Revised: November 28, 2012
Accepted: December 23, 2012
Published online: February 27, 2013
AIM: To identify blood donors with occult hepatitis B virus (HBV) infection (OBI) to promote safe blood donation.
METHODS: Descriptive cross sectional study was conducted on 3167 blood donors negative for hepatitis B surface antigen (HBsAg), hepatitis C antibody (HCV Ab) and human immunodeficiency virus Ab. They were subjected to the detection of alanine aminotransferase (ALT) and aspartate transaminase (AST) and screening for anti-HBV core antibodies (total) by two different techniques; [Monoliza antibodies to hepatitis B core (Anti-HBc) Plus-Bio-Rad] and (ARC-HBc total-ABBOT). Positive samples were subjected to quantitative detection of antibodies to hepatitis B surface (anti-HBs) (ETI-AB-AUK-3, Dia Sorin-Italy). Serum anti-HBs titers > 10 IU/L was considered positive. Quantitative HBV DNA by real time polymerase chain reaction (PCR) (QIAGEN-Germany) with 3.8 IU/mL detection limit was estimated for blood units with negative serum anti-HBs and also for 32 whose anti-HBs serum titers were > 1000 IU/L. Also, 265 recipients were included, 34 of whom were followed up for 3-6 mo. Recipients were investigated for ALT and AST, HBV serological markers: HBsAg (ETI-MAK-4, Dia Sorin-Italy), anti-HBc, quantitative detection of anti-HBs and HBV-DNA.
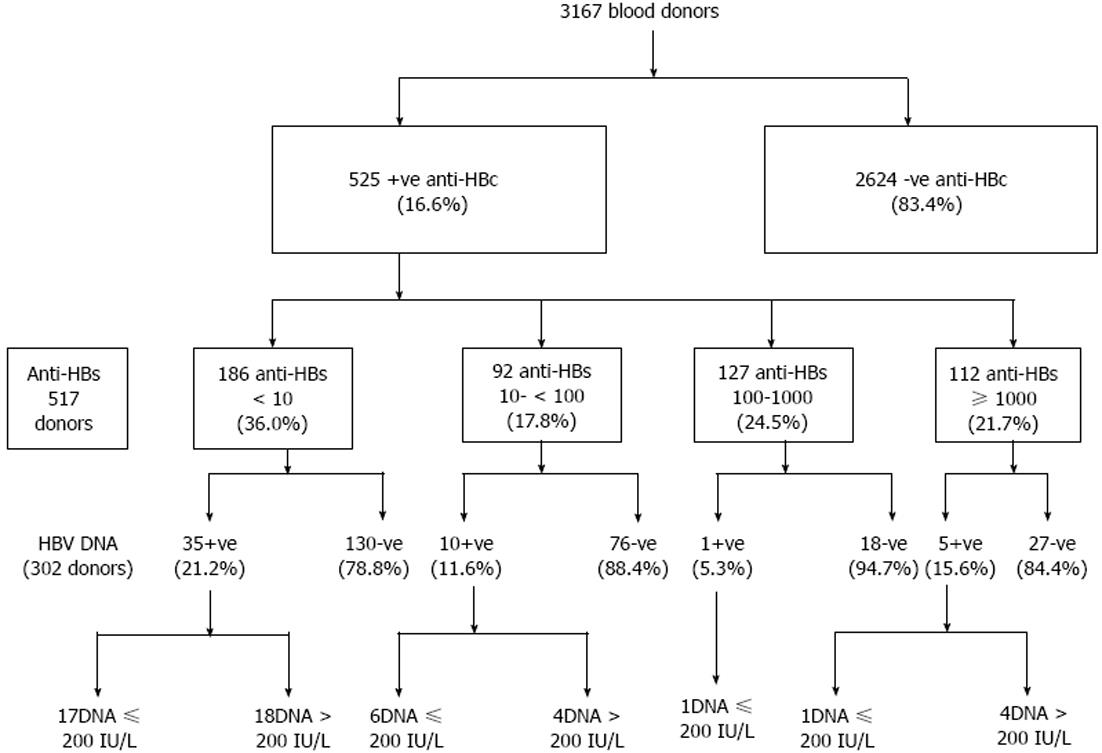
RESULTS: 525/3167 (16.6%) of blood units were positive for total anti-HBc, 64% of those were anti-HBs positive. Confirmation by ARCHITECT anti-HBc assay were carried out for 498/525 anti-HBc positive samples, where 451 (90.6%) confirmed positive. Reactivity for anti-HBc was considered confirmed only if two positive results were obtained for each sample, giving an overall prevalence of 451/3167 (14.2%) for total anti-HBc. HBV DNA was quantified by real time PCR in 52/303 (17.2%) of anti-HBc positive blood donors (viral load range: 5 to 3.5 x 105 IU/mL) with a median of 200 IU/mL (mean: 1.8 x 104± 5.1 x 104 IU/mL). Anti-HBc was the only marker in 68.6% of donors. Univariate and multivariate logistic analysis for identifying risk factors associated with anti-HBc and HBV-DNA positivity among blood donors showed that age above thirty and marriage were the most significant risk factors for prediction of anti-HBc positivity with AOR 1.8 (1.4-2.4) and 1.4 (1.0-1.9) respectively. Other risk factors as gender, history of blood transfusion, diabetes mellitus, frequent injections, tattooing, previous surgery, hospitalization, Bilharziasis or positive family history of HBV or HCV infections were not found to be associated with positive anti-HBc antibodies. Among anti-HBc positive blood donors, age below thirty was the most significant risk factor for prediction of HBV-DNA positivity with AOR 3.8 (1.8-7.9). According to HBV-DNA concentration, positive samples were divided in two groups; group one with HBV-DNA ≥ 200 IU/mL (n = 27) and group two with HBV-DNA < 200 IU/mL (n = 26). No significant difference was detected between both groups as regards mean age, gender, liver enzymes or HBV markers. Serological profiles of all followed up blood recipients showed that, all were negative for the studied HBV markers. Also, HBV DNA was not detected among studied recipients, none developed post-transfusion hepatitis (PTH) and the clinical outcome was good.
CONCLUSION: OBI is prevalent among blood donors. Nucleic acid amplification/HBV anti core screening should be considered for high risk recipients to eliminate risk of unsafe blood donation.
- Citation: Said ZN, Sayed MHE, Salama II, Aboel-Magd EK, Mahmoud MH, Setouhy ME, Mouftah F, Azzab MB, Goubran H, Bassili A, Esmat GE. Occult hepatitis B virus infection among Egyptian blood donors. World J Hepatol 2013; 5(2): 64-73
- URL: https://www.wjgnet.com/1948-5182/full/v5/i2/64.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i2.64
Hepatitis B virus (HBV) remains a major public health problem[1]. It is estimated that approximately 400 million people worldwide are chronically infected with HBV, where Egypt is considered as an area of intermediate endemicity[2].
Although, the incidence of transfusion-transmitted hepatitis B has been steadily reduced over the last four decades[3], HBV still remains the most frequent transfusion-transmitted viral infection[4-6]. Being the first-line of blood screening for HBV[7], different hepatitis B surface antigen (HBsAg) assays showed a range of sensitivity between < 0.1 and 0.62 ng of HBsAg per mL; 1 ng/mL corresponds to approximately 2 IU/mL[8,9], However, there is clear evidence that transmission by HBsAg-negative components occurs, in part, during the serologically negative window period, but more so during the late stages of infection[1], the later is referred to as occult HBV infection (OBI).
Occult HBV infection initially described in the late 1970 by Tabor et al[10] is characterized by the presence of HBV DNA in blood or tissues with undetectable HBsAg, with or without antibodies to hepatitis B core (anti-HBc) or hepatitis B surface (anti-HBs), outside the pre seroconversion window period[11,12].
Detection of OBI requires assays of the highest sensitivity and specificity with a lower limit of detection of less than 10 IU/mL for HBV DNA and < 0.1 ng/mL for HBsAg[13]. Most OBI are asymptomatic and would only be detected by systematic screening of large populations[14].No published guidelines are provided for categorizing those who should be screened for OBI[15]. Continuous progress in molecular biology techniques has led to greater recognition and diagnosis of OBI. It was reported in healthy blood donors, patients with chronic liver disease, and patients with hepatocellular carcinoma[13], in viral reactivation following immunosuppression, accidental transmission through transplantation, transfusion or experimental transmission to chimpanzees[16].
Published data reporting the infectivity of OBIs by transfusion are rare[17]. Pre-seroconversion window period (WP) infections are most likely transmit HBV but transmission from occult HBV infection remains debated[18]. In immunocompetent recipients, there is no evidence that anti-HBs-containing components (even at low titer) are infectious. Anti-HBc only, with HBV DNA, can be associated with infectivity, as can rare cases of HBV DNA without any serological HBV marker[11]. Addition of anti-HBc testing for donor screening, although will lead to rejection of a large number of donor units, will definitely eliminate HBV infected donations and help in reducing HBV transmission with its potential consequences, especially among the immunocompromised population[19].
The aim of this work is to determine the prevalence of occult HBV among Egyptian healthy blood donors and highlight the residual risks of transmitting HBV in blood banks through blood transfusion.
A cross sectional study was undertaken including two main blood transfusion centers. Only donors negative for anti- hepatitis C antibody (HCV), anti-human immunodeficiency virus (HIV), and HBsAg were included in this study. All donors did not receive HBV vaccine. They were medically assessed and any known risk factors for viral infection listed in the questionnaire were recorded. Recipients’ samples were collected before transfusion and a follow up sample was recollected whenever possible from those whose donor was found to be positive for total anti-HBc.
Serum HBV total anti-HBc was performed by ELIZA technique [Monoliza Anti-hepatitis B core Plus-Bio-Rad] according to manufacturer’s instructions. Sera were also investigated for alanine aminotransferase (ALT) and aspartate transaminase (AST) (Spectrum, Egypt). Anti-HBc positivity was confirmed with (ARC-Hepatitis B core total-ABBOT). Positive samples were subjected to quantitative detection of anti-HBs with commercially available kits (ETI-AB-AUK-3, Dia Sorin-Italy). Serum anti-HBs titer > 10 IU/L was considered positive. HBV DNA level was estimated mainly for blood units with baseline low or undetectable serum anti-HBs levels and also for 32 whose anti-HBs serum titers were > 1000 IU/L. All available recipients’ samples as well as follow-up samples were investigated for ALT and AST HBV serological markers: HBsAg (ETI-MAK-4, Dia Sorin-Italy), anti-HBc, quantitative detection of anti-HBs as well as HBV-DNA.
Real-time polymerase chain reaction quantification of HBV genome: HBV-DNA quantification by real-time polymerase chain reaction (PCR) was performed using automated system. Viral DNA was extracted from serum samples using QIAxtractor®, and VX kit as recommended by the manufacturer. QIAGEN- Germany. PCR setup was automated via QIAgility (QIAGEN, Germany). HBV real-time assays were performed in combination of Artus HBV RG PCR Kit (Artus™ GmbH, Hamburg Germany) and the Real time PCR instrument, Rotor-Gene Q (QIAGEN, Germany). Thermal profile was set according to manufacturer’s guideline. Detection limit of HBV DNA in the current study assay is 3.8 IU/mL assessed by the World Health Organization (WHO) international standard (97/750)[20]. At least two negative controls, one non template control, and four standards (provided by the manufacturer) were added per run. Strict precautions were taken to avoid possible contamination. Only data that revealed no false-positive results in the negative controls and that were reproducible were used.
From the available archival sera of the recipients, AST, ALT, HBV markers as well as the HBV DNA was examined as mentioned above.
The study has been approved by the Al-Azhar University Ethical Committee and the procedures have been performed according to the World Medical Association Declaration of Helsinki, 1979; http://www.wma.net/e/policy/b3.htm. Informed, written consent at the time of sampling has been obtained from both donors and recipients.
Data entry and statistical analysis was done using SPSS under windows, version 13. χ2 test was used in order to detect the difference in frequency for categorical variables. Fisher’s exact test was used, instead of χ2, when there was a cell in the 2 × 2 table with an expected frequency below 5. t test was used to assess the difference between two means of continuous variables. All tests were 2-sided and a P value < 0.05 was considered statistically significant. Multiple stepwise logistic analyses were done to predict the most important risk factors associated anti-HBc and HBV-DNA positivity.
A descriptive cross sectional study was conducted on 3167 blood donors negative for HBsAg, HCV Ab and HIV Ab. The study included 491 blood donors from The National Blood Transfusion Center and 2676 blood donors as well as 265 blood recipients from the blood bank of Ain-Shams Maternity and Women’s University hospital.
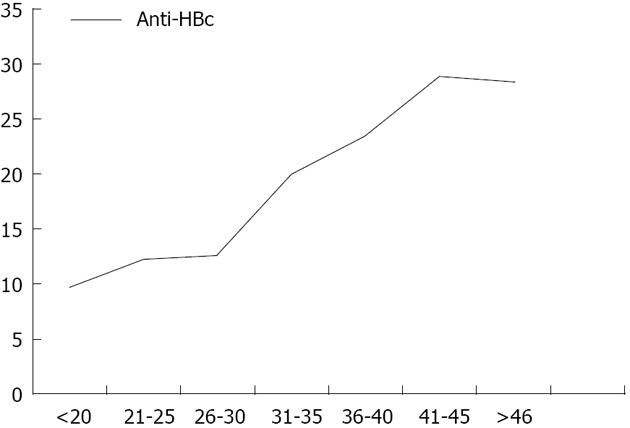
Total anti-HBc antibodies was positive in 525/3167 (16.6%) blood donors; 64% of those were positive for anti-HBs antibodies. Confirmation by ARCHITECT anti-HBc assay was carried out for 498/525 anti-HBc positive samples, where 451 (90.6%) were found positive. Reactivity for anti-HBc was considered confirmed only if two positive results were obtained, giving an overall prevalence of 451/3167 (14.2%) for total anti-HBc. The sensitivity of the assay was evaluated and 100 total anti-HBc ELIZA negative samples were retested by ARCHITECT for confirmation, three were positive, and only one showed HBV-DNA positivity by real time PCR. The prevalence of positive anti-HBc was significantly increased with increasing age (Figure 1). Other risk factors as gender, blood transfusion, diabetes mellitus, frequent injections, tattooing, previous surgery, hospitalization, Bilharziasis or positive family history of HBV or HCV infections were not found to be associated with positive anti-HBc antibodies (P > 0.05). Moreover, mean level of ALT and AST were found to be significantly higher among anti-HBc negative blood donors (42.7 ± 25.0 IU/L and 29.1 ± 17.1 IU/L) compared to anti-HBc positive blood donors (38.7 ± 20.0 IU/L and 26.0 ± 13.9 IU/L), respectively.
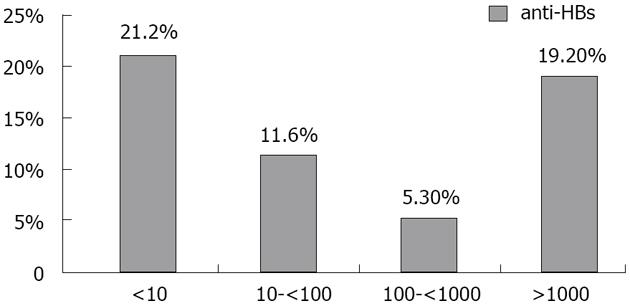
Five hundred and seventeen anti-HBc-positive samples were tested for anti-HBs and in eight cases samples were not enough to perform the assay. Anti-HBs was negative in 186 (36%) and positive in 331 (64%), where 92 (27.8%), 127 (38.4%) and 112 (33.8%) of them had anti-HBs levels of 10-99 IU/L, 100.0-999.9 IU/L and ≥ 1000 IU/L respectively.
HBV DNA was quantified in 303 samples with real-time PCR assay. It was detected in 52/303 (17.2 %) of anti-HBc positive blood donors. They were 88.2% males and 11.8% females with a mean age of 29.2 years (range, 20-47). Viral load range: 5 to 3.5 × 105 IU/mL with a median of 200 IU/mL and a mean of 1.8 × 104± 5.1 × 104 IU/mL; 49% had a viral load < 200 IU/mL (low level of viremia that correlates with chronic resolving infections). According to the anti-HBs marker, the 51 occult cases could be divided into two groups: 35 subjects (68.6%) were only anti-HBc positive without detectable antibodies to the surface antigen, whereas 16 (30.8%) were positive for both anti-HBc and anti-HBs. Figure 2 shows the prevalence of HBV-DNA among blood donors with different anti-HBs levels (P > 0.05). All results are summarized in Figure 3.
Complete laboratory investigations were available for only 280 blood donors. Their results are summarized in Table 1.
| Anti-HBc positive donors n (%) | Anti-HBc Architec | Anti-HBs | HBV-DNA |
| 102 (36.4) | +ve | -ve | -ve |
| 29 (10.4) | +ve | -ve | +ve |
| 97 (34.6) | +ve | +ve | -ve |
| 16 (5.7) | +ve | +ve | +ve |
| 24 (8.6) | -ve | -ve | -ve |
| 10 (3.6) | -ve | +ve | -ve |
| 2 (0.7) | -ve | -ve | +ve |
Multivariate logistic analysis revealed that age above thirty and marriage were the most significant risk factors for prediction anti-HBc positivity among blood donors with AOR 1.8 (1.4-2.4) and 1.4 (1.0-1.9) respectively. Among anti-HBc positive blood donors, age below thirty was the most significant risk factor for prediction of HBV-DNA positivity with AOR 3.8 (1.8-7.9) (Table 2).
| Risk factor | n (%) | Crude odds ratio (95%CI) | Adjusted odds ratioΩAOR (95%CI) |
| For anti-HBc +ve | |||
| Age > 30 yr | 196/853 (23.0) | 2.1 (1.7-2.7)b | 1.8 (1.4-2.4)b |
| Age ≤ 30 yr | 151/1233 (12.2) | ® | ® |
| Marital status | |||
| Married | 287/1530 (18.8) | 2 (1.5-2.5)b | 1.4 (1.0-1.9) |
| Single | 94/898 (10.5) | ® | ® |
| For HBV-DNA+ve | |||
| Age ≤ 30 yr | 29/95 (30.5) | 3.6 (1.7-7.4)b | 3.8 (1.8-7.9)b |
| Age > 30 yr | 13/119 (10.9) | ® |
According to HBV-DNA concentration, positive samples were divided in two groups; group one is with HBV-DNA ≥ 200 IU/mL (n = 27) and group two is with HBV-DNA < 200 IU/mL (n = 26). No significant difference was detected between both groups as regards to mean age, gender, liver enzymes or HBV markers (Table 3).
| Variable | HBV-DNA≥200 | HBV-DNA < 200 | P value |
| (n = 27) | (n = 26) | ||
| Age (mean ± SD) | 29.1 ± 6.7 | 29.2 ± 7.1 | 0.972 |
| Gender | |||
| Males | 26 (57.8) | 19 (42.2) | 0.088 |
| Females | 1 (16.7) | 5 (83.3) | |
| Marital status | |||
| Single | 7 (50.0) | 7 (50.0) | 0.75 |
| Married | 16 (55.2) | 13 (44.8) | |
| Donation site | |||
| Ain Shams Hospital | 27 (54.0) | 23 (46.0) | 0.11 |
| Central blood bank | 0 (0.0) | 3 (100.0) | |
| Previous blood donation | 16 (64.0) | 9 (36.0) | 0.12 |
| ALT (mean ± SD) | 33.2 ± 14.7 | 34.4 ± 17.6 | 0.868 |
| AST (mean ± SD) | 25.5 ± 13.5 | 26.1 ± 10.8 | 0.671 |
| Anti-HBs (mean ± SD) | 156.1 ± 359.3 | 59.9 ± 203.8 | 0.634 |
| Architect HBV | |||
| +ve | 23 (51.1) | 22 (48.9) | 0.99 |
| -ve | 2 (66.7) | 1 (33.3) | |
| Anti-HBs level | |||
| < 10 | 18 (51.4) | 17 (48.6) | 0.447 |
| 10- | 5 (50.0) | 5 (50.0) | |
| 100- | 0 (0.0) | 1 (100) | |
| ≥ 1000 | 4 (80.0) | 1 (20.0) |
At baseline, total anti-HBc was positive in the sera of 49/265 (18.5%) recipients. Confirmation for anti-HBc by ARCHITECT revealed that 47 (95.9%) were positive. HBsAg were found positive in the sera of 6 blood recipients, 4 had also positive anti-HBc antibodies and 2 were negative. None of the recipients had HBV DNA.
It is noteworthy that getting follow-up samples from the recipients was not an easy task, mostly because they often refused to come back to give a new sample and also due to communication problems. This was reflected in lack of full characterization of the studied OBI cases. However, follow-up samples were acquired from 34 out of 216 recipients. Serological profiles of all followed up blood recipients showed that, all of them were negative for the studied HBV markers. No HBV DNA was detected among these recipients. No one developed post-transfusion hepatitis and the clinical outcome was good. It has to be mentioned that only 11/34 recipients received blood from anti-HBc positive blood donors. These 11 blood donors had total anti-HBc positive by both assays, 9 were negative for HBV-DNA (81.8%), and two were HBV-DNA positive (18.2%); one was with HBV DNA concentration of 8 IU/mL and the other was of 3.3 × 104 IU/mL.
Occult hepatitis B infection is one of the most challenging topics in the field of viral hepatitis. The frequency of detection of OBI is directly dependent on the sensitivity of assays of either or both HBV markers[14]; however, detection of virus specific nucleic acid does not always translate into infectivity[13]. In humans, HBV transmission was reported from donors in the window period and OBI donors showing HBV DNA load ≤ 200 IU/mL[7,21].
In Egypt, HBV screening in blood banks relies only on detection of HBsAg; however, introducing NAT in some major blood banks is under implementation. Discrepancies in analytical sensitivity and specificity have been recorded among commonly used EIAs for the detection of HBsAg from viruses of different genotypes[22,23]. Failure to detect HBsAg antigen and differences in signal intensity were mainly associated with mutations in the preS/S gene outside the “a” determinant[23].
In this study, the overall prevalence of total anti-HBc antibodies among HBsAg negative blood donors was 14.2% (451/3167). This is comparable to the older anti-HBc prevalence rates reported among HBsAg-negative blood donors in the Mediterranean region and Arab Peninsula; 15.03% in Greece[24] and 16.4% in Saudi Arabia[25], respectively. A previous Egyptian study, however, reported a prevalence of 10.9% in volunteer HBsAg-negative blood donors, where, HBV-DNA was detected in 11.54% of the anti-HBc positive units[26]. A more recent study among Egyptian healthy male HBsAg-negative donors showed that 80/1026 (7.8%) were reactive to anti-HBc. Of those, 5 (6.25%) had HBV-DNA as detected by real-time polymerase chain[27].
It was shown that low levels of viremia are detectable in 1.6% to 38% of HBsAg-negative/anti-HBc-positive donors when highly sensitive techniques for the detection of HBV-DNA were used[28-30]. Using a very sensitive real time PCR assay, 52/302 (17.2%) (95%CI: 14.7%-19.7%) anti-HBc positive blood donors were positive for HBV-DNA. This relatively high prevalence could be attributed to the high sensitivity of the assay. Furthermore, among 165 anti-HBs negative donors (all anti-HBs negative donors except 23) who were assayed for HBV-DNA by real time PCR, 35 (21.2%) tested positive.
Thus it could be estimated that 2.8% of blood donor units have been viremic and otherwise transferable (525 anti-HBc-positives out of 3167 tested donors times 451 confirmed anti-HBc-positive out of 498 anti-HBc-initially reactive times 45 HBV-DNA-positive out of 244 confirmed anti-HBs positive tested for viremia). This equals to the release of one unit containing HBV-DNA in 36 donations or 27 in one thousand units eligible for transfusion.
It was previously shown that approximately 90% of blood donors carrying anti-HBc also carried anti-HBs signaling recovered HBV infection[31]. The remaining 10% were termed anti-HBc alone, the significance of which was extensively studied by many authors. It may be in part false positive anti-HBc due to poor assay specificity or true anti-HBc[32] that may originate either from recovered infections having lost detectable anti-HBs or from late stage chronic infections having lost detectable HBsAg[7].
In the current study, 17% of the HBsAg negative, anti-HBc positive blood donors were HBV-DNA positive with 6% also anti-HBs positive (Table 1). On the other hand, only 38% of anti-HBc positive donors who were HBV-DNA negative had anti-HBs denoting past infection. An earlier study similarly showed that up to 16% of anti-HBc/anti-HBs-positive donors have circulating HBV-DNA unbound to anti-HBs in their sera and thus in a potentially infective form[28]. Also, these results are in accordance with studies of Brojer et al[1], Candotti et al[33] and Katsoulidou et al[12] where, it was found that nearly 50% of OBI are asymptomatic, apparently healthy, blood donors carries anti-HBs. Satake et al[34] in Japan emphasized that no evidence of HBV infections was found when donations contained both anti-HBc and anti-HBs. Experiments in chimpanzees showed no HBV infection in animals transfused with blood from three anti-HBs-positive human plasmas, despite exposure to an HBV DNA dose known to be infectious in the absence of anti-HBs[35]. However, Levicnik-Stezinar et al[17] identified a case report of an OBI carrier who transmitted HBV to two immunocompetent transfusion recipients despite anti-HBs and concluded that the neutralizing capacity of low-level anti-HBs is limited and reinforced the validity of considering anti-HBs below 100 IU/L to be poorly protective from infectivity when HBV DNA is present.
A recent study conducted by Launay et al[36] showed that a small proportion of patients with “anti-HBc alone” have high viral loads, revealing the occurrence of infection with HBV mutants that escape detection even by multivalent HBsAg assays. Only 24 (8.6 %) donors’ samples were false positive in our study, while 102 (36%) were “anti-HBc alone” positive by both evaluated assays (Table 1). A Lebanese study demonstrated that 56 out of 2505 (22%) blood donors screened for HBV markers were “anti-HBc alone” positive[37].
HBV nucleic acid amplification testing (NAT) is effective in reducing the risk of HBV transmission if performed on individual donations; nevertheless, the costs of such a strategy could be prohibitive until multiplex NAT testing for blood-borne viruses is available everywhere[38]. In addition, it was previously emphasized that the marginal yield of HBV NAT does not justify its implementation in routine screening of blood donors. Thus, anti-HBc assays represent a second safeguard that may further reduces the need for HBV NAT implementation[39].
Different risk factors are known to be associated with OBI including detectable HBV DNA, anti-HBc, antibodies to HBsAg < 100 IU/mL, young age and male gender. On the other hand, a study by Minuk et al[40], demonstrated that age, gender and liver biochemistry findings do not identify those with OBI. The current study does not show an association between any of the former risk factors and OBI except for age below thirty which was the most significant risk factor for prediction of HBV-DNA positivity among anti-HBc positive blood donors (AOR 3.8; 1.8-7.9). This is recently reported by Allain et al[41] who found that OBI donors are generally older than 45 years except in Africa, carry very low viral load (median 11-25 IU/mL) and have normal alanine transaminase levels.
A recent study conducted in Egypt showed that HBV transmission is community rather than iatrogenic-acquired[42]. In several studies, low educational attainment had been associated with higher prevalence of hepatitis B in both developed and developing countries[43,44].
It is noteworthy, in this study, that only one subject was HBV-DNA positive when 100 HBV markers’ negative blood donors were evaluated. Minuk et al[40] studied the prevalence of occult HBV in 487 HBsAg negative sera with/without serologic evidence of previous HBV infection. They concluded that the prevalence of OBI was 18% in those with serologic evidence of previous HBV infection and 8.1% in HBV seronegative individuals. HBsAg diagnostic failure and low viral replication in OBI carriers could possibly be attributed to multiple changes in envelope and polymerase regions, respectively[12]. Inhibition of HBsAg mRNA production and export are potential mechanisms of OBI occurrence[41].
The use of HBV anti core testing to eliminate the residual transfusion risk of transmission of HBV has not been evaluated in Egypt. The results of this study proved that anti-HBc testing would cause the exclusion of a consistent number of donors, more than 80% of whom are HBV-DNA negative. It was also shown that nearly half of anti core positive blood donors were also positive for HBs antibodies. A study conducted in Italy by Manzini et al[38] detected HBV-DNA among donors with an anti-HBs titer > 100 IU/L. They raise doubt about whether high titer anti-HBs blood may guarantee against HBV transmission, where countries such as Germany, Austria and Japan allow transfusion of units with anti-HBs titers higher than 100 IU/L[38].
Allain et al[11] reported that average HBV-DNA detection rates of 7% and 13% were observed in anti-HBc positive subjects with or without anti-HBs respectively, and in blood donors the rates ranged from 0% to 17%. Cases carrying anti-HBc alone are more infectious than those with low level of anti-HBs[41].
As for other viral infections, HBV infectivity depends on three main factors: the infectious dose, the level of immune complexing by neutralizing anti-HBs, and the immunocompetence of the host[45]. It is of note that proper donor selection, using a highly sensitive assay to rule out suspicious blood units together with application of quality control measures will aid in blood safety. In addition, it is important to measure the incidence rate of HBV infections in donor groups and to determine the major risk factors that cause an HBV infection in each country[46].
In 2008, an expert report meeting introduced a cutoff value for serum HBV DNA < 200 IU/mL for OBI[47]. In the current study, nearly half of HBV DNA viremic blood donors (27/53) were having DNA level ≥ 200 IU/mL. This could be possibly attributed to escape mutations which could have altered the target epitope(s) of the HBsAg assay[48]. A recent study of OBI in Asian blood donors showed a viral load range between unquantifiable and 3670 IU/mL with a median 11 IU/mL[49]. In humans, HBV transmission was reported from donors in the window period and OBI donors showing HBV DNA load < 20 IU/mL[7,34,50].
There is preliminary evidence that immunocompromised patients are not only more susceptible to lower infectious dose in the presence of anti-HBs but also at higher risk of developing chronic infection[51]. The prevalence of occult HBV in children and adolescents with haematological disorders and malignancies was 21%, with a significantly increased frequency of HBV-DNA in the HBsAg negative (HCV-RNA positive-63.2%) compared with patients negative for HCV-RNA (25%)[52].
In the current study, none of the followed up recipients showed HBV infection when their sera were examined for HBV markers/HBV DNA within a period of 3-6 mo and none seroconverted to HBsAg positivity. These results are comparable to a recent Taiwanese study[53]. Tani et al[54] identified one case of post-transfusion HBV infection (rate, 0·0004675; 95%CI for the risk of transmission, 1 in 451-41 841). The background rates of HBV, infections in patients prior to transfusion was 3·4% (72/2139), Sixty-four anti-HBc- and/or anti-HBs-reactive blood components were transfused to 52 patients non-reactive for anti-HBc or anti-HBs before and after transfusion (rate, 0; 95%CI for the risk of transmission, < 1 in 22).
As confirmed by Prati et al[55], nosocomial sources should be carefully excluded before speculating that blood donors with OBI were involved in viral transmission. A decrease of HBV infection incidence was observed following HBV vaccine implementation in many countries with moderate to high HBV endemicity[56,57].
In conclusion, on the basis of available data, our findings proved that OBI exists among Egyptian blood donors. Thorough understanding of the infectivity of OBI in immunocompetent and immunosuppressed recipients and molecular characterization by sequencing is strongly indicated. New screening policy to further increase the safety of blood transfusion and exclude all HBV DNA-positive donations has to be thoroughly evaluated. The cost effectiveness of eliminating potentially infectious donors with OBI by further assays is a major concern and has to be investigated.
The study, however, has some limitations. The post-transfusion follow-up schedule was not adequately fulfilled, as the sample size of recipients was small. It is of note that iatrogenic sources of infection have to be excluded by adequate donor follow-up together with pre- and post-transfusion testing of recipients. Molecular analysis by sequencing of virus infecting both donor and recipient is required for confirmation of transmission and consequence infectivity. HBV-anti core screening would possibly eliminate potentially infectious blood donations. Nucleic acid amplification should be considered as the primary screening method for high risk recipients. The provided data encourage further studies aimed at preventing HBV spread where specific management strategy for OBI should be implemented.
These investigations received technical and financial support from the joint WHO Eastern Mediterranean Region, Division of Communicable Disease and the WHO special program for Research and Training in Tropical Diseases: The EMRO/TDR Small Grants Scheme for Operational research in Tropical and other communicable Disease. Also, Real time PCR is cofunded by QIAGEN through its distributor in Egypt.
Among the important clinical impacts of occult hepatitis B virus (HBV) infection (OBI) is transmission of HBV from OBI donors to recipients. Proper diagnosis will promote safety blood donation especially for high risk recipients.
Hepatitis is a major worldwide public health problem. World Health Organization reports considered Egypt as an area of high hepatitis C virus (HCV) prevalence but of intermediate prevalence for HBV. The question is how a single environment like Egyptian environment promotes infection with HCV but not HBV, both have the same route of transmission, even though, the concentration of HBV in serum is much higher than that of HCV. Occult HBV infection has been reported in several clinical entities. Therefore, we conducted a series of researches to study the situation of occult HBV infection in Egypt (Chronic liver disease patients, polytransfused children and renal dialysis patients), and we come to conclusion that occult HBV do exist in our community. Blood donors with OBI have the risk of HBV transmission to their corresponding recipients. HBV transmission was previously reported from OBI donors who had circulating HBV DNA at a low level. Thus this study was carried out to identify apparently healthy blood donors with OBI in order to promote safe blood donation. The possibility of transmission of such infection through blood donation was also evaluated.
Among those who were positive for anti-HBs. Detection of HBV DNA does not always translated into infectivity.
The cost effectiveness of eliminating potentially infectious donors with OBI by further assays is a major concern and has to be investigated. New screening policy to further increase the safety of blood transfusion and exclude all HBV DNA-positive donations has to be thoroughly evaluated. Thorough understanding of the infectivity of OBI in immunocompetent and immunosuppressed recipients and molecular characterization by sequencing is strongly indicated.
The authors addressed an interesting topic “Occult hepatitis B virus infection among egyptian blood donors”. The manuscript is well written, and the manuscript is acceptable for publication.
P- Reviewer Huang CH S- Editor Cheng JX L- Editor A E- Editor Yan JL
| 1. | Brojer E, Grabarczyk P, Liszewski G, Mikulska M, Allain JP, Letowska M. Characterization of HBV DNA+/HBsAg- blood donors in Poland identified by triplex NAT. Hepatology. 2006;44:1666-1674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Available from: www.who.int. [Cited in This Article: ] |
| 3. | Liu Y, Li P, Li C, Zhou J, Wu C, Zhou YH. Detection of hepatitis B virus DNA among accepted blood donors in Nanjing, China. Virol J. 2010;7:193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Niederhauser C, Mansouri Taleghani B, Graziani M, Stolz M, Tinguely C, Schneider P. Blood donor screening: how to decrease the risk of transfusion-transmitted hepatitis B virus? Swiss Med Wkly. 2008;138:134-141. [PubMed] [Cited in This Article: ] |
| 5. | Calderón GM, González-Velázquez F, González-Bonilla CR, Novelo-Garza B, Terrazas JJ, Martínez-Rodríguez ML, Cortés-Márquez SR, Blanco-Flores JP, Rodríguez-Rodríguez A, Del Campo MA. Prevalence and risk factors of hepatitis C virus, hepatitis B virus, and human immunodeficiency virus in multiply transfused recipients in Mexico. Transfusion. 2009;49:2200-2207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Kafi-abad SA, Rezvan H, Abolghasemi H, Talebian A. Prevalence and trends of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus among blood donors in Iran, 2004 through 2007. Transfusion. 2009;49:2214-2220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Candotti D, Allain JP. Transfusion-transmitted hepatitis B virus infection. J Hepatol. 2009;51:798-809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Biswas R, Tabor E, Hsia CC, Wright DJ, Laycock ME, Fiebig EW, Peddada L, Smith R, Schreiber GB, Epstein JS. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion. 2003;43:788-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Scheiblauer H, Soboll H, Nick S. Evaluation of 17 CE-marked HBsAg assays with respect to clinical sensitivity, analytical sensitivity, and hepatitis B virus mutant detection. J Med Virol. 2006;78 Suppl 1:S66-S70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. |
Tabor E, Hoofnagle JH, Smallwood LA, Drucker JA, Pineda-Tamondong GC, Ni LY, Greenwalt TJ, Barker LF, Gerety RJ; Studies of donors who transmit posttransfusion hepatitis. |
| 11. | Allain JP. Occult hepatitis B virus infection: implications in transfusion. Vox Sang. 2004;86:83-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Katsoulidou A, Paraskevis D, Magiorkinis E, Moschidis Z, Haida C, Hatzitheodorou E, Varaklioti A, Karafoulidou A, Hatzitaki M, Kavallierou L. Molecular characterization of occult hepatitis B cases in Greek blood donors. J Med Virol. 2009;81:815-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Hollinger FB, Sood G. Occult hepatitis B virus infection: a covert operation. J Viral Hepat. 2010;17:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Allain JP. Occult hepatitis B virus infection. Hepatitis B Annual. 2009;2: 14-30. [Cited in This Article: ] |
| 15. | Said ZN. An overview of occult hepatitis B virus infection. World J Gastroenterol. 2011;17:1927-1938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 112] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (2)] |
| 16. | Chemin I, Trépo C. Clinical impact of occult HBV infections. J Clin Virol. 2005;34 Suppl 1:S15-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Levicnik-Stezinar S, Rahne-Potokar U, Candotti D, Lelie N, Allain JP. Anti-HBs positive occult hepatitis B virus carrier blood infectious in two transfusion recipients. J Hepatol. 2008;48:1022-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 18. | Hollinger FB. Hepatitis B virus infection and transfusion medicine: science and the occult. Transfusion. 2008;48:1001-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Panigrahi R, Biswas A, Datta S, Banerjee A, Chandra PK, Mahapatra PK, Patnaik B, Chakrabarti S, Chakravarty R. Anti-hepatitis B core antigen testing with detection and characterization of occult hepatitis B virus by an in-house nucleic acid testing among blood donors in Behrampur, Ganjam, Orissa in southeastern India: implications for transfusion. Virol J. 2010;7:204. [PubMed] [Cited in This Article: ] |
| 20. | Baylis SA, Heath AB, Chudy M, Pisani G, Klotz A, Kerby S, Gerlich W. An international collaborative study to establish the 2nd World Health Organization International Standard for hepatitis B virus DNA nucleic acid amplification technology-based assays. Vox Sang. 2008;94:358-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Dow BC, Peterkin MA, Green RH, Cameron SO. Hepatitis B virus transmission by blood donation negative for hepatitis B surface antigen, antibody to HBsAg, antibody to hepatitis B core antigen and HBV DNA. Vox Sang. 2001;81:140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Weber B, Dengler T, Berger A, Doerr HW, Rabenau H. Evaluation of two new automated assays for hepatitis B virus surface antigen (HBsAg) detection: IMMULITE HBsAg and IMMULITE 2000 HBsAg. J Clin Microbiol. 2003;41:135-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Olinger CM, Weber B, Otegbayo JA, Ammerlaan W, van der Taelem-Brulé N, Muller CP. Hepatitis B virus genotype E surface antigen detection with different immunoassays and diagnostic impact of mutations in the preS/S gene. Med Microbiol Immunol. 2007;196:247-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Zervou EK, Dalekos GN, Boumba DS, Tsianos EV. Value of anti-HBc screening of blood donors for prevention of HBV infection: results of a 3-year prospective study in Northwestern Greece. Transfusion. 2001;41:652-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Bernvil SS, Andrews V, Kuhns MC, McNamara AL. Hepatitis B core antigen antibody as an indicator of a low grade carrier state for hepatitis B virus in a Saudi Arabian blood donor population. Transfus Sci. 1997;18:49-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | El-Zayadi AR, Ibrahim EH, Badran HM, Saeid A, Moneib NA, Shemis MA, Abdel-Sattar RM, Ahmady AM, El-Nakeeb A. Anti-HBc screening in Egyptian blood donors reduces the risk of hepatitis B virus transmission. Transfus Med. 2008;18:55-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Antar W, El-Shokry MH, Abd El Hamid WA, Helmy MF. Significance of detecting anti-HBc among Egyptian male blood donors negative for HBsAg. Transfus Med. 2010;20:409-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 28. | Yotsuyanagi H, Yasuda K, Moriya K, Shintani Y, Fujie H, Tsutsumi T, Nojiri N, Juji T, Hoshino H, Shimoda K. Frequent presence of HBV in the sera of HBsAg-negative, anti-HBc-positive blood donors. Transfusion. 2001;41:1093-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Hennig H, Puchta I, Luhm J, Schlenke P, Goerg S, Kirchner H. Frequency and load of hepatitis B virus DNA in first-time blood donors with antibodies to hepatitis B core antigen. Blood. 2002;100:2637-2641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Dreier J, Kröger M, Diekmann J, Götting C, Kleesiek K. Low-level viraemia of hepatitis B virus in an anti-HBc- and anti-HBs-positive blood donor. Transfus Med. 2004;14:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Allain JP, Candotti D, Soldan K, Sarkodie F, Phelps B, Giachetti C, Shyamala V, Yeboah F, Anokwa M, Owusu-Ofori S. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood. 2003;101:2419-2425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Katz L, Strong DM, Tegtmeier G, Stramer S. Performance of an algorithm for the reentry of volunteer blood donors deferred due to false-positive test results for antibody to hepatitis B core antigen. Transfusion. 2008;48:2315-2322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Candotti D, Grabarczyk P, Ghiazza P, Roig R, Casamitjana N, Iudicone P, Schmidt M, Bird A, Crookes R, Brojer E. Characterization of occult hepatitis B virus from blood donors carrying genotype A2 or genotype D strains. J Hepatol. 2008;49:537-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Satake M, Taira R, Yugi H, Hino S, Kanemitsu K, Ikeda H, Tadokoro K. Infectivity of blood components with low hepatitis B virus DNA levels identified in a lookback program. Transfusion. 2007;47:1197-1205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Prince AM, Lee DH, Brotman B. Infectivity of blood from PCR-positive, HBsAg-negative, anti-HBs-positive cases of resolved hepatitis B infection. Transfusion. 2001;41:329-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Launay O, Masurel J, Servant-Delmas A, Basse-Guérineau AL, Méritet JF, Laperche S, Sogni P, Rosenberg AR. High levels of serum hepatitis B virus DNA in patients with ‘anti-HBc alone’: role of HBsAg mutants. J Viral Hepat. 2011;18:721-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Ramia S, Ramlawi F, Kanaan M, Klayme S, Naman R. Frequency and significance of antibodies against hepatitis B core (anti-HBc) antigen as the only serological marker for hepatitis B infection in Lebanese blood donors. Epidemiol Infect. 2005;133:695-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Manzini P, Girotto M, Borsotti R, Giachino O, Guaschino R, Lanteri M, Testa D, Ghiazza P, Vacchini M, Danielle F. Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica. 2007;92:1664-1670. [PubMed] [Cited in This Article: ] |
| 39. | Stramer SL. Pooled hepatitis B virus DNA testing by nucleic acid amplification: implementation or not. Transfusion. 2005;45:1242-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Minuk GY, Sun DF, Uhanova J, Zhang M, Caouette S, Nicolle LE, Gutkin A, Doucette K, Martin B, Giulivi A. Occult hepatitis B virus infection in a North American community-based population. J Hepatol. 2005;42:480-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Allain JP, Cox L. Challenges in hepatitis B detection among blood donors. Curr Opin Hematol. 2011;18:461-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Paez Jimenez A, El-Din NS, El-Hoseiny M, El-Daly M, Abdel-Hamid M, El Aidi S, Sultan Y, El-Sayed N, Mohamed MK, Fontanet A. Community transmission of hepatitis B virus in Egypt: results from a case-control study in Greater Cairo. Int J Epidemiol. 2009;38:757-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Bovet P, Yersin C, Herminie P, Lavanchy D, Frei PC. Decrease in the prevalence of hepatitis B and a low prevalence of hepatitis C virus infections in the general population of the Seychelles. Bull World Health Organ. 1999;77:923-928 [10612888]. [Cited in This Article: ] |
| 44. | Camejo MI, Mata G, Díaz M. [Prevalence of hepatitis B, hepatitis C and syphilis in female sex workers in Venezuela]. Rev Saude Publica. 2003;37:339-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Liu CJ, Chen DS, Chen PJ. Epidemiology of HBV infection in Asian blood donors: emphasis on occult HBV infection and the role of NAT. J Clin Virol. 2006;36 Suppl 1:S33-S44. [PubMed] [Cited in This Article: ] |
| 46. | Yugi H, Mizui M, Tanaka J, Yoshizawa H. Hepatitis B virus (HBV) screening strategy to ensure the safety of blood for transfusion through a combination of immunological testing and nucleic acid amplification testing - Japanese experience. J Clin Virol. 2006;36 Suppl 1:S56-S64. [PubMed] [Cited in This Article: ] |
| 47. | Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, Craxì A, Donato F, Ferrari C, Gaeta GB. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 574] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 48. | Bremer CM, Saniewski M, Wend UC, Torres P, Lelie N, Gerlich WH, Glebe D. Transient occult hepatitis B virus infection in a blood donor with high viremia. Transfusion. 2009;49:1621-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Candotti D, Lin CK, Belkhiri D, Sakuldamrongpanich T, Biswas S, Lin S, Teo D, Ayob Y, Allain JP. Occult hepatitis B infection in blood donors from South East Asia: molecular characterisation and potential mechanisms of occurrence. Gut. 2012;61:1744-1753. [PubMed] [Cited in This Article: ] |
| 50. | Inaba S, Ito A, Miyata Y, Ishii H, Kajimoto S, Tanaka M, Maruta A, Saito S, Yugi H, Hino M. Individual nucleic amplification technology does not prevent all hepatitis B virus transmission by blood transfusion. Transfusion. 2006;46:2028-2029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Roche B, Feray C, Gigou M, Roque-Afonso AM, Arulnaden JL, Delvart V, Dussaix E, Guettier C, Bismuth H, Samuel D. HBV DNA persistence 10 years after liver transplantation despite successful anti-HBS passive immunoprophylaxis. Hepatology. 2003;38:86-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 52. | Said ZN, El-Sayed MH, El-Bishbishi IA, El-Fouhil DF, Abdel-Rheem SE, El-Abedin MZ, Salama II. High prevalence of occult hepatitis B in hepatitis C-infected Egyptian children with haematological disorders and malignancies. Liver Int. 2009;29:518-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Su TH, Chen PJ, Chen TC, Cheng HR, Li L, Lin KS, Kao JH, Chen DS, Liu CJ. The clinical significance of occult hepatitis B transfusion in Taiwan--a look-back study. Transfus Med. 2011;21:33-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Tani Y, Aso H, Matsukura H, Tadokoro K, Tamori A, Nishiguchi S, Yoshizawa H, Shibata H. Significant background rates of HBV and HCV infections in patients and risks of blood transfusion from donors with low anti-HBc titres or high anti-HBc titres with high anti-HBs titres in Japan: a prospective, individual NAT study of transfusion-transmitted HBV, HCV and HIV infections. Vox Sang. 2012;102:285-293. [PubMed] [Cited in This Article: ] |
| 55. | Prati D, Gerosa A, Porretti L. Occult HBV infection and blood transfusion. J Hepatol. 2006;44:818; author reply 819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Ni YH, Huang LM, Chang MH, Yen CJ, Lu CY, You SL, Kao JH, Lin YC, Chen HL, Hsu HY. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology. 2007;132:1287-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 57. | Nardone A, Anastassopoulou CG, Theeten H, Kriz B, Davidkin I, Thierfelder W, O’Flanagan D, Bruzzone B, Mossong J, Boot HJ. A comparison of hepatitis B seroepidemiology in ten European countries. Epidemiol Infect. 2009;137:961-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |