the prophylactic treatment postoperative recurrence.
We are indebted to the late Professor Takayuki Matsumoto (died on October 21, 2012) for his support and guidance throughout our study.
After surgery for Crohn’s disease (CD), patients often develop endoscopic inflammatory lesions before clinical recurrence occurs. The current gold standard for the evaluation of postoperative endoscopic recurrence is to perform ileocolonoscopy. However, authors cannot rule out the possibility that the inflammatory lesions detected by ileocolonoscopy in the neoterminal ileum within 1 year after surgery represents lesions that were left unresected during the operation rather than new lesions that developed after surgery. It is essential to establish a diagnostic technique that enables the precise judgment about the presence of postoperative recurrence.
The usefulness of capsule endoscopy (CE) in diagnosing recurrent small bowel lesions after surgery for CD has not yet been sufficiently established. In this prospective study that included 19 patients who underwent ileocolectomy or partial ileal resection for CD, CE was performed 2-3 wk after surgery and was repeated after 6-8 mo, and the findings were compared to judge the presence of progressive recurrence.
This study revealed that many inflammatory lesions were already present throughout residual small bowel shortly after surgery for CD, thus indicating of an active stage of the disease on the basis of the total Lewis score in 77.8% of the patients. The authors concluded that the CE findings shortly after surgery can be used as a baseline for comparison against the findings from additional CE sessions over time and that this method can be used to objectively evaluate the postoperative recurrence of small bowel lesions after surgery for CD.
CE was safe, well tolerated and useful in the evaluation of entire small bowel after surgery for CD, regardless of the operative procedure applied. Comparing the CE findings collected at multiple time points after surgery with the CE findings at baseline (shortly after surgery) may enable an objective evaluation of true endoscopic recurrence, the natural history of CD after surgery, and the efficacy of the prophylactic treatment postoperative recurrence.
CE: CE is a diagnostic imaging device for the small bowel. CE has been shown to be superior over other radiologic and endoscopic modalities, in terms of the effectiveness in diagnosing small bowel lesions associated with the non-structuring type of CD. Lewis score: Lewis score is the scoring index to quantify the inflammatory small bowel lesions detected via CE.
This is an interesting study evaluate the use of CE soon after surgery and half year later. It provides important information about the history of small bowel Crohn’s disease.
P- Reviewer: Yen HH S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38-45. [Cited in This Article: ] |
| 2. | Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237-241. [Cited in This Article: ] |
| 3. | Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol. 2005;11:3971-3979. [Cited in This Article: ] |
| 4. | Lazarev M, Ullman T, Schraut WH, Kip KE, Saul M, Regueiro M. Small bowel resection rates in Crohn’s disease and the indication for surgery over time: experience from a large tertiary care center. Inflamm Bowel Dis. 2010;16:830-835. [Cited in This Article: ] |
| 5. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956-963. [Cited in This Article: ] |
| 6. | Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665-672. [Cited in This Article: ] |
| 7. | Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331-335. [Cited in This Article: ] |
| 8. | Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, Harrison J, Plevy SE. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology. 2009;136:441-50.e1; quiz 716. [Cited in This Article: ] |
| 9. | Yoshida K, Fukunaga K, Ikeuchi H, Kamikozuru K, Hida N, Ohda Y, Yokoyama Y, Iimuro M, Takeda N, Kato K. Scheduled infliximab monotherapy to prevent recurrence of Crohn’s disease following ileocolic or ileal resection: a 3-year prospective randomized open trial. Inflamm Bowel Dis. 2012;18:1617-1623. [Cited in This Article: ] |
| 10. | Yamamoto T, Umegae S, Matsumoto K. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn’s disease: a prospective pilot study. Inflamm Bowel Dis. 2009;15:1460-1466. [Cited in This Article: ] |
| 11. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol. 2006;101:954-964. [Cited in This Article: ] |
| 12. | Bourreille A, Jarry M, D’Halluin PN, Ben-Soussan E, Maunoury V, Bulois P, Sacher-Huvelin S, Vahedy K, Lerebours E, Heresbach D. Wireless capsule endoscopy versus ileocolonoscopy for the diagnosis of postoperative recurrence of Crohn’s disease: a prospective study. Gut. 2006;55:978-983. [Cited in This Article: ] |
| 13. | Pons Beltrán V, Nos P, Bastida G, Beltrán B, Argüello L, Aguas M, Rubín A, Pertejo V, Sala T. Evaluation of postsurgical recurrence in Crohn’s disease: a new indication for capsule endoscopy? Gastrointest Endosc. 2007;66:533-540. [Cited in This Article: ] |
| 14. | Biancone L, Calabrese E, Petruzziello C, Onali S, Caruso A, Palmieri G, Sica GS, Pallone F. Wireless capsule endoscopy and small intestine contrast ultrasonography in recurrence of Crohn’s disease. Inflamm Bowel Dis. 2007;13:1256-1265. [Cited in This Article: ] |
| 15. | Endo H, Kondo Y, Inamori M, Ohya TR, Yanagawa T, Asayama M, Hisatomi K, Teratani T, Yoneda M, Nakajima A. Ingesting 500 ml of polyethylene glycol solution during capsule endoscopy improves the image quality and completion rate to the cecum. Dig Dis Sci. 2008;53:3201-3205. [Cited in This Article: ] |
| 16. | Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146-154. [Cited in This Article: ] |
| 17. | Rutgeerts P. Review article: recurrence of Crohn’s disease after surgery - the need for treatment of new lesions. Aliment Pharmacol Ther. 2006;24 Suppl 3:29-32. [Cited in This Article: ] |
| 18. | Lescut D, Vanco D, Bonnière P, Lecomte-Houcke M, Quandalle P, Wurtz A, Colombel JF, Delmotte JS, Paris JC, Cortot A. Perioperative endoscopy of the whole small bowel in Crohn’s disease. Gut. 1993;34:647-649. [Cited in This Article: ] |
| 19. | Tytgat GN, Mulder CJ, Brummelkamp WH. Endoscopic lesions in Crohn’s disease early after ileocecal resection. Endoscopy. 1988;20:260-262. [Cited in This Article: ] |
| 20. | Angerson WJ, Allison MC, Baxter JN, Russell RI. Neoterminal ileal blood flow after ileocolonic resection for Crohn’s disease. Gut. 1993;34:1531-1534. [Cited in This Article: ] |
| 21. | Wagtmans MJ, Verspaget HW, Lamers CB, van Hogezand RA. Clinical aspects of Crohn’s disease of the upper gastrointestinal tract: a comparison with distal Crohn’s disease. Am J Gastroenterol. 1997;92:1467-1471. [Cited in This Article: ] |
| 22. | Petruzziello C, Onali S, Calabrese E, Zorzi F, Ascolani M, Condino G, Lolli E, Naccarato P, Pallone F, Biancone L. Wireless capsule endoscopy and proximal small bowel lesions in Crohn’s disease. World J Gastroenterol. 2010;16:3299-3304. [Cited in This Article: ] |
| 23. | Cesarini M, Angelucci E, Fiorino G, Crudeli A, Vernia P, Caprilli R. Postoperative recurrence of Crohn’s disease and videocapsule endoscopy: it is necessary to leave no stone unturned. Inflamm Bowel Dis. 2008;14:1165-1166. [Cited in This Article: ] |
| 24. | Leal-Valdivieso C, Marín I, Mañosa M, Naves JE, Zabana Y, Piñol M, Cabré E, Domènech E. Should we monitor Crohn’s disease patients for postoperative recurrence after permanent ileostomy? Inflamm Bowel Dis. 2012;18:E196. [Cited in This Article: ] |
| 25. | Vadlamudi N, Alkhouri N, Mahajan L, Lopez R, Shen B. Ileoscopy via stoma after diverting ileostomy: a safe and effective tool to evaluate for Crohn’s recurrence of neoterminal ileum. Dig Dis Sci. 2011;56:866-870. [Cited in This Article: ] |
| 26. | Koulaouzidis A, Douglas S, Plevris JN. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn’s Disease Activity Index. Dig Dis Sci. 2012;57:987-993. [Cited in This Article: ] |
| 27. | Sorrentino D, Terrosu G, Paviotti A, Geraci M, Avellini C, Zoli G, Fries W, Danese S, Occhipinti P, Croatto T. Early diagnosis and treatment of postoperative endoscopic recurrence of Crohn’s disease: partial benefit by infliximab--a pilot study. Dig Dis Sci. 2012;57:1341-1348. [Cited in This Article: ] |